-
Medical journals
- Career
Is Electrophysiology Useful in the Differential Diagnostics of Lumbar Spinal Stenosis and Diabetic Polyneuropathy?
Authors: B. Adamová 1,2; R. Kopacik 1,2; S. Voháňka 1,2; L. Dusek 3; J. Bednarik 1,2
Authors‘ workplace: Department of Neurology, Masaryk University and University Hospital Brno 1; CEITEC – Central European Institute of Technology, Masaryk University, Brno 2; Institute of Biostatistics and Analyses, Masaryk University, Brno 3
Published in: Cesk Slov Neurol N 2014; 77/110(6): 684-690
Category: Original Paper
Overview
Aim:
To evaluate validity of electrophysiological examination in the differential diagnostics of lumbar spinal stenosis (LS) and diabetic polyneuropathy (DPN). Methods: Electrophysiological parameters were examined in 68 patients with clinically symptomatic LS, in 28 patients with DPN, and in a group of 32 healthy volunteers. Results: Electrophysiological parameters evaluated from the upper extremities (F-wave latency of the ulnar nerve and radial SNAP amplitude), all the latencies measured in the lower extremities (tibial F-wave, soleus H-reflex and spinal latency of MEP), and the sural SNAP amplitude contributed significantly to distinguishing the LS from DPN patients. ROC analysis, however, disclosed only two electrophysiological parameters as effective in independent discrimination between the LS and DPN patient groups: ulnar F-wave latency (cut-off point at 24.2 ms, sensitivity 82.7% and specificity 63.9%); and radial SNAP amplitude (cut-off point at 10.5 µV, sensitivity 75.5%, specificity 58.2%). Multivariate discrimination provided a canonical score with the most powerful predictive value of all. Conclusions: Electrophysiological examination evaluated from the upper extremities proved very useful in the differential diagnostics of LS and DPN, but the most powerful predictive value was obtained by using a canonical score involving several electrophysiological parameters.Key words:
lumbar spinal stenosis – diabetic polyneuropathy – electrophysiological examination – electromyographyUsed abbreviations
DPN Diabetic PolyNeuropathy
EMG ElectroMyoGraphy
LS Lumbar Spinal stenosis
MEP Motor Evoked Potential
NCS Nerve Conduction Study
SNAP Sensory Nerve Action Potential
Introduction
Degenerative lumbar spinal stenosis (LS) is a painful and potentially disabling condition, often encountered by older adults. It is now more commonly diagnosed, possibly in relation to increasingly better access to advanced imaging and to demographic increases in the ageing population [1,2]. Kalichman et al. noted that the prevalence of relative and absolute acquired stenosis increases with age and is 47.2% and 19.4%, respectively, in the 60 to 69 years age group [3]. Diabetic polyneuropathy (DPN) is also a common disease of advancing age and many people suffer from both at the same time. The prevalence of known cases of diabetes mellitus are 6 to 7% for persons aged 45 to 64 years, and 10 to 12% for those aged 65 years and older [4]. The prevalence of diabetic peripheral neuropathy in patients with diabetes is around 50% [5]. Symptoms of LS are sometimes similar to those of DPN, for example numbness and tingling of the feet, and helpful clinical clues such as back pain, proximal weakness, or radiating pain in the legs may be absent; clinical differentiation between lumbosacral polyradicular disease (typical of LS) and diffuse peripheral neuropathy of the distal-axonal type (typical for diabetic patients) in such patients can be difficult [6,7]. Electrophysiological examination may be useful in these circumstances.
The purpose of this prospective study was to evaluate usefulness of electrophysiological examination in the differential diagnostics of LS and DPN and to identify electrophysiological parameters with the highest diagnostic validity.
Methods
This study was approved by the Institutional Ethics Committee and an informed consent was given by all subjects. Fig. 1 summarizes subject recruitment.
1. Study flowchart including subject recruitment. 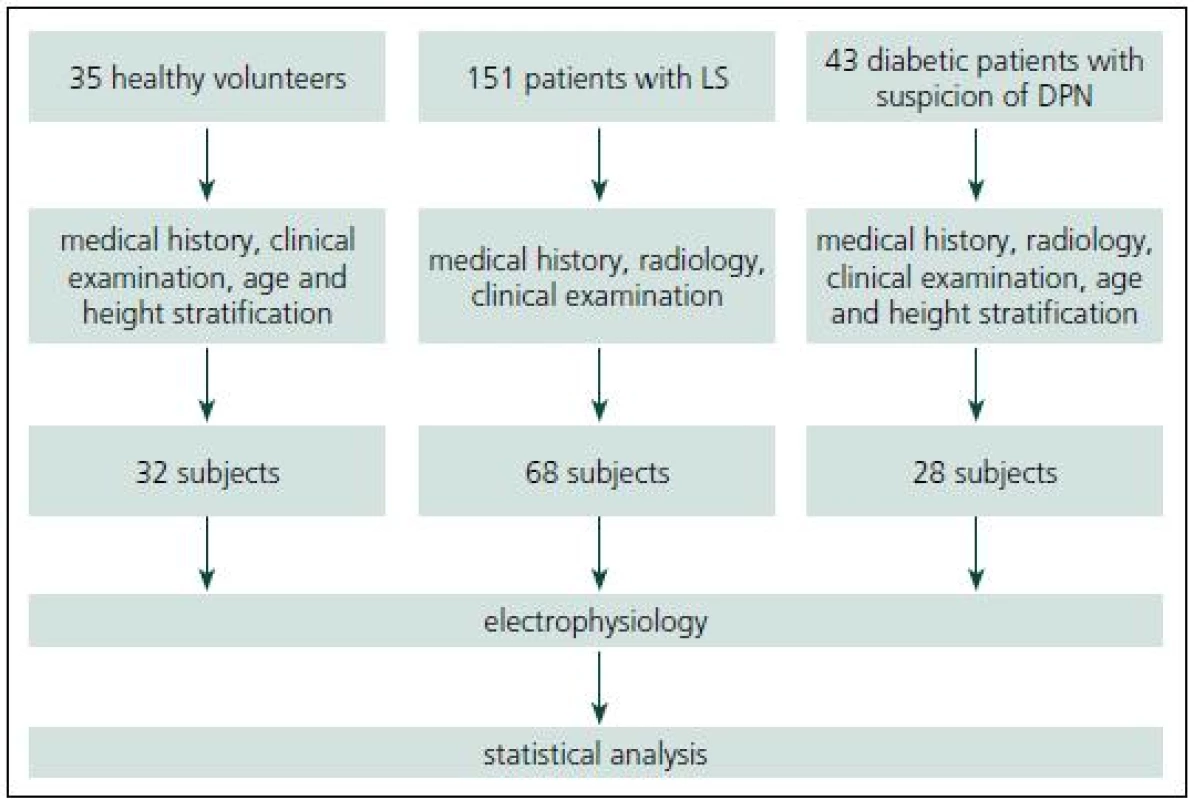
Patients with LS
Sixty-eight patients (32 men, 36 women) were recruited consecutively from a total of 151 patients with clinically symptomatic LS.
The patients were considered for inclusion if they fulfilled the following criteria:
- Clinically symptomatic LS (neurogenic claudication and/ or radicular pain, e. g. low back pain radiating below the knee to one or both lower limbs).
- Presence of central LS (an osteoligamentous narrowing of the lumbar spinal canal) at at least one level, established by computed tomography or magnetic resonance imaging. The radiological examination was described in detail in our previous publications [8,9].
- Absence of isolated herniated nucleus pulposus and isolated lateral or foraminal stenosis.
- Absence of diabetes mellitus or other disease causing polyneuropathy.
Patients with DPN
The group was recruited from consecutive subjects referred to an EMG laboratory because of suspected distal symmetric DPN. Patients with DPN underwent a detailed clinical neurological examination, including full medical history and radiological examination (computed tomography or magnetic resonance imaging of the lumbar spine). Patients with DPN were prospectively stratified according to age and height categories in order to establish a sample fully comparable with the LS patients.
Finally, 28 patients with distal symmetric DPN (18 men, 10 women) were included who fulfilled the following criteria:
- Diabetes mellitus type I or II.
- Symptoms (sensory and/ or motor) and signs of polyneuropathy in the distal part of lower extremities. Signs of polyneuropathy included abnormalities of primary sensory modalities and/ or motor signs such as weakness and atrophy and/ or depressed or non-elicitable tendon reflexes.
- No attack of radicular lumbosacral syndrome, and absence of neurogenic claudication in the medical history.
- Normal diameter of the lumbar spinal canal.
- Absence of another disease associated with polyneuropathy.
Control group
Healthy volunteers were recruited among the employees of the Department of Neurology, their relatives and patients of the Department of Ophthalmology (patients awaiting cataract surgery). Thirty-two healthy volunteers (11 men, 21 women) with no history of radicular lumbosacral syndrome or neurogenic claudication, without diabetes mellitus or another disease associated with polyneuropathy, and with normal clinical neurological findings from the lower extremities were included. The study sample was prospectively stratified according to age and height categories in order to establish a sample fully comparable with LS and DPN patients.
Electrophysiological examination
The following tests were examined bilaterally in all groups:
Soleus H-reflex
The subject lays down in a fully prone position. The active electrode was placed 2 cm distal to the insertion of the gastrocnemius on the Achilles tendon, and the reference electrode 3 cm further distally. Submaximal stimuli (duration 0.5 ms) with increasing voltage were delivered at the popliteal fossa and facilitation was used to provide maximum H-reflex amplitude. We determined:
- H-reflex latency measured from the stimulus artefact to the first deflection from baseline;
- H-reflex amplitude measured from the base to peak of the negative phase.
Tibial F-wave
Ten supramaximal stimuli at a frequency of 1 Hz were delivered to the tibial nerve at the malleolus, while the recording electrode was placed over the abductor hallucis muscle. The following parameters were determined:
- minimum F-M latency, i.e. central latency: this is calculated as a minimum F-wave latency minus the latency of the M response. This parameter provides conduction time from stimulus point to and from the spinal cord [10];
- chronodispersion: the difference between the maximum and minimum F-wave latencies;
- persistence: occurrence of recordable F-wave responses to 10 stimuli expressed as a percentage.
MEP to abductor hallucis muscle
Magstim 200 was used for transcranial and spinal stimulation. In the course of spinal stimulation, the coil was located one centimetre to one side of the centre of the lower lumbar spine; for transcranial stimulation, the coil was located above the motor cortex and facilitation was employed. Supramaximal stimulation was applied. We determined:
- spinal latency (peripheral motor conduction time), obtained by stimulating the spinal roots at the point at which they exit the intervertebral foramen;
- cortical latency, obtained by transcranial stimulation;
- central motor conduction time, calculated as cortical latency minus spinal latency;
- amplitude of the cortical response, measured from baseline to negative peak.
In patients with LS and DPN, electrophysiological examination was extended by motor and sensory conduction studies and needle EMG of the lower extremities, radial sensory NCS and ulnar F-wave. Sensory NCSs of the sural and radial nerves were performed using the antidromic surface technique. The sural nerve was examined bilaterally, the radial nerve on the right side. The nerves were stimulated using 0.1 ms electrical impulses at an intensity sufficient to elicit maximal SNAP amplitude, quantified by peak-to-peak measurement. Responses were averaged to obtain the best response. The sural/ radial amplitude ratio was calculated by dividing the right sural by the right radial SNAP amplitudes. The right ulnar F-waves were recorded, with 10 supramaximal stimuli at a frequency of 1 Hz delivered to the ulnar nerve at the wrist and the recording electrode placed over the abductor digiti minimi muscle. The minimum F-M latency was calculated as minimum F-wave latency minus the latency of M response.
A temperature of ≥ 32 °C was maintained for all NCSs. If parameters were measured bilaterally, the results from both sides were included in subsequent statistical analysis. Non-elicitability of responses precluded the use of parameters measured in milliseconds (latencies, conduction times and chronodispersion) in statistical analysis. This study was not focused on the needle examination.
Statistical approaches
Standard robust summary statistics were used to describe primary data: relative and absolute frequencies, median, 10% and 90% quantiles. The binomial test was employed to compare relative frequencies and the non-parametric Mann-Whitney U test was used for comparison of variants on the basis of continuous variables. A value of p < 0.05 was taken as the universal indicative limit for statistical significance in all analyses. Standard one-way ANOVA technique, followed by Tukey multiple-range testing was applied to compare values of more than two parameters. In addition to univariate analyses, multivariate strategy was applied for the assessment of risk associated with individual factors. Multivariate differences in the twelve electrophysiological parameters examined were summarized in principal component analysis (PCA) effectively simplifying the correlation structure through linear transformations of the original variables. Based on these two-dimensional plots, multivariate discrimination analysis was employed in order to separate LS patients and patients with DPN. The values of single electrophysiological parameters, as well as the multivariate case-specific canonical score obtained, were then tested for specificity and sensitivity in diagnostic decisions.The best maximum likelihood estimates for cut-off values were obtained by receiver operating characteristic curve analysis.
Results
Basic characteristics of study groups are presented in Tab. 1, with all groups fully comparable with respect to the age and height; the difference in gender structure has no impact on bias in the differential tests.
1. Basic characteristics of groups. 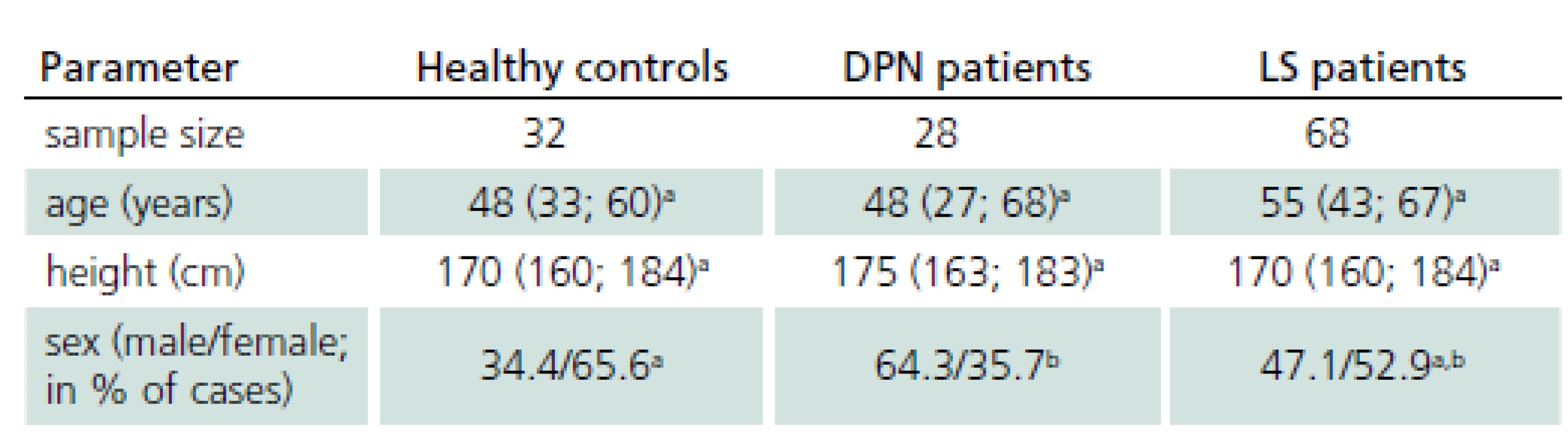
Quantitative parameters are expressed as median estimates, with 10–90% quantiles in parentheses. a,b Letters indicate statistical significance within one row: groups marked with the same letter are not significantly different (p > 0.05; Mann-Whitney U test for quantitative parameters, binomial test for relative frequencies). Contribution of electrophysiological parameters to differential diagnostics of patients with LS and DPN
A diagnostic set of 12 electrophysiological parameters was applied in univariate classification of all the groups examined (Fig. 2). All the lower extremity latencies (tibial F-wave latency, soleus H-reflex latency and spinal MEP latency) reliably discriminated between all subject groups (LS patients, diabetic patients and healthyvolunteers); latencies in DPN patients were more prolonged than in LS patients. Electrophysiological parameters evaluated from the upper extremity (ulnar F-wave latency and radial SNAP amplitude) and sural SNAP amplitude significantly contributed to distinguishing patients with LS from DPN patients, and the study confirms a contribution of the sural/ radial amplitude ratio to differential diagnostics in this respect. The chronodispersion of the tibial F-wave, central motor conduction time of MEP, the amplitude of the soleus H-reflex, the amplitude of the cortical MEP response and persistence of the tibial F-wave did not discriminate between DPN patients and LS patients, but the amplitude of the soleus H-reflex separated DPN patients and LS patients from healthy volunteers, and central motor conduction time of MEP and chronodispersion of the tibial F-wave separated LS patients from healthy volunteers.
2. Healthy volunteers, DPN patients and LS patients were separated on the basis of initial values for electrophysiological parameters. Data are expressed as an arithmetical mean supplied by 95% confidence limits; p value – significance level of ANOVA test (one-way model); letters indicate statistical significance, while groups marked with the same letter are not significantly different (Tukey multiple range test; p > 0.05). 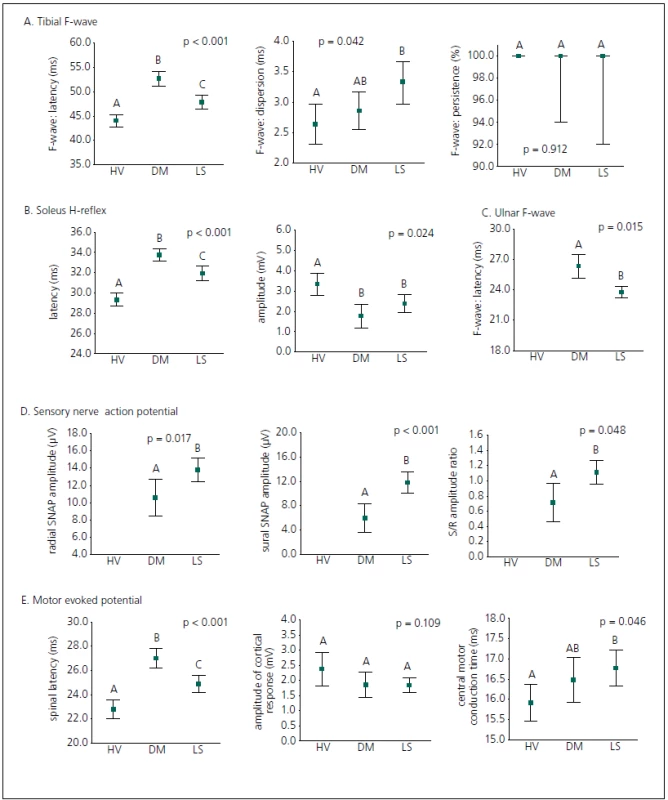
Groups compared: HV – healthy volunteers, DM – patients with diabetic polyneuropathy, LS – patients with lumbar spinal stenosis. Receiver operating characteristic curves were then employed in order to define the best cut-off values of single potential electrophysiological predictors (Tab. 2). Receiver operating characteristic analysis disclosed only two electrophysiological parameters as effective discriminating factors between DPN patients and LS patients: the ulnar F-wave latency (sensitivity 82.7% and specificity 63.9% when using the optimal discriminating cut-off point of 24.2 ms) and the radial SNAP amplitude (sensitivity 75.5%, specificity 58.2% when using the optimal discriminating cut-off point of 10.5 µV).
2. Electrophysiological parameters – cut-off values of single parameters based on the analysis of receiver operating characteristic (ROC) curves (LS vs DPN patients). 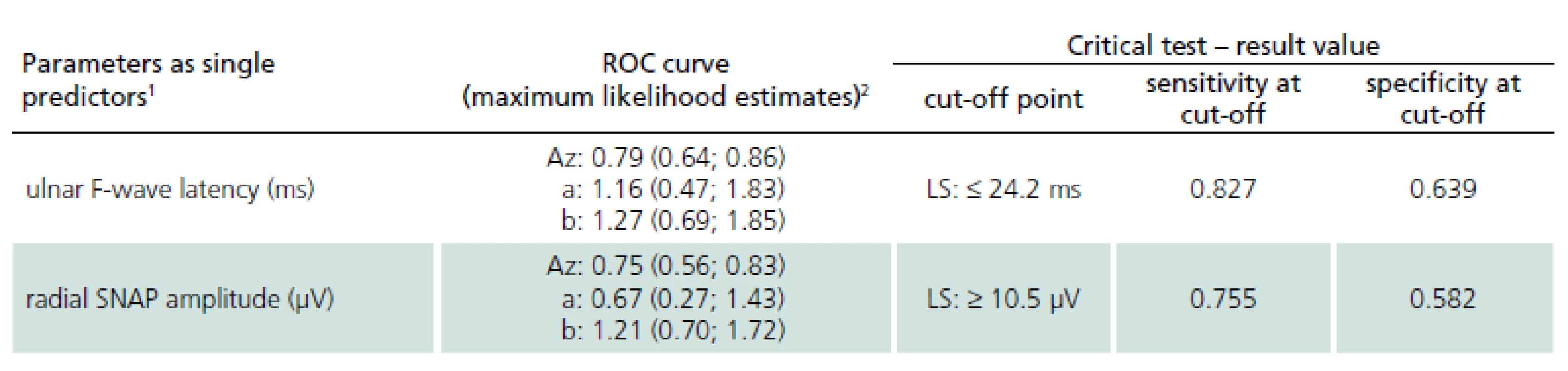
1 Only electrophysiological parameters providing sufficient ROC estimates of cut-off points were included, i.e. area under the curve ≥ 0.65 and sensitivity or specificity of critical test at least 0.6. 2 Parameters of binormal ROC curve: a – vertical of ROC curve; b – slope of the fitted ROC curve when plotted as a straight line on normal deviate axis; both a and b were supplied with 95% confidence intervals (in parentheses). Az – area under the ROC curve, supplied with asymmetrical 95% confidence limits for binormal area estimate (in parentheses). Multivariate discrimination of LS and DPN
Multivariate discrimination was also employed. Principal component analysis led to a two-dimensional projection of original values that enabled effective separation of the two patient groups (LS patients and DPN patients) using multivariate discrimination (Fig. 3).
3. Two-dimensional biplot projection identifying the association between electrophysiology and the position of patient groups: case-sensitive canonical score for two statistically significant discrimination roots. 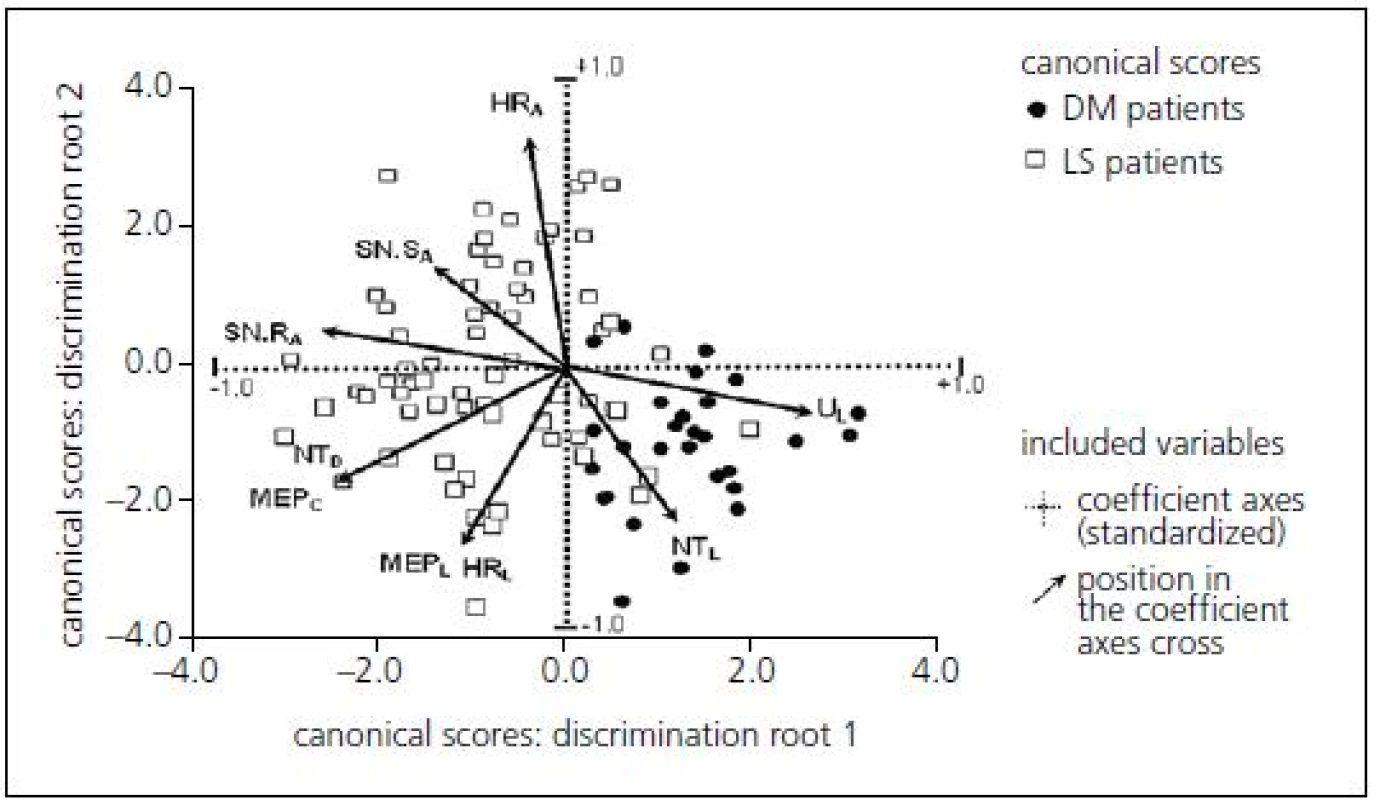
HRA – amplitude of soleus H-reflex, HRL – latency of soleus H-reflex, UL – ulnar F-wave latency, SN.RA – radial SNAP amplitude, SN.SA – sural SNAP amplitude, NTD -chronodispersion of tibial F-wave, NTL – tibial F-wave latency, MEPC – central motor conduction time of MEP to abductor hallucis muscle, MEPL – spinal latency of MEP to abductor hallucis muscle, DM – diabetes mellitus. Multivariate discrimination including several electrophysiological parameters provided a canonical score with more powerful predictive values: sensitivity 79.8%, specificity 73.5% when using the optimal cut-off point of 1.04 (Tab. 3).
3. Multivariate discrimination of DPN and LS patients based on initial electrophysiological examination – analysis of final canonical score. 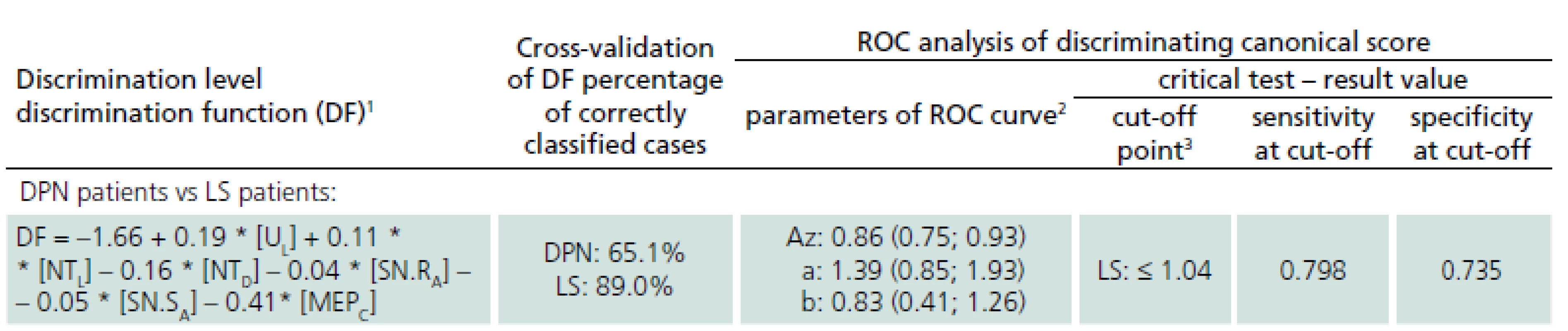
1 Only electrophysiological parameters making a significant contribution to the correct classification of discriminated cases were included in the equations (stepwise forward algorithm). 2 Parameters of binormal ROC curves estimated on discrimination canonical scores: a – vertical of ROC curve; b – slope of the fitted ROC curve when plotted as a straight line on normal deviate axis; both a and b were supplied with 95% confidence intervals (in parentheses). Az – area under the ROC curve, supplied with asymmetrical 95% confidence limits for binormal area estimate (in parentheses). 3 Cut-off point estimated for canonical score given by discrimination function. UL – ulnar F-wave latency, SN.RA – radial SNAP amplitude, SN.SA – sural SNAP amplitude, NTD – chronodispersion of tibial F-wave, NTL – tibial F-wave latency, MEPC – central motor conduction time of MEP to abductor hallucis muscle. Cross-validation of the defined discrimination function assessed that 89% of LS patients and 65% of patients with DPN were classified correctly, so it may be recommended as an effective and diagnostically relevant prognostic index.
Discussion
Although data on the differential diagnostics of LS and DPN is sparse in the literature, the problem is relatively frequent in routine clinical and electrophysiological practice. Large numbers of elderly people suffer from both diseases at the same time but differentiation on the basis of clinical neurological examination may be difficult. This study evaluated selected electrophysiological parameters and their contribution to the differential diagnostics of LS and DPN. The distinction between DPN patients and patients with LS by ulnar F-wave latency and radial SNAP amplitude was highly significant. We defined cut-off values for each of these parameters with sensitivity around 80% and specificity about 60%. Discrimination between patients with DPN and LS patients using electrophysiological parameters from an upper extremity may be anticipated, because DPN is a diffuse disorder also affecting upper extremity nerves, while LS only affects lower extremity nerves. We would like to draw attention to performing NCSs from upper extremities in the differential diagnostics of LS and DPN. The NCSs from upper extremities are also included in a protocol recommended for identification of distal symmetrical polyneuropathy [11].
Berger et al. reported that tibial F-waves in patients with axonal polyneuropathy are characterised by prolongation of the minimum latency with a lower persistence. In contrast, prolonged chronodispersion rather than abnormalities of minimum latency or persistence appears to be the most frequent F-response abnormality in radiculopathies [6]. In our study, the latencies of the tibial F-wave, as well as the soleus H-reflex and spinal response of MEP to the abductor hallucis muscle, were only slightly prolonged in LS patients but considerably prolonged in patients with polyneuropathy. On the other hand, chronodispersion was prolonged significantly in patients with LS only, thus confirming the results of Berger et al. [6]. Persistence of the tibial F-wave showed no statistically significant difference among the groups. Egli et al. have also reported that tibial F-wave persistence is usually normal in LS patients [12].
Prolongation of F-wave latencies (and also soleus H-reflex latency, and spinal MEP latency) in diabetic patients reflects reduced conduction velocity. Minimum F-wave latency is considered to be the most sensitive measure for detection of nerve pathology in diabetic patients [13]. In LS patients, normal or only slightly prolonged F-wave latency for the tibial nerve may occur; a long segment of normally conducting nerve compensates for impaired conduction across a shorter radicular segment, thus balancing the overall latency. The increased chronodispersion of F-wave in patients with radiculopathy is probably a consequence of non-uniform segmental demyelination, more common in radiculopathy than axonal polyneuropathy [6,14].
The amplitude of sural SNAP contributes significantly to differentiation between patients with LS and DPN, and the same trend is demonstrated for a parameter known as the sural/ radial amplitude ratio [15]. Decrease in amplitude of SNAPs in DPN patients probably reflects axonal loss [13]. Values of sural SNAP amplitude and the sural/ radial amplitude ratio appear lower in DPN patients, because a typical impairment sensory neurogram exhibits a distal-to-proximal gradient of severity in DPN [15].
It proved useful to combine several electrophysiological parameters into one discriminatory function that provides a canonical score of more powerful predictive value. The defined function correctly identified 89% of LS patients. It has been described in the literature that a combination of several electrophysiological parameters significantly improves diagnostic yield [16].
The present study has some limitations. The non-elicitability of responses precluded the use of parameters that are measured in milliseconds, and such results are frequent, especially in severe forms of the two diseases. The choice of electrophysiological parameters for evaluation was limited and, therefore, some parameters useful in the differential diagnostics of LS and DPN may have been omitted (for example, compound motor action potentials in the lower extremity were not analysed). The needle EMG including paraspinal EMG, a sensitive method in the diagnosis of LS, has also been omitted in our study [17,18]. However, the selected parameters have already been described as useful in the diagnostics of one or both of the diseases. Sensory NCSs and F-wave studies have a high sensitivity in polyneuropathies [19 – 21], and the value of soleus H-reflex in the diagnosis of DPN has been described [22]. Due to the proximal location of the pathology in LS, late responses such as tibial F-wave and soleus H-reflex are utilized in the evaluation of radiculopathies. The soleus H-reflex is a sensitive indicator of S1 radiculopathy and can often be the first abnormality to show in the earlier stages of LS [23].
A possible source of error may lie in electrophysiological data not being corrected for age and height. The groups we compared, however, were height - and age-matched in this study, with defined cut-off values for these groups at about 50 years, height 170 – 175 cm. Care must be exercised, however, in the interpretation of findings in patients beyond this age and height category.
The electrophysiological parameters defined, the canonical score and the estimated cut-off values are especially useful in mild forms of impairment. We presume the main contribution of these parameters to lie in diabetic patients with a narrow lumbar spinal canal, in whom common clinical and electrophysiological findings are inconclusive.
Conclusions
Electrophysiological examination is useful in the differential diagnostics of lumbar spinal stenosis and diabetic polyneuropathy. Upper extremity parameters are very helpful, but the canonical score combining several electrophysiological parameters have been proven to have the best discriminating effect.
Blanka Adamová, MD, PhD
Department of Neurology
University Hospital and Masaryk University
Jihlavska 20
625 00 Brno
e-mail: badamova@fnbrno.cz
Accepted for review: 11. 8. 2014
Accepted for print: 2. 9. 2014
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Sources
1. Fritz JM, Delitto A, Welch WC, Erhard RE. Lumbar spinal stenosis: a review of current concepts in evaluation, management, and outcome measurements. Arch Phys Med Rehabil 1998; 79(6): 700 – 708.
2. Genevay S, Atlas SJ. Lumbar spinal stenosis. Best Pract Res Clin Rheumatol 2010; 24(2): 253 – 265. doi: 10.1016/ j.berh.2009.11.001.
3. Kalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J 2009; 9(7): 545 – 550. doi: 10.1016/ j.spinee.2009.03.005.
4. Roman SH, Harris MI. Management of diabetes mellitus from a public health perspective. Endocrinol Metab Clin North Am 1997; 26(3): 443 – 474.
5. Pop-Busui R, Lu J, Lopes N, Jones TL. Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J Peripher Nerv Syst 2009; 14(1): 1 – 13. doi: 10.1111/ j.1529-8027.2009.00200.x.
6. Berger AR, Sharma K, Lipton RB. Comparison of motor conduction abnormalities in lumbosacral radiculopathies and axonal polyneuropathy. Muscle Nerve 1999; 22(8): 1053 – 1057.
7. Adamova B, Vohanka S, Dusek L. Differential diag-nostics in patients with mild lumbar spinal stenosis: the contributions and limits of various tests. Eur Spine J 2003; 12(2): 190 – 196.
8. Micankova Adamova B, Vohanka S, Dusek L, Jarkovsky J, Bednarik J. Prediction of long-term clinical outcome in patients with lumbar spinal stenosis. Eur Spine J 2012; 21(12): 2611 – 2619. doi: 10.1007/ s00586-012-2424-7.
9. Adamova B, Vohanka S, Dusek L, Jarkovsky J, Chaloupka R, Bednarik J. Outcomes and their predictors in lumbar spinal stenosis: a 12-year follow-up. Eur Spine J 2014. doi: 10.1007/ s00586-014-3411-y.
10. Kimura J. The F wave and the A wave. In: Kimura J (ed). Electrodiagnosis in diseases of nerve and muscle: principles and practice. 3rd ed. New York: Oxford University Press 2001 : 439 – 465.
11. England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005; 64(2): 199 – 207.
12. Egli D, Hausmann O, Schmid M, Boos N, Dietz V, Curt A. Lumbar spinal stenosis: assessment of cauda equina involvement by electrophysiological recordings. J Neurol 2007; 254(6): 741 – 750.
13. Andersen H, Stålberg E, Falck B. F-wave latency, the most sensitive nerve conduction parameter in patients with diabetes mellitus. Muscle Nerve 1997; 20(10): 1296 – 1302.
14. Shahani BT. Modern approach to nerve conduction studies. In: Delwaide PJ, Gorio A (eds). Clinical neurophysiology in peripheral neuropathies. Amsterdam: Elsevier Science Publishers 1985 : 17 – 38.
15. Rutkove SB, Kothari MJ, Raynor EM, Levy ML, Fadic R, Nardin RA. Sural/ radial amplitude ratio in the diagnosis of mild axonal polyneuropathy. Muscle Nerve 1997; 20(10): 1236 – 1241.
16. Rajabally YA, Beri S, Bankart J. Electrophysiological markers of large fibre sensory neuropathy: a study of sensory and motor conduction parameters. Eur J Neurol 2009; 16(9): 1053 – 1059. doi: 10.1111/ j.1468-1331.2009.02651.x.
17. Haig AJ, Tong HC, Yamakawa KS, Quint DJ, Hoff JT, Chiodo A et al. The sensitivity and specificity of electrodiagnostic testing for the clinical syndrome of lumbar spinal stenosis. Spine 2005; 30(23): 2667 – 2676.
18. Yagci I, Gunduz OH, Ekinci G, Diracoglu D, Us O, Akyuz G. The utility of lumbar paraspinal mapping in the diagnosis of lumbar spinal stenosis. Am J Phys Med Rehabil 2009; 88(10): 843 – 851. doi: 10.1097/ PHM.0b013e3181b333a9.
19. Pastore C, Izura V, Geijo-Barrientos E, Dominguez JR. A comparison of electrophysiological tests for the early diagnosis of diabetic neuropathy. Muscle Nerve 1999; 22(12): 1667 – 1673.
20. Johnsen B, Fuglsang-Frederiksen A. Electrodiagnosis of polyneuropathy. Neurophysiol Clin 2000; 30(6): 339 – 351.
21. Perkins BA, Bril V. Diabetic neuropathy: a review emphasizing diagnostic methods. Clin Neurophysiol 2003; 114(7): 1167 – 1175.
22. Millán-Guerrero R, Trujillo-Hernández B, Isais-Millán S, Prieto-Díaz-Chávez E, Vásquez C, Caballero-Hoyos JR et al. H-reflex and clinical examination in the diagnosis of diabetic polyneuropathy. J Int Med Res 2012; 40(2): 694 – 700.
23. Cho SC, Ferrante MA, Levin KH, Harmon RL, So YT. Utility of electrodiagnostic testing in evaluating patients with lumbosacral radiculopathy: an evidence-based review. Muscle Nerve 2010; 42(2): 276 – 282. doi: 10.1002/ mus.21759.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2014 Issue 6-
All articles in this issue
- Reducing the Door-to-needle Interval, Experience from the Stroke Centre in Kladno
- WHO Grade II Ependymomas of the Fourth Ventricle in Adults – Single Institution Experience
- Diazepam i. m. – the Most Common, but Inappropriate Medication for Management of Acute Anxiety, Agitation and Aggression
- Sporadic Use of Decompressive Hemicraniectomy in a Patient with Brain Abscess – a Case Report
- Missile Head Injury with a Replica of a Historical Weapon – Pathophysiology and a Case Study
- Adult Form of Pompe Disease
- Cognitive Rehabilitation in Patients with Multiple Sclerosis
- Effectiveness of the Temporary Splinting after Carpal Tunnel Release
- Increasing the Sensitivity of Brain Death Confirmation Using the Combination of Auditory and Somatosensory Evoked Potentials
- The Five Point Test – a Test of Nonverbal Fluency: Normative Data for Adults
- Molecular Diagnostics of NF1 in Slovakia Using cDNA and MLPA Analysis
- The Effect of Suboptimal Surgical Treatment of Spinal Fractures on the Course of Spinal Cord Injury
- The Use of Electromagnetic Navigation in Surgeries for Hydrocephalus and Arachnoid Cysts in Children under One Year of Age
- Is Electrophysiology Useful in the Differential Diagnostics of Lumbar Spinal Stenosis and Diabetic Polyneuropathy?
- Epidemiological Study of Neurodegenerative Parkinsonism in “Hornacko”, a Specific Region of the South-eastern Moravia, Czech Republic
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Diazepam i. m. – the Most Common, but Inappropriate Medication for Management of Acute Anxiety, Agitation and Aggression
- Missile Head Injury with a Replica of a Historical Weapon – Pathophysiology and a Case Study
- The Five Point Test – a Test of Nonverbal Fluency: Normative Data for Adults
- Adult Form of Pompe Disease
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

