-
Medical journals
- Career
The inhibition activity of selected ß‑carboline alkaloids on enzymes of acetylcholinesterase and butyrylcholinesterase
Authors: Zuzana Kršková; Jan Martin; Jaroslav Dušek
Authors‘ workplace: Department of Pharmacognosy, Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Czech Republic
Published in: Čes. slov. Farm., 2011; 60, 125-131
Category: Original Articles
Overview
This thesis deals with testing of inhibition activity ß-carboline alkaloids on activity of enzymes acetylcholinesterase (ACHE) and butyrylcholinesterase (BUCHE) using test „Fast Blue B salt“ at TLC desk and Ellman’s test using spectrophotometer. It was also investigated how dimethylsulfoxide used as a solvent in combination with water affects activity of enzymes and alkaloids. Results show harmine in form of base and salt in water and in mixture of DMSO and water has the hightest inhibition activity on ACHE using eserine as reference substance. Harmalol in form of salt in water and harmine in form of base and salt in mixture of DMSO and water has the hightest activity on BUCHE. It was find out that DMSO considerably affects activity of enzymes and alkaloids.
Key words:
ß-carboline alkaloids – ACHE – BUCHE – DMSO – Ellmanęs test – test „Fast Blue B salt“Introduction
The inhibitors of acetycholinesterase are substances used recently to treat primarily Alzheimer’s disease. The occurrence of this disease in human population on increase and mainly older people of the age of 65 and up are affected. Alzheimer’s disease is accompanied by deficits and failures of many neurotransmitter systems, namely of the cholinergic system. At present, the inhibitors of acetylcholinesterase (ACHE) and butyrylcholinesterase (BUCHE) appear to be the most promising therapeutic substances. Not till long ago, the ideal inhibitors were considered those that selectively block the ACHE function, easily permeate the brain-barrier membrane and, at the same time, they do not inhibit the peripheral BUCHE since that could bring undesirable side effects such as cramps. The latest research shows that in the last stages of Alzheimer’s disease the level of butyrylcholinesterase is increasing; therefore the patient in this stage may benefit from the inhibition activity on butyrylcholinesterase 1, 2).
Many inhibitors used in therapy have their disadvantages. These disadvantages include either too short or too long duration of the half–life period, skeletal muscles cramps, and interaction with other medicaments regarding their metabolism in liver 3). The discovery of new structures is thus still a pressing topic. ß-carboline alkaloids are one of the groups under research.
ß-carboline alkaloids are abundant group of substances that are present in various plant families, for instance in the Apocynaceae, Eleagnaceae, Fabaceae, Passifloraceae, Zygophyllaceae families 4). These alkaloids are also sometimes called harmala alkaloids because they were first isolated from the Peganum harmala plant (Zygophyllaceae) 1). In addition to their occurrence in plants, ß-carboline alkaloids can also be found in cigarette smoke, grilled food, and wine 5–8). Some of them are even commonly present in human and animal tissues and body fluids. They can be found in cerebral cortex and other brain tissues, in liver, and in adrenal gland 9, 10).
ß-carboline alkaloids have wide spectrum of effects, namely on muscular, cardio-vascular, and central nervous systems. These include the inhibition of enzymes (for instance, of acetylcholinesterases and butyrylcholinesterases and monoaminooxidases A and B), also the bond to different types of receptors (for instance, benzodiazepine, serotonine and imidazoline receptors) which is related to the convulsive and anticonvulsive effects, to the anxiolytic and tremorgen effects; they are also able to intercalate into DNA, cause mutations in various types of organisms; their antioxidant, neuroprotective and immunomodulatory effects must not be left out to mention here either 11).
The families of plants which contain ß-carboline alkaloids and are used for therapeutic purposes include Anadenanthera spp. (Fabaceae), Banisteriopsis spp., Terapteris spp. (Malpighiaceae) and Passiflora spp. (Passifloraceae). In Brazil, they are used for their antispastic and sedative effects 12). The Desmodium spp. (Fabaceae) family of plants that contain ß-carboline alkaloids is used in traditional Indian medicine to treat eye ailments and intestinal malnutrition 1). In addition to their therapeutic effects, some drugs that contain ß carboline alkaloids are used in South America and Africa to enhance the effects of hallucinogens during religious ceremonies. To name one of the hallucination-inducing venues, it would be drink called „ayahuasca“ which is prepared from rainforest lianas Banisteriopsis caapi (Malpighiaceae) and Psychotria viridis (Rubiaceae). ß-carboline alkaloids themselves probably also have certain hallucinogenic effects 13–15).
The objective of this work was to find out the inhibition activity of selected ß-carboline alkaloids on acetylcholinesterases and butyrylcholinesterases while using eserine (physostigmine) as reference inhibitor. At the same time, we studied how dimethyl sulfoxide-water solution, used as dissolvent of alkaloids, influenced the inhibition activity. Two tests – “Fast Blue B Salt” and “Ellman’s Test” – were performed to determine the inhibition activities of substances on ACHE and BUCHE.
EXPERIMENTAL PART
Chemicals
- acetylcholinesterase from Electrophorus electricus (electric eel) (0,77 mg, 658 U.mg-1, 1210 U.mg-1 of protein, type V-S), acetylthiocholine iodide (ATCHI) ≥ 99 %, 1-naphthyl acetate ≥ 98%, bovine serum albumin (BSA) 98%, butyrylcholinesterase from equine serum (526,32 mg, 11,4 U.mg-1, 19 U.mg-1 of protein), butyrylthiocholine iodide (BUTCHI) ≥ 98%, dimethylsulfoxide (DMSO) ≥ 99,6%, 5,5’-dithiobis(2-nitrobenzoic acid) (DTNB) 99 %, eserine, eserine salicylate ≥ 97%, „Fast Blue B salt“ 95%, harmaline p. a., harmaline hydrochloride 95%, harmane 98%, harmine ≥ 98%, harmalol hydrochloride dihydrate ≥ 98%, norharmane crystalline, norharmane hydrochloride crystalline: Sigma-Aldrich Chemie, Steinheim;
- acetone p. a., potassium phosphate p. a., monosodium phosphate dodecahydrate p. a., ethanol 96%, potassium iodide p. a., hydrochloric acid 36%, acetic acid 99%, methanol p. a., sodium acetate anhydrous p. a.: Penta, Chrudim;
- bismuth nitrate basic p. a.: Lach - Ner, s. r. o., Neratovice;
- (harmane hydrochloride and harmine hydrochloride salts were not commercially available; they were thus prepared from harmane and harmine in laboratory).
Preparation of solutions
The selection of concentrations of solutions was based on experiments that had been performed by the teams of Martson A. et al. 16), Nino J. et al. 17) , and Darvesh S. et al. 18).
To perform the “Fast Blue B Salt” test, the alkaloids (eserine, harmane, harmine, harmaline, harmalol hydrochloride, and norharmane) were dissolved in methanol. To determine the inhibition activity during the Ellman’s test, the alkaloids (eserine, eserine salicylate, harmane, harmane hydrochloride, harmine, harmine hydrochloride, harmaline, harmaline hydrochloride, harmalol hydrochloride, norharmane, and norharmane hydrochloride) were dissolved in water (alkaloid salts) or in the DMSO and water solution in the ratio of 5 ml DMSO to 2.6 ml of water (salts and bases). The selection of this ratio was based on the solubility test of individual alkaloids and the ratio for the least soluble alkaloid (harmane) was used. The highest concentration of solution used was 0.01 M and other concentrations were diluted either with water or with DMSO/water solution; the decisions were based on results that were used to prepare the inhibition activity curve.
The “Fast Blue B Salt” detection solution was prepared as follows: 62.5 mg 1-naphtyl acetate was dissolved in 25 ml of ethanol. 100 mg of “Fast Blue B Salt” was dissolved in 40 ml distilled water. 5 ml of 1 naphtyl acetate solution and 20 ml of “Fast Blue B Salt” was mixed just before application to the TLC plate 16).
ATCHI and BUTCHI solutions of the concentration of 14 mM were prepared by dissolving ATCHI and BUTCHI in distilled water. DNTB solution of the concentration of 3 mM was prepared by dissolving DTNB and CH3COON additive in distilled water.
ACHE and BUCHE were dissolved in pH 7.9 phosphate buffer and the same buffer solution was used to dilute them further. The buffer solution that was used to work with ACHE was further enriched with BSA in the concentration of 1 mg.ml-1 to maintain the stability of enzyme. ACHE as well as BUCHE concentrations of 3.38 U.ml-1 were used during the “Fast Blue B Salt” test. In case of Ellman test, the concentration of ACHE was 0.3 U.ml-1 and BUCHE was 0.5 U.ml-1.
Dragendorff reagent to detect alkaloids was prepared as follows:
Basic solution: 0.85 mg of bismuth nitrate basic was dissolved in mixture of 40 ml of distilled water and 10 ml of concentrated acetic acid. Later, 50 ml of KI (500 g.l-1) was added and agitated until dissolved. At the time of use, 1 ml of the basic solution was mixed with 2 ml of concentrated acetic acid and with 10 ml of distilled water 19).
Procedures
“Fast Blue B Salt” test
Two TLC plates were prepared. Both were first developed with acetone and then they were freely let dry in the air. The solution of eserine was applied with microsyringe on the first plate and the solutions of other alkaloids on the other plate. To achieve the results, the first plate contained eserine in four different amounts (1–0.001 μg) and the other plate held 20 μg of harmane, norharmane, harmine, harmaline, and harmalol hydrochloride. The plates were then allowed to develop in the CHCl3 system: MeOH in the ratio of 9 : 1. The solvent was let to evaporate freely. Then the ACHE (BUCHE) solution, tempered in incubator to 30 °C, was applied and the plates were let dry freely at the room temperature for 5 minutes. After that, the detection solution which consisted of solutions of naphthyl acetate and „Fast Blue B Salt” was applied. The results were taken after 3 minutes – after the reaction, the plate changed color to purple and the determination of white spots in places where alkaloids were located was carried out 16).
Ellman’s test
The inhibition activity of control was measured first. 150 μl ATCHI (BUTCHI), 750 μl DTNB and 150 μl of the tested alkaloid were pipetted into test tube as needed in different concentrations. At the same time, another test tube was filled with control solution that contained 150 μl ATCHI (BUTCHI), 750 μl DTNB, and 150 μl of solvent of alkaloid (water or DMSO/water mixture). The contents of both test tubes were allowed to incubate at 30 °C in an incubator. Then 150 μl of ACHE (BUCHE) was added into the first test tube and 150 μl of buffer into the other one. The solutions were transferred into semi-microcells and the change in the absorbance of solutions at 412 nm was measured during 3 minutes span of time.
Similar method was used to measure the inhibition activity of samples. 150 μl ATCHI (BUTCHI), 750 μl DTNB and 150 μl of the tested alkaloid solution was pipetted into both test tubes. After 15 minutes of incubation in an incubator, 150 μl of ACHE (BUCHE) was added into the first test tube and 150 μl of buffer into the other one. The solutions were transferred into semi-microcells and the change in the absorbance of solutions at 412 nm was measured during 3 minutes span of time.
For every alkaloid sample, there was a control measurement with water. 150 μl ATCHI (BUTCHI), 750 μl DTNB and 150 μl of the highest alkaloid concentration used was pipetted into test tube number one. 1200 μl of distilled water was pipetted into test tube number two. After 15 minutes of incubation in the incubator, 150 μl of buffer was added to the test tube number one and 150 μl of distilled water into test tube number two; the solutions were transferred to semi-microcells and the absorbance at 412 nm was measured again for the duration of 3 minutes 17).
The results were depicted in graphs, the inhibition curves of individual alkaloids were created and GrafPad sofware was used to read the IC50 values.
RESULTS
The inhibition activity of each control and alkaloid concentration in the Ellman’s test was measured, in total, three times. At the end of each measuring procedure, the activity of control was measured again to verify the stability of the enzyme. The IC50 values of individual alkaloids are listed in Tables 1 and 2.
1. IC<sub>50</sub> of measured alkaloids for ACHE inhibition 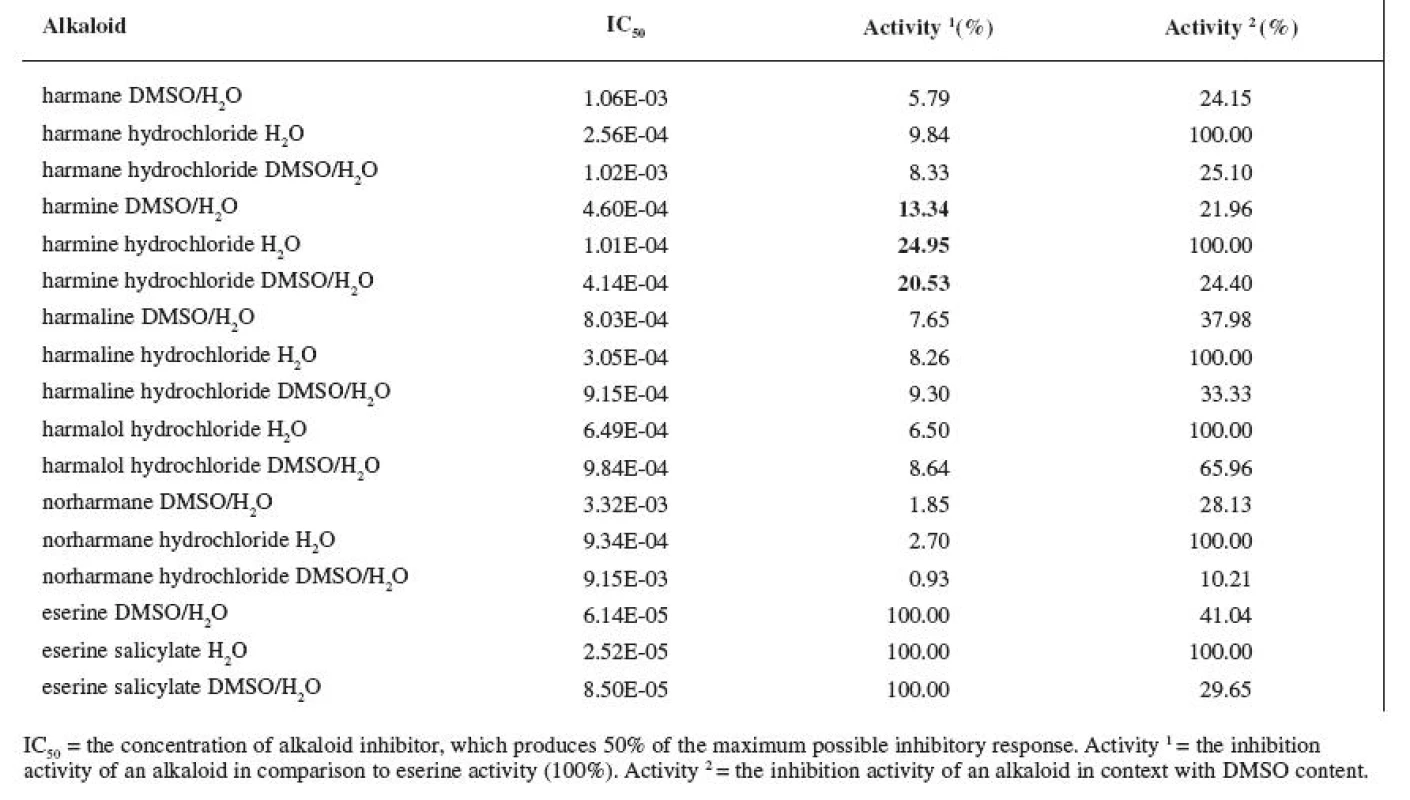
2. IC<sub>50</sub> of measured alkaloids for BUCHE inhibition 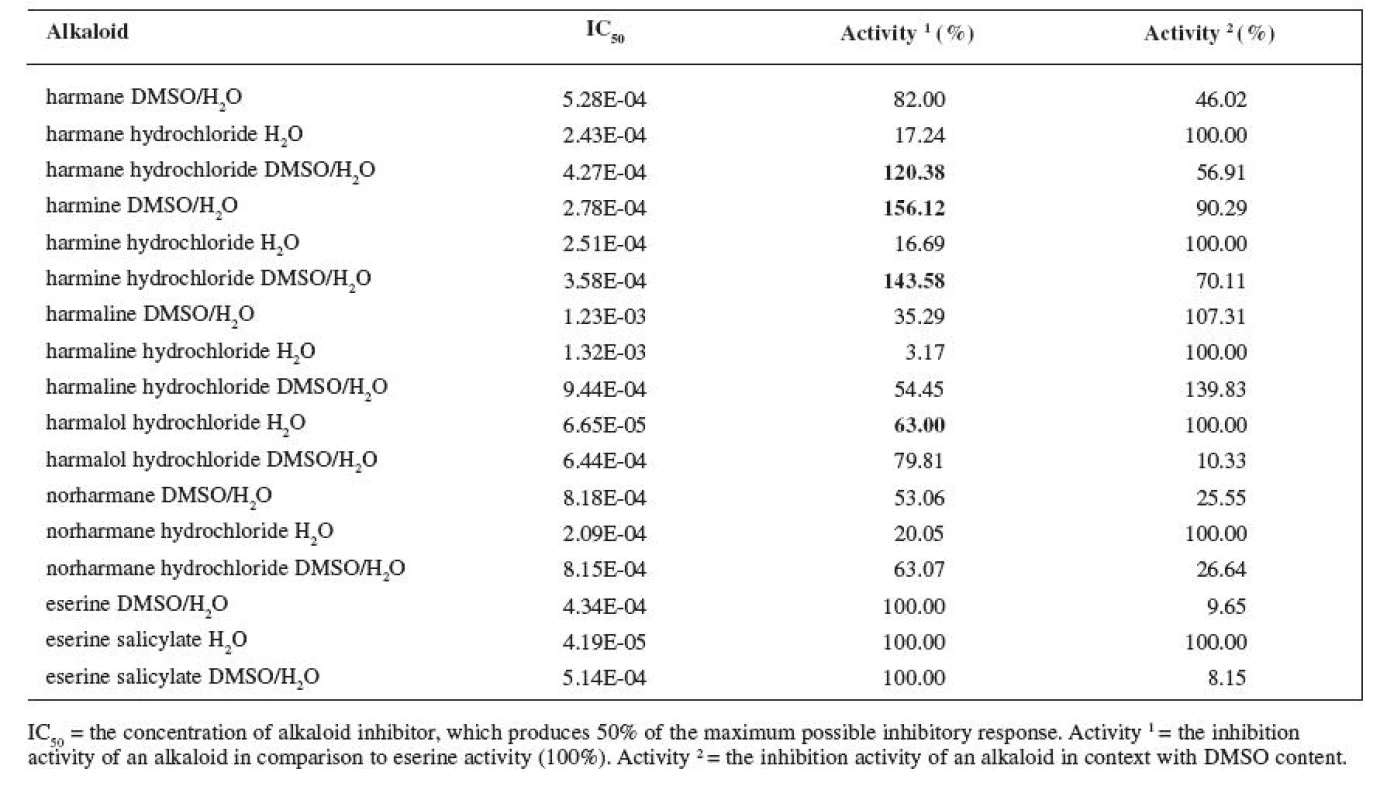
DISCUSSION
„Fast Blue B salt“ – TLC method was used in the initial, orientation detection of tested alkaloids. In the event that the tested substance possesses inhibition activity, it will show as a white spot on purple background of the TLC plate.
The sensitivity of reference inhibitor – in our case it was eserine (physostigmine) – was determined first. The inhibition activity results indicated that the inhibition activity of ACHE and BUCHE was still TLC noticeable at 0.01 μg of eserine on the plate. Then the inhibition activity of individual alkaloids was tested. Harman, norharman, harmine, and harmalol alkaloids showed weak inhibition activity on BUCHE; harmaline in the concentration used did not show any activity. The detection of alkaloid activity on ACHE was somehow more difficult; the reagents did not produce unbroken, coherent purple color. Nevertheless, white spots were noticeable in places where there were TLC alkaloids. However, it cannot be stated with certainty that this was indeed due to the enzyme inhibition.
The detection with Dragendorff reagent confirmed that the ACHE and BUCHE inhibition had indeed been caused by alkaloids. The alkaloids changed color to orange after having been sprayed with the detection reagent.
Next step was testing the activity of extracts and alkaloids in modified Ellman’s test in which a spectrophotometer was used. The reaction of reagents without the addition of inhibitors shows as yellow color of the solutions. The spectrophotometer measures the change in the solution absorbance which corresponds with the velocity of hydrolysis of the substrate catalyzed with enzyme. In the event that the solution is enriched with an inhibitor of enzyme, the hydrolysis is lesser and the resulting value of the absorbance change is lower. All alkaloids were tested in spite of the fact that not all of them showed inhibition activity on the TLC plate during the “Fast Blue B Salt” test.
Eserine was used as reference substance in determining the alkaloid inhibition activity in the Ellman’s test. We tested the activity of alkaloid bases dissolved in DMSO/H2O mixture. DMSO is one of organic solvents that are commonly used in Ellman’s tests. However, there was a discovery indicating that it does not only influence the activity of enzyme but also the alkaloids themselves 20). Part of this work was to determine how DMSO influences the inhibition activity in case of ß-carboline alkaloids.
The resulting values of the inhibition activity of individual ß-carboline alkaloids on ACHE and BUCHE were then related to eserine whose activity was marked to be 100% (Tables 1 and 2).
First, we carried out the ACHE tests in which we used alkaloid salts dissolved in H2O. The measured value of IC50 eserine on ACHE per the conditions mentioned was 2.52E-05 M. The highest activity recorded was at harmine hydrochloride, IC50 = 1.01E-04 M, which represents 24.95% of the activity of eserine salicylate. The rest of the salts of alkaloids showed activity lower than 10% of the reference substance.
The inhibition activity of eserine in DMSO/H2O on ACHE was measured to be IC50 = 6.14E-05 M. The inhibition activity thus decreased to 41.04% of the original activity of the salt in H2O. Harmine again showed the highest activity, IC50 = 4.60E-04 M, which represents 13.34% of the activity of eserine. The rest of the alkaloid bases again showed lower then 10% of eserine activity. Percent wise, their inhibition activity was even lower in comparison with the activity of salts in H2O.
IC50 of eserine salicylate in DMSO/H2O was 8.50E--05 M, which represents 29.65% of the activity of eserine salicylate in H2O. Harmine again showed the highest activity, IC50 = 4.14E-04 M, which represents 20.53% of the reference substance activity. The inhibition activity of the rest of the alkaloids was again lower than 10% of eserine salicylate activity in DMSO/H2O.
The inhibition activity of eserine as reference substance was lower in all three cases. Of all tested alkaloids, harmine showed the highest inhibition activity. However, its inhibition activity is rather weak for use in AD therapy. The rest of the alkaloids showed activity under 10% in comparison with the reference substance and these values are not very significant either when compared with the inhibition activity of other alkaloids. For instance, alkaloid waltherine, isolated from a Brazilian plant Walteria brachypellata (Sterculiaceae), in 100 μg/ml concentration reaches 72.20% of the activity of eserine 21) and IC50 of huperzine A is three times lower than the one of eserine 22).
The solution to the low activity of ß-carboline alkaloids might rest in changing their basic structures and synthesizing more effective derivates. The research on these issues is currently in progress.
Also M. Skupa’s team was involved in measuring the activity of ß-carboline alkaloids on ACHE. In case of harman, the inhibition activity of 66.6% in the concentration of 1E-04 was determined, and, in case of norharman in the same concentration, it was 59.1%. These results, when compared with the results given in this work, vary quite a bit (the inhibition activity of harman was determined at 30.87%; the one of norharman at 9.33%). The reason for this is probably the fact that acetylcholinesterase isolated from bovine erythrocytes was used (ACHE from Electrophorus electricus was used in this work) and, also, the conditions for reaction were different 23).
The results of the inhibition activities of alkaloids in the “Fast Blue B Salt” test were not conclusively positive. As Ellman’s test showed, the inhibition activity of alkaloids was probably so low that it did not show in the “Fast Blue B Salts” test.
The results of the inhibition of alkaloids with BUCHE were more various. IC50 of eserine salicylate in H2O on BUCHE was measured at 4.19E-05 M. The substance with the highest inhibition activity was harmalol hydrochloride with IC50 6.65E-05 M, which represents 63% of the activity of eserine salicylate. Other alkaloids showed activity around 20% of the activity of eserine salicylate, only harmaline hydrochloride showed mere 3.17%.
IC50 of eserine in DMSO/H2O was 4.34E-04 M, which is 9.65% of activity when compared with eserine salicylate in H2O. IC50 of harmine was even at 2.28E-04 M, which is 156.12% of activity of eserine under same conditions. Other alkaloids showed relatively high activity as well. The lowest activity was observed with harmaline, IC50 = 1.23E-03 M, which represents 35.29% of the activity of eserine.
IC50 of eserine salicylate in DMSO/H2O was 5.14E-04 M, which is 8.15% of the eserine salicylate activity in H2O. Harmine hydrochloride showed the highest activity, IC50 = 3.58E-04 M, which is 143.58% of the activity of eserine salicylate. Harman hydrochloride showed higher activity than eserine salicylate too, 4.27E-04 M, which is 120.38% % of the activity of eserine salicylate. Harmaline hydrochloride had again the lowest activity, IC50 = 9.44E-04 M, which is 54.45% of the activity of eserine salicylate.
Harmalol showed to be the best inhibitor on BUCHE in aqueous environment and harmine in the DMSO/H2O mixture. As it has already been mentioned, the BUCHE inhibition is considered rather undesirable in the AD treatment, with the exception of late stages. The lowest inhibition activity in aqueous environment was recorded at harmaline – as BUCHE inhibitor, it is roughly hundred times poorer than eserine. For the sake of comparison, the inhibition activity of huperzine A is sixty times poorer when compared with eserine 22). Harmaline is thus substance that features low inhibition activity on BUCHE and, at the same time, is, unfortunately, a very poor ACHE inhibitor (8.26% when compared with eserine salicylate). Thus, it is not adequate for standard AD therapy.
The results gained on the inhibition activity of alkaloids on BUCHE in Ellman’s test correspond with the results of orientation inhibition activities on TLC plate during the “Fast Blue B Salt” test. The inhibition activity of harmaline proved to be very poor and this is the reason why this alkaloid did not show inhibition on the TLC plate.
It is evident that the DMSO preparation substantially changed the inhibition activity. DMSO acts as ACHE and BUCHE inhibitor and an increased inhibition activity of substances dissolved in this solvent can be expected. The article by Giovanni S et al. describes the research of the DMSO effect on the inhibition activity of substances. DMSO was proven to act as an inhibitor. DMSO also influenced the activity of added inhibitors. Even at the concentration of 1.6% of DMSO in mixture, the activity of alkaloids was not influenced to a greater extent, nevertheless, there was a slight decrease in IC5020).
Quite opposite effect was observed during the ACHE tests in this work; the DMSO additions increased the IC50 values. The DMSO concentration used in this work amounted to 65.79%. The most stable against the influence of DMSO proved to be harmalol hydrochloride dissolved in DMSO/H2O; the activity decreased, in comparison with harmalol hydrochloride dissolved in plain water, to 65.96%. Harmine, whose activity decreased to 21.96%, was least stable.
The effect described in the article was evident during the BUCHE tests; harmaline increased its inhibition activity (harmaline 107.31%, harmaline hydrochloride in DMSO/H2O 139.83%). Harmaline thus seems to be most resistant against the DMSO influence. The activity of all other alkaloids again decreased; the biggest decrease in activity was recorded at eserine (eserine salicylate in DMSO/H2O 8.15%); the least stable of all tested alkaloids was harmalol hydrochloride whose activity decreased in the DMSO/H2O mixture to 10.33%.
The differences in the activity of bases and salts in the DMSO/H2O were very slight. The differences were more obvious only in cases of norharman and norharman hydrochloride with ACHE; the IC50 value of norharman was 3.32E-03 M and IC50 of norharman hydrochloride was 9.15E-03 M.
The reason for the increase in IC50, which leads to the decrease in the inhibition activity, is probably the fact that DMSO partially decomposed the reaction components (the inhibitor and substrate might be considered).
DMSO, regarding its rather extensive influence on the inhibition activity of ß-carboline alkaloids, was thus proven not to be the most suitable solvent. It would be of an advantage to find a solvent that would influence the inhibition activity of ß-carboline alkaloids to the least possible extent and, at the same time, it would dissolve them well. Alcohol based solvents (methanol and ethanol), or their mixtures with water or other solvents, are an option to be considered.
This study was supported by the Charles University in Prague (Project SVV 2010-261-002) and by MSM 0021620822 research project.
Address for correspondence:
PharmDr. Jan Martin, Ph.D.
Katedra farmakognozie FaF UK
Heyrovského 1203, 500 05 Hradec Králové
e-mail: martin@faf.cuni.cz
Sources
1. Schott, Y. et al.: 6-Hydroxy - and 6-methoxy-ß-carbolines as acetyl - and butyrylcholinesterase inhibitors. Bioorg. Med. Chem. Lett. 2006; 16, 5840–5843.
2. Kamal, M. A. et al.: Kinetic analysis of the inhibition of human butyrylcholinesterase with cymserine. Biochim. Biophys. Act. 2006; 1760, 200–206.
3. Höschl, C. a kol.: Symposium 1 „Alzheimerova choroba“. Praha: Galén 1999; 10 s.
4. Allen, J. R. F., Holmested, B. R.: The simple ß‑carboline alkaloids. Phytochemistry 1980; 19, 1573-1582.
5. Adachi, J. et al.: Determination of ß-carbolines in foodstuffs by high-performance liquid chromatography and high-performance liquid chromatography – mass spectrometry. J. Chromat. A 1991; 538, 331–339.
6. Gross, G. A. et al.: Heterocyclic aromatic amine formation in grilled bacon, beef and fish and in grill scrapings. Carcinogenesis 1993; 14, 2313–2318.
7. Herraiz, T., Huang, Z. and Ough, C. S.: 1,2,3,4--Tetrahydro-ß-carboline-3-carboxylic acid and 1-methyl-1,2,3,4-tetrahydro-ß-carboline-3-carboxylic acid in wines. J. Agric. Food Chem. 1993; 41, 455–459.
8. Totsuka, Y. et al.: Quantification of the co-mutagenic ß-carbolines, norharman and harman, in cigarette smoke condensates and cooked fous. Cancer Lett. 1999; 143, 139–143.
9. Zheng, W. et al.: Determination of Harmane and Harmine in Human Blood Using Reversed-Phased High-Performance Liquid Chromatography and Fluorescence Detection. Anal. Biochem. 2000; 279, 125-–129.
10. May, T. et al.: Comparison of the in vitro binding characteristics of the ß-carbolines harman and norharman in rat brain and liver and in bovine adrenal medulla. Arch. Pharmacol. 1994; 349, 308–317.
11. Moura, D. J. et al.: Antioxidant properties of ß-carboline alkaloids are related to their antimutagenic and antigenotoxic activities. Mutagenesis 2007; 22, 293–302.
12. Jiménez, J. et al.: Cytotoxicity of the ß-carboline alkaloids harmine and harmaline in human cell assays in vitro. Experiment. Toxicol. Pathol. 2008; 60, 381–389.
13. Freedland, C. S. and Mansbach, R. S.: Behavioral profile of constituents in ayahuasca, an Amazonian psychoactive plant mixture. Drug Alcohol Depend. 1999; 54, 183–194.
14. Boeira, J. M. et al.: Genotoxic and recombinogenic activities of the two ß-carboline alkaloids harman and harmine in Saccharomyces cervisie. Mutat. Res. 2002; 500, 39–48.
15. Glennon, R. A. et al.: Binding of ß-carbolines and related agents at serotonin (5-HT2 and 5-HT1A), dopamine (D2) and benzodiazepine receptors. Drug and Alcohol Depend. 2000; 60, 121–132.
16. Martson, A., Kissling, J. and Hostettmann, K.: A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. 2002; 13, 51–54.
17. Nino, J. et al.: In vitro inhibition of acetylcholinesterase by crude plant extracts from Colombian flora. Mem. Inst. Oswaldo Cruz 2006; 101, 783–785.
18. Darvesh, S. et al.: Differential effects of lipid-lowering agents on human cholinesterases. Clin. Biochem. 2004; 37, 42–49.
19. Composite authors: Československý lékopis 4, Praha: Avicenum 1987, 360 s., 370 s.
20. Di Giovanni, S. et al.: In vitro screening assays to identify natural or synthetic acetylcholinesterase inhibitors: Thin layer chromatography versus microplate methods. Eur. J. Pharmacol. Sci. 2008; 33, 109–119.
21. Lima, M. M. C. et al.: Acetylcholinesterase Activity of Alkaloids from the Leaves of Waltheria brachypetala. Planta Med. 2009; 75, 335–337.
22. Ma, X. and Gang, D. R.: The Lycopodium alkaloids. Nat. Prod. Rep. 2004; 21, 752–772.
23. Skup, M., Oderfeld-Nowak, B., Rommelspacher, H.: In Vitro Studies on the Effect of ß-Carbolines on the Activities of Acetylcholinesterase and Choline Acetyltransferase and on the Muscarinic Receptor Binding of the Rat Brain. J. Neurochem. 1983; 41, 62–68.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2011 Issue 3-
All articles in this issue
-
Effects of health-service reform in the field of medicinal drugs
I. Analysis from the standpoint of a pharmacy -
Effects of health-service reform in the field of medicinal drugs
II. Analysis from the standpoint of financial participation of the patient in pharmacotherapy - Clinical significance of cytochrome P450 genetic polymorphism – Part I. Enzymatic system of cytochrome P450 and cytochrome P450 1A2
- Farmaceutické aspekty živočíšnej lipoxygenázy
- Influence of membranes on alaptide permeation from hydrogels
- Synthesis and basic physicochemical properties of 1-[3-(Y-alkoxyphenylcarbamoyloxy)-2--hydroxypropyl]-4-(2-methylphenyl)piperazinium chlorides
- The inhibition activity of selected ß‑carboline alkaloids on enzymes of acetylcholinesterase and butyrylcholinesterase
-
Effects of health-service reform in the field of medicinal drugs
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Clinical significance of cytochrome P450 genetic polymorphism – Part I. Enzymatic system of cytochrome P450 and cytochrome P450 1A2
- Influence of membranes on alaptide permeation from hydrogels
- Farmaceutické aspekty živočíšnej lipoxygenázy
- Synthesis and basic physicochemical properties of 1-[3-(Y-alkoxyphenylcarbamoyloxy)-2--hydroxypropyl]-4-(2-methylphenyl)piperazinium chlorides
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

