-
Medical journals
- Career
COMPUTER TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING OF THE ORBIT IN THE DIAGNOSIS AND TREATMENT OF THYROID-ASSOCIATED ORBITOPATHY – EXPERIENCE FROM PRACTICE. A REVIEW
Authors: M. Karhanová 1,2; J. Čivrný 3,4; J. Kalitová 1; J. Schovánek 5,6; B. Pašková 1,2; Z. Schreiberová 1,2; P. Hübnerová 1,2
Authors‘ workplace: Oční klinika, Fakultní nemocnice Olomouc 1; Oční klinika, Lékařská fakulta Univerzity Palackého Olomouc 2; Radiologická klinika, Fakultní nemocnice Olomouc 3; Radiologická klinika, Lékařská fakulta Univerzity Palackého Olomouc 4; III. interní klinika - nefrologická, revmatologická a endokrinologická, Fakultní nemocnice Olomouc 5; III. interní klinika - nefrologická, revmatologická a endokrinologická, Lékařská fakulta Univerzity Palackého Olomouc 6
Published in: Čes. a slov. Oftal., 3, 2023, No. Ahead of Print, p. 1001-1010
Category: Review Article
Overview
The purpose is to acquaint readers with the contribution of imaging methods (IMs) of the orbit, specifically computer tomography (CT) and magnetic resonance imaging (MRI), in the diagnosis of thyroid-associated orbitopathy (TAO).
Methods: IMs of the orbit are an indispensable accessory in the clinical and laboratory examination of TAO patients. The most frequently used and probably most accessible method is an ultrasound examination of the orbit (US), which, however, has a number of limitations. Other methods are CT and MRI. Based on the published knowledge implemented in our practice and several years of experience with the diagnosis and treatment of TAO patients, we would like to point out the benefits of CT and MRI in the given indications: visualisation of the extraocular muscles, assessment of disease activity, diagnosis of dysthyroid optic neuropathy and differential diagnosis of other pathologies in the orbit. Our recommendation for an ideal MRI protocol for disease activity evaluation is also included.
Conclusion: IMs play an irreplaceable role not only in the early diagnosis of TAO, but also in the monitoring of the disease and the response to the applied treatment. When choosing a suitable IM for this diagnosis, a number of factors must always be taken into account; not only availability, cost and burden for the patient, but especially the sensitivity and specificity of the given method for the diagnosis of TAO.
Keywords:
activity – extraocular muscles – magnetic resonance imaging – thyroid-associated orbitopathy – computer tomography
INTRODUCTION
Thyroid-associated orbitopathy (TAO) is a chronic ocular disease with a demonstrated link to thyroid autoimmunity. The consequences of this disease may be relatively serious, causing a pronounced deterioration of patients’ quality of life. As a result, timely diagnosis of TAO is of major importance for the further course of the disease. However, determining the correct TAO diagnosis from the clinical picture and laboratory values may be challenging. Diagnostic problems appear especially if the condition is markedly asymmetrical, with the affliction of only one eye, and if there is a lack of data about previous or ongoing thyroid disease in the patient’s medical history. In these cases, determining the correct diagnosis is often the result of a relatively extensive investigation, in which valuable assistance is provided by imaging methods (IMs) of the orbit. The advantages and disadvantages of the most commonly used IMs of the orbit in TAO patients are frequently discussed in the literature [1,2]. Ultrasound diagnosis is a quick, non-invasive, and widely used method. It enables not only an assessment of the width of the extraocular muscles but also provides information about the degree of edema in the muscles. However, its disadvantages are low reproducibility and dependency on the examiner's experience [3]. Computed tomography (CT) is a widely available and relatively quick method. However, it represents a burden by exposing the patient to ionizing radiation. The advantage of magnetic resonance imaging (MRI) when compared to CT is better differentiation of the orbital soft-tissue structures, the possibility of evaluating the activity of TAO and the zero-radiation dose. Nevertheless, MRI examination is often more time consuming and more expensive than CT. Scintigraphy of the orbit is used only rather in exceptional cases.
In the overwhelming majority of today’s clinical practice, patients are observed with ultrasonography (US). Nevertheless, US of the orbit and extraocular muscles requires considerable experience, and as a result in practice we often encounter situations in which the ophthalmologist or endrocrinologist refer patients for MRI or CT examination of the orbit. A correctly formulated request, and therefore also choice of IM (examination protocol for IM) should guarantee a clinically satisfactory result.
This review aims to provide readers with a comprehensible overview of the current possibilities of imaging methods of the orbit, specifically CT and MRI, in diagnosing TAO. We shall deal with the issue of US in another, follow-up study. Briefly stated, it is possible to find indications, advantages and disadvantages of both CT and MRI in the diagnosis of TAO in the Recommended Procedure for the Diagnosis and Treatment of Thyroid-Associated Orbitopathy, amendment 3/2022, which is freely available for download on the pages of the Czech Endocrinology Society and Czech Ophthalmology Society of J. E. Purkyně [4,5]. We aim to elaborate upon this issue in further detail, and in particular to highlight the benefits of the individual methods in the given indications: imaging of the width of the extraocular muscles, evaluation of the activity of the disease, diagnosis of possible compression of the optic nerve, differential diagnosis of other pathological conditions within the orbit, and also to draw attention to the limitations of these methods. Also included in the review are our recommendations for an appropriate MRI protocol to evaluate the disease’s activity, which we are also now using in our clinical practice. In the text we start out from both published observations and from long-term experiences with the diagnosis and treatment of TAO at our centre. The text is designated not only for ophthalmologists and endocrinologists, who most often refer patients for these examinations, but also for radiologists, who by choosing the proper examination protocol can make a significant contribution to the successful treatment of this serious pathology.
MAGNETIC RESONANCE IMAGING
Examination principle, used methods
Magnetic resonance imaging (MRI) uses a physical phenomenon known as nuclear magnetic resonance (NMR). This phenomenon has been described in the literature since 1940 [7]. Imaging with nuclear magnetic resonance appeared after 1970, and to ensure better acceptance on the part of the lay public, the word nuclear was omitted, and the title MRI was adopted [7].
In magnetic resonance imaging, we determine changes of the magnetic moment of the nuclei of elements with an odd proton number exposed to a strong static magnetic field followed by the application of radio frequency pulses. As a consequence of the rotation of the atom nuclei around their axis (spin), a magnetic field (magnetic moment) is created around the nuclei with an odd proton number. The hydrogen atom 1H contains a single proton in its nucleus, which is widespread in the human body and is used in MRI. If we insert the examined tissue into a strong external magnetic field, the spins of the protons are arranged in one predominant direction. In this state, the magnetic moment of the protons performs two types of movement – firstly it rotates on its axis (spin), and secondly along the perimeter of an imaginary cone shape, which is referred to as precession. Application of a radiofrequency pulse (electromagnetic wave within a bandwidth of very short radio waves) at a frequency that is identical to the frequency of precession of the proton leads to a development of a physical phenomenon called magnetic resonance. In that case, a deflection of the magnetic moment from the original direction takes place by a certain angle, as well as a synchronisation of the precession of all protons (induction of phase coherence). After the application of the pulse has been discontinued, there is a gradual return to the original state. The time required to restore 63% of the original value of longitudinal magnetisation is termed the T1 relaxation time. The time required for harmonisation of precession, leading to a decrease of transverse magnetisation to 37% of the baseline value is termed the T2 relaxation time [8]. Both depend primarily on the mass's composition in the vicinity of the examined protons. These times are not measured directly in MRI, but their differences in the individual tissues are compared in individual sequences. T1 - and T2-weighted images rank among the basic types of imaging in MRI. They are used in imaging of the orbits, frequently in combination with sequences with selective suppression of the signal from adipose tissue. Selective fat suppression is possible by several methods [9]. Upon imaging of the orbit, the most commonly used method is inversion recovery, termed TIR (Turbo Inversion Recovery), or also STIR (Short Tau Inversion Recovery). Another frequently used method is “Fat-Sat”, i.e. Fat Saturation (FS). In this method, the signal from adipose tissue is suppressed by means of saturation pulse targeted at the slightly different resonance frequency of the hydrogen in fat tissue compared h the hydrogen in the other tissues and fluids. In principle, the methods from the Inversion Recovery group are less susceptible to the lack of homogeneity in the magnetic fields that typically occurs near stomatological material, the interface of air, bones and soft tissues. However, fat suppression by the Inversion Recovery method is not specific only for fat, but causes the suppression of all tissues that have the same T1 relaxation curve as fat. This does not occur in the case of non-contrast imaging, because fat relaxes very rapidly in contrast with other tissues. In contrast imaging, the T1 shortening due to the influence of the gadolinium contrast medium may result in the undesirable suppression of tissues other than fat. As a result, methods from the Inversion Recovery group are not usually used post-contrast. In comparison, methods using a saturation pulse are specific for fat, and are used upon the administration of a contrast medium. However, their disadvantage is susceptibility to the homogeneity of the magnetic field, which, if not optimal, causes failure of fat supression, which may lead to confusion with pathological tissue saturation in contrast examination [9]. In the case of strong artefacts, we encounter the failure of suppression of the signal from fat tissue also in the sequences of Inversion Recovery.
Upon orbital MRI, layers with a width of 1 to 3 mm are most frequently used in the transverse and coronal plane, and along the optic nerve in the parasagittal plane. Our current MRI protocol for the examination of patients with TAO is described below in the section focusing on the assessment of TAO activity.
Indications, advantages and disadvantages of MRI for patients with TAO
The main advantage of MRI is indisputably its capacity for differentiation of individual soft-tissue structures and the possibility of excellent spatial resolution. The main indications for MRI examination of the orbit in patients with TAO are summarised in Table 1. In most cases, we first perform a US examination of the orbit and extraocular rectus muscles in patients with TAO (or suspected TAO). In the case of an unequivocal clinical finding, confirmed thyroid disease, positive laboratory findings, and a corresponding finding on the US, it is not necessary to refer the patient for further IMs of the orbit. However, it is not at all exceptional that with the aid of US we are unable to unequivocally verify conditions such as expansion of the extraocular inferior rectus muscle in a high supraorbital arch, if the clinical signs of activity are questionable and the US finding is unconvincing, or if the clinical ocular finding is atypical. In such cases we always prefer to indicate MRI rather than CT, in particular due to the possibility of describing the activity of the pathology. The advantages and disadvantages of MRI are summarised in Table 2.
1. Indications for magnetic resonance imaging in thyroid-associated orbitopathy 
2. Advantages and disadvantages of magnetic resonance imaging of the orbit in patients with thyroid-associated orbitopathy 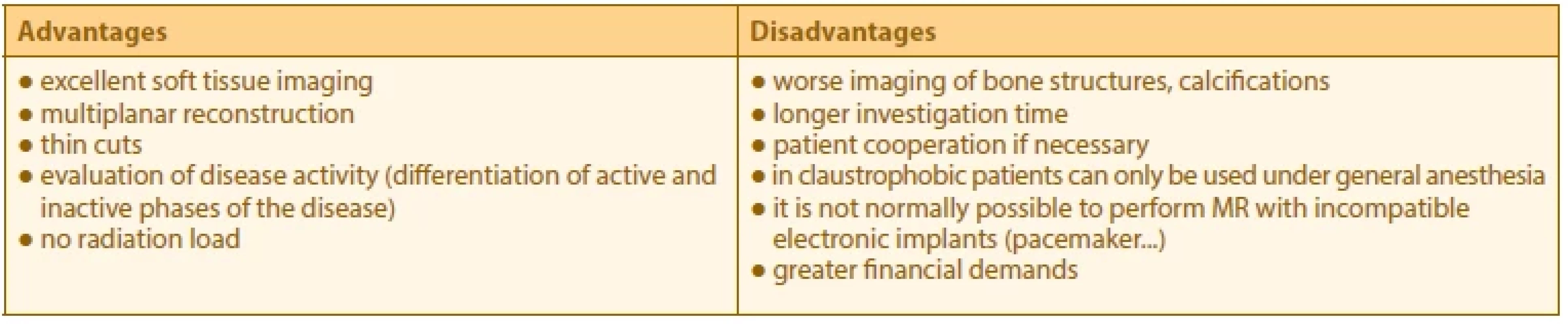
Evaluation of morphological changes in the orbit in TAO by the use of MRI
For an evaluation of morphological changes, the axial and coronal T1-weighted sequences are particularly clear without fat suppression. However, it is also possible to use T2-weighted sequences or proton density sequences. For an evaluation of morphological changes, the optimum method is “isotropic” imaging with thin, contiguous cross-sections in which the voxel (volume unit of tissue) is of a cube shape. This kind of imaging not only achieves excellent spatial resolution, but the source data can be reformatted in order to obtain a cross - -section in any plane. It is therefore possible to evaluate the extraocular muscles either in the anatomical coronal plane or in a diagonal plane perpendicular to the long axis of the orbit. Typical morphological changes in TAO detected on MRI include a finding of a bilateral spindle-shaped expansion of the belly of the extraocular rectus muscles (Fig. 1,2), in which the muscle tendon remains relatively unaffected. This symptom is considered of fundamental significance in differential diagnosis of TAO and orbital myositis; nevertheless, affliction of the tendon does not exclude a diagnosis of TAO [10]. An increase of the intra - and extraconal volume of fat may also be evident [11]. In the axial cross-section it is possible to verify the degree of protrusion of the eyeball in front of the interzygomatic line [12]. MRI is also beneficial in the diagnosis of potential optic nerve damage upon a background of TAO [13]. Typical symptoms include “apical crowding syndrome” – a markedly increased diameter of the extraocular muscles, “flattening” of the optic nerve in the apex region, proptosis, expansion of the upper ophthalmic vein and dislocation of the lacrimal gland in an anterior direction. Another possible symptom of neuropathy upon a background of TAO is intracranial prolapse of orbital fat across the upper orbital aperture. We assess the risk of optic neuropathy by measuring the width of the extraocular muscles and the orbit’s size at the halfway point between the dorsal margin of the eyeball and the orbital apex with the aid of the “Barrett’s index” [14]. The measurement methodology is illustrated in Fig. 3. A value of the index lower than 50% virtually excludes optic neuropathy. In comparison, by contrast values of the index above 60% are highly sensitive and specific for neuropathy [15]. It is necessary to point out here that the original studies started out with measurement based on CT. Still, concerning the quality of the current MRI instruments and good spatial resolution, it is possible to apply the methodology also to MRI without any difficulties, and this is the method we routinely use in practice.
1. Magnetic resonance – axial image at the level of orbits of a patient with thyroid-associated orbitopathy, nonenhanced T1-weighted image: left medial rectus muscle is enlarged, with tendinous insertion spared (arrow) 
2. Magnetic resonance – coronal image at the level of orbits of a patient with thyroid- -associated orbitopathy, fat-suppressed nonenhanced T1-weighted image: bilateral intraocular muscles enlargement more pronounced on right side 
3. Thin MR coronal slice at a point halfway between the orbital apex and posterior margin of the globe: technique for measuring Barrett's index based on measurements of thickness of extraocular muscles (A, B) and orbital width/height (C) through the optic nerve to assess the risk of dysthyroid optic neuropathy. The index is calculated for each eye separately and both horizontal and vertical indices are measured, in which only the larger of the two indices is taken into account. An index value of less than 50% almost excludes optical neuropathy – calculation formula (A+B)/Cx100 
MRI evaluation of the activity of the disease
A particular advantage of MRI is the possibility of evaluating the disease’s activity upon a TAO background. The presence of edema and inflammatory changes in the muscles is indicated by prolonging the T2-relaxation time independently of the degree of their enlargement [16]. It has been demonstrated that a prolonging of the T2-relaxation time correlates with the activity of TAO expressed with the aid of the CAS (Clinical Activity Score) [17,18], and that it is therefore a potential predictor of the probable effect of administered immunosuppressant treatment [19,20].
In order to differentiate active from inactive disease, several different authors have stipulated cut-off values of the relative signal intensity (RIS) on T2-weighted images. We refer to relative values because the measurement of the intensity of the signal in MRI is always dimensionless and is not standardised. The value depends on the specific configuration of the sequence and on the technical parameters of the given MRI scanner. We obtain the RIS as the ratio of the signal of the affected muscle and the referential tissue, which may for example be the temporalis muscle or the thalamus. The RIS should be similar on various different MRI scanners. Kirsch et al. demonstrated that an RIS value of the most heavily affected muscle on T2 STIR/TIRM higher than 2.5 correlates with CAS ≥ 4 (sensitivity 75%, specificity 100%) [18]. However, they did not demonstrate a correlation with CAS ≥ 3. The temporalis muscle was used as a referential tissue. Politi et al. determined a cut-off value of 2.23 for the extraocular medial rectus muscle in comparison with the temporalis muscle in order to determine active disease (CAS ≥ 3, sensitivity 100%, specificity 78%) [21].
Another possibility for analysis, instead of evaluating the T2 signal intensity, is to assess the course of the T2 relaxation curve, termed relaxometry. Its output is the actual T1 relaxation time stated in milliseconds [22,23].
A further option for assessing activity is to evaluate the degree of diffusivity of the water molecules in the affected muscles. In active muscles there is an increase of diffusivity, which we refer to as facilitation of diffusion. It has been demonstrated that the degree of diffusivity correlates with CAS [21]. Another alternative is dynamic contrast examination, in which we evaluate the time course of the signal intensity on T1 following the intravenous administration of a gadolinium contrast medium. The shapes of the contrast saturation curves of the extraocular muscles have been described, enabling differentiation between active and inactive disease [24].
In addition to the possibilities mentioned above for detecting activity, new and advanced methods are being investigated, such as T2 mapping or the use of artificial intelligence [25,26]. A disadvantage of MRI in our experience, is the relatively frequent occurrence of susceptibility artefacts, which we find near the ethmoids or the maxillary cavity, which may distort the intensity of the signal of the adjacent extraocular muscle, and thereby also the evaluation of the activity.
A disadvantage of dynamic contrast examination is the intravenous application of gadolinium, which means a lower level of comfort for the patient and a higher examination price. The administration of a contrast medium for patients with TAO is unnecessary, because if we evaluate the activity based on T2 and diffusion-weighted images, we do not administer a contrast medium. Administration of a contrast medium is necessary in the case of suspicion of neoplasia.
The protocol we use at present is focused primarily on the evaluation of activity and is described in Table 3. With the protocol, we recommend including one of the gradient T2-weighted sequences, with isotropic imaging and thin contiguous cross-sections, which is suitable for assessing the anatomical conditions, but is not appropriate for evaluating the signal intensity to assess activity. For example, on our scanner this concerns a CISS sequence (Constructive Interference in Steady State). An example of MRI focused on the activity of the disease is presented in Fig. 4, where expansion of the left inferior rectus muscle is evident, with signs of the pathology. An image of a patient with this MRI finding and corresponding motility disorder in the left eye is presented in Fig. 5.
3. Our MRI protocol aimed at evaluation of disease activity 
FS – fat saturation, STIR – short tau inversion recovery, DWI – diffusion-weighted imaging, GRE – gradient echo 4. Magnetic resonance - coronal image of orbits, T2 weighted fat-suppressed STIR image (short tau inversion recovery) showing increased signal of left inferior rectus muscle 
5. Patient with active form of thyroid-associated orbitopathy, already with diplopia in forward gaze (A), in which slight hypotropia of the left eye is evident when looking up (B), when limitation of elevation is noticeable in the left eye 
This protocol has proven its worth in our practice, and has made a significant contribution especially in contentious cases, which we have encountered with increasing frequency in recent times. This, in particular, concerns insidious progressive myopathic forms in which the main symptom of TAO is slowly manifesting diplopia. Patients frequently report to our centre with unclear information about the duration of the complaint, they have only small clinical activity (CAS <3) throughout the entire duration of the symptoms, and therefore the indication for application of pulse therapy with corticosteroids is borderline. If we unequivocally confirm signs of activity of the disease in this case on MRI, in our experience pulse therapy has a substantial effect. However, this issue still requires a further prospective study, on which we are currently working.
COMPUTED TOMOGRAPHY
CT is a diagnostic method that enables differentiation between normal and abnormal tissue structures based on different absorption of X-rays. Orbital fat and water absorb fewer X-rays than the optic nerve or bone structures. As a result, water and fat are shown on CT as hypodense, i.e. darker than the optic nerve or bones.
CT examination is quickly and widely available. It enables detailed imaging of the orbital region and confirmation of a diagnosis of TAO, or exclusion of other pathologies in the orbit. In comparison with MRI, however, CT does not have such quality contrast resolution in the imaging of soft tissues. CT is able to distinguish fat tissues from muscles and normal fat from edematous fat effectively, but it is unable to evaluate edema of the muscles or optic nerve. Good simultaneous visualisation of the bone structures and soft tissues is one of the reasons why CT examination is preferred to MRI before a planned decompression of the orbit. On the other hand, it is necessary to consider the radiation burden that this examination places upon the patient [27]. Indications for CT examination are presented in summary in Table 4, and the main advantages and disadvantages of this method in Table 5. An example of CT before decompression of the orbit is presented in Fig. 6, and a finding after decompression of the orbit in Fig. 7.
4. Indications for computed tomography in thyroid-associated orbitopathy 
5. Advantages and disadvantages of computed tomography of the orbit in patients with thyroid-associated orbitopathy 
6. Computer tomography –finding before decompression of orbit, excellent depiction of bone structures 
7. Computer tomography – finding after orbital decompression on left (arrow) 
In patients with suspected thyroid disease and TAO, it is recommended that only non-enhanced CT is indicated, without administering an iodinated contrast medium (ICM) [4], which could lead to a better differentiation of soft-tissue structures. The high quantity of iodine contained in the contrast medium could lead to “contrast-induced thyroid dysfunction”. This alteration of function of the thyroid gland may be in the sense of the development of hyper and hypofunction. In the literature the incidence is stated within a wide range of 1–15 %. Hyperfunctions are more commonly described in regions with an iodine deficiency and in patients with multinodular goitre or latent Graves-Basedow disease. Patients with autoimmune (Hashimoto’s) thyroiditis and patients in countries with sufficient iodine supplementation are more commonly at risk of contrast-induced hypofunctions of the thyroid gland.
The recently recommended procedures do not recommend routine laboratory examination of thyroid hormones before each CT examination with the use of an ICM; nevertheless, the possibility of untreated thyroid disorder should be clinically evaluated. However, examination with the use of an ICM is not recommended for patients with known and uncontrolled thyroid hyperfunction (therefore in certain patients examined for TAO). In this case other available imaging methods should be chosen. However, for patients with adequately treated hyperfunction or hypofunction, the use of an ICM is not contraindicated. Following the examination with an ICM it is not necessary to conduct a routine laboratory assessment of thyroid function; nevertheless, patients should be notified of the potential symptoms of thyroid disorders so that they are able to seek professional assistance in case of necessity. In risk patients with a known thyroid disorder, it is possible to expect a hormonal change approximately 3–4 weeks after the use of the ICM. Therefore, if we were to refer a patient with thyroid disease for a CT examination with an ICM, it would be appropriate to consult an endocrinologist beforehand [28].
CONCLUSION
IMs play an irreplaceable role not only in the early diagnosis of TAO, but also in the monitoring of the disease and the response to the applied treatment. When choosing a suitable IM for this diagnosis, several factors must always be considered; not only availability, cost and burden for the patient, but especially the sensitivity and specificity (yield) of the given method for the diagnosis of TAO. If we are choosing between CT and MRI, it is always necessary to know what we should expect from the given method and whether we are indicating it in order to 1) exclude another pathology in the orbit (here it is sufficient primarily in the first phase CT examination, although upon suspicion of untreated thyroid disease without administration of an ICM), 2) due to verification of a finding on the extraocular muscles in TAO (in these cases we prefer MRI due to the possibility of evaluating the activity of the disease), or 3) before decompression of the orbit (here it is necessary to perform CT examination).
The study’s authors declare that no conflict of interest exists in the compilation, theme, and subsequent publication of this professional communication and that it is not supported by any pharmaceuticals company. The authors further declare that the study has not been submitted to any other journal or printed elsewhere except for congress abstracts and recommended procedures.
This work was supported by the Ministry of Health of the Czech Republic - grant no. NU21J-01-00017 and DRO (FNOL, 00098892). All rights reserved.
MUDr. Marta Karhanová, Ph.D.,
FEBO
Oční klinika, Fakultní nemocnice
Olomouc
Oční klinika, Lékařská fakulta
Univerzity Palackého OlomoucReceived: 9 October 2022
Accepted: 17 November 2022
Available on-line: 20 February 2023Čes. a slov. Oftal., 79, 2023, No.x, p. x–xx
I. P. Pavlova 6
775 20 Olomouc
E-mail: marta.karhanova@fnol.cz
Sources
1. Kahaly G. Imaging in thyroid-associated orbitopathy. Eur J Endocrinol. 2001 : 107-118. https://doi.org/10.1530/eje.0.1450107
2. Rabinowitz MP, Carrasco JR. Update on advanced imaging options for thyroid-associated orbitopathy. Saudi J Ophthalmol. 2012;26 : 385-392. https://doi.org/10.1016/j.sjopt.2012.07.006
3. Karhanova M, Kovar R, Frysak Z et al. Correlation between magnetic resonance imaging and ultrasound measurements of eye muscle thickness in thyroid-associated orbitopathy. Biomed Pap. 2015;159 : 307-312. https://doi.org/10.5507/bp.2014.001
4. Jiskra J, Gabalec F, Diblík P et al. Doporučený postup pro diagnostiku a léčbu endokrinní orbitopatie, NOVELIZACE. 3/2022 n.d.
5. Česká oftalmologická společnost ČLS JEP. Doporučený postup pro diagnostiku a léčbu endokrinní orbitopatie [internet]. Available from: www.oftalmologie.com/cs/doporucene-postupy/doporuceny - postup-pro-diagnostiku-a-lecbu-endokrinni-orbitopatie.html Czech. n.d.
6. Shampo MA, Kyle RA, Steensma DP. Isidor Rabi-1944 Nobel Laureate in Physics. Mayo Clin Proc. 2012;87:e11. https://doi.org/ 10.1016/j.mayocp.2011.11.012
7. Pohost GM, Elgavish GA, Evanochko WT. Nuclear magnetic resonance imaging: With or without nuclear? J Am Coll Cardiol. 1986;7 : 709-710. https://doi.org/10.1016/S0735-1097(86)80486-7
8. Bloch F. Nuclear Induction. Phys Rev. 1946;70 : 460-474. https://doi. org/10.1103/PhysRev.70.460
9. Bley TA, Wieben O, François CJ, Brittain JH, Reeder SB. Fat and water magnetic resonance imaging: Fat and Water MRI. J Magn Reson Imaging. 2010;31 : 4-18. https://doi.org/10.1002/jmri.21895
10. Rana K, Juniat V, Patel S, Selva D. Extraocular muscle enlargement. Graefes Arch Clin Exp Ophthalmol. 2022. https://doi.org/10.1007/ s00417-022-05727-1
11. Kuriyan AE, Woeller CF, O’Loughlin CW, Phipps RP, Feldon SE. Orbital Fibroblasts From Thyroid Eye Disease Patients Differ in Proliferative and Adipogenic Responses Depending on Disease Subtype. Investig Opthalmology Vis Sci. 2013;54 : 7370. https://doi. org/10.1167/iovs.13-12741
12. Ozgen A, Ariyurek M. Normative measurements of orbital structures using CT. Am J Roentgenol. 1998;170 : 1093-1096. https://doi. org/10.2214/ajr.170.4.9530066
13. Dodds NI, Atcha AW, Birchall D, Jackson A. Use of high-resolution MRI of the optic nerve in Graves’ ophthalmopathy. Br J Radiol. 2009;82 : 541-544. https://doi.org/10.1259/bjr/56958444
14. Barrett L, Glatt JH, Burde RM. Ronald, Gado H. Optic nerve dysfunction in thyroid eye disease: CT. Head Neck Radiol. 1988;167 : 503 - 507. https://doi.org/10.1148/radiology.167.2.3357962.
15. Monteiro MLR, Gonçalves ACP, Silva CTM, Moura JP, Ribeiro CS, Gebrim EMMS. Diagnostic Ability Of Barrett’s Index to Detect Dysthyroid Optic Neuropathy Using Multidetector Computed Tomography. Clinics. 2008;63 : 301-306. https://doi.org/10.1590/S1807 - 59322008000300003
16. Nagy E, Toth J, Kaldi I et al. Graves’ ophthalmopathy: eye muscle involvement in patients with diplopia. Eur J Endocrinol. 2000;142 : 591-597. https://doi.org/10.1530/eje.0.1420591
17. Majos A, Pajak M, Stefanczyk L. Magnetic Resonance evaluation of disease activity in Graves’ ophthalmopathy: T2-time and signal intensity of extraocular muscles. Med Sci Monit. 2007;13 : 44-48.
18. Kirsch EC, Kaim AH, De Oliveira MG, von Arx G. Correlation of signal intensity ratio on orbital MRI-TIRM and clinical activity score as a possible predictor of therapy response in Graves’ orbitopathy - —a pilot study at 1.5 T. Neuroradiology. 2010;52 : 91-97. https://doi. org/10.1007/s00234-009-0590-z
19. Mayer EJ, Fox DL, Herdman G et al. Signal intensity, clinical activity and cross-sectional areas on MRI scans in thyroid eye disease. Eur J Radiol. 2005;56(1):20-24. https://doi.org/10.1016/j.ejrad. 2005.03.027
20. Yokoyama N, Nagataki S, Uetani M, Ashizawa K, Eguchi K. Role of Magnetic Resonance Imaging in the Assessment of Disease Activity in Thyroid-Associated Ophthalmopathy. Thyroid. 2002;12 : 223 - 227. https://doi.org/10.1089/105072502753600179
21. Politi LS, Godi C, Cammarata G et al. Magnetic resonance imaging with diffusion-weighted imaging in the evaluation of thyroid-associated orbitopathy: getting below the tip of the iceberg. Eur Radiol. 2014;24 : 1118-1126. https://doi.org/10.1007/s00330-014-3103-3
22. Prummel MF, Gerding MN, Zonneveld FW, Wiersinga WM. The usefulness of quantitative orbital magnetic resonance imaging in Graves’ ophthalmopathy: Quantitative orbital MRI in Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 2001;54 : 205-209. https:// doi.org/10.1046/j.1365-2265.2001.01220.x
23. Cheng HLM, Stikov N, Ghugre NR, Wright GA. Practical medical applications of quantitative MR relaxometry. J Magn Reson Imaging. 2012;36 : 805-824. https://doi.org/10.1002/jmri.23718
24. Jiang H, Wang Z, Xian J, Li J, Chen Q, Ai L. Evaluation of rectus extraocular muscles using dynamic contrast - enhanced MR imaging in patients with Graves’ ophthalmopathy for assessment of disease aktivity. Acta Radiol. 2012;53(1):87-94. https://doi.org/ 10.1258/ ar.2011.110431
25. Das T, Roos JCP, Patterson AJ, Graves MJ, Murthy R. T2-relaxation mapping and fat fraction assessment to objectively quantify clinical activity in thyroid eye disease: an initial feasibility study. Eye. 2019;33 : 235-243. https://doi.org/10.1038/s41433-018-0304-z
26. Lin C, Song X, Li L et al. Detection of active and inactive phases of thyroid-associated ophthalmopathy using deep convolutional neural network. BMC Ophthalmol. 2021;21 : 39. https://doi. org/10.1186/s12886-020-01783-5
27. Yuan MK, Tsai DC, Chang SCet al. The Risk of Cataract Associated With Repeated Head and Neck CT Studies: A Nationwide Population - Based Study. Am J Roentgenol. 2013;201 : 626-630. https://doi. org/10.2214/AJR.12.9652
28. Bednarczuk T, Brix TH, Schima W, Zettinig G, Kahaly GJ. 2021 European Thyroid Association Guidelines for the Management of Iodine-Based Contrast Media-Induced Thyroid Dysfunction. Eur Thyroid. J 2021;10 : 269-284. https://doi.org/10.1159/000517175
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology

2023 Issue Ahead of Print-
All articles in this issue
- CURRENT VIEW OF THE SPECTRUM OF PACHYCHOROID DISEASES. A REVIEW
- ULTRASOUND EXAMINATION OF THE ORBIT IN PATIENTS WITH THYROIDASSOCIATED ORBITOPATHY – EXAMINATION GUIDE AND RECOMMENDATIONS FOR EVERYDAY PRACTICE. A REVIEW
- COMPUTER TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING OF THE ORBIT IN THE DIAGNOSIS AND TREATMENT OF THYROID-ASSOCIATED ORBITOPATHY – EXPERIENCE FROM PRACTICE. A REVIEW
- DETERMINATION OF CORNEAL POWER AFTER REFRACTIVE SURGERY WITH EXCIMER LASER: A CONCISE REVIEW
- Eyelid Schwannoma. A Case Report
- The Current State of Artificial Intelligence in Neuro-Ophthalmology. A Review
- Central Serous Chorioretinopathy. A Review
- Therapy for Vitreous Seeding Caused by Retinoblastoma. A Review
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Central Serous Chorioretinopathy. A Review
- ULTRASOUND EXAMINATION OF THE ORBIT IN PATIENTS WITH THYROIDASSOCIATED ORBITOPATHY – EXAMINATION GUIDE AND RECOMMENDATIONS FOR EVERYDAY PRACTICE. A REVIEW
- CURRENT VIEW OF THE SPECTRUM OF PACHYCHOROID DISEASES. A REVIEW
- COMPUTER TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING OF THE ORBIT IN THE DIAGNOSIS AND TREATMENT OF THYROID-ASSOCIATED ORBITOPATHY – EXPERIENCE FROM PRACTICE. A REVIEW
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


