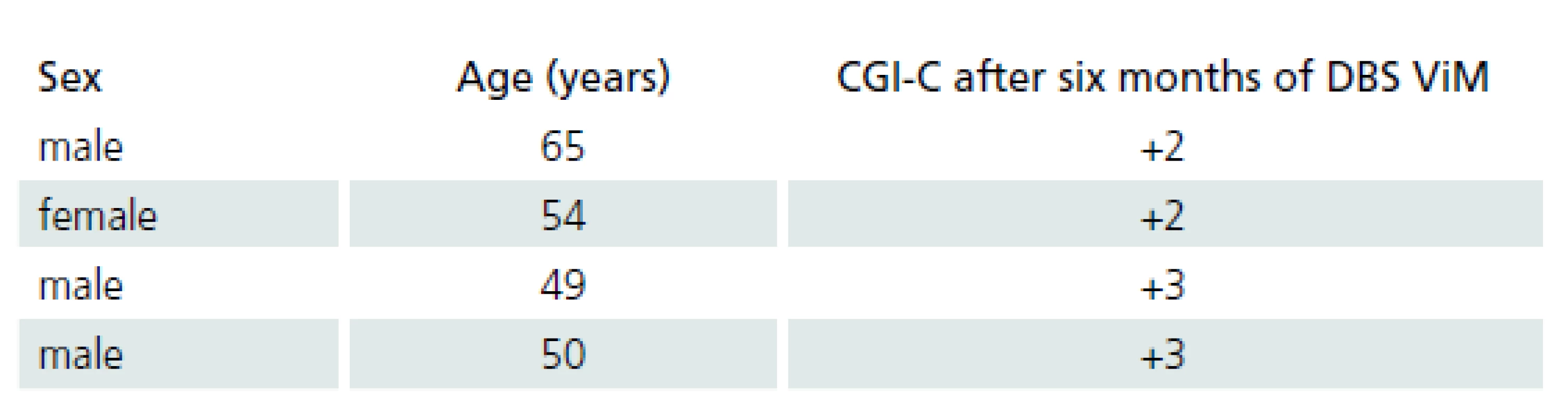-
Medical journals
- Career
Deep Brain Stimulation in Olomouc – Techniques, Electrode Locations, and Outcomes
Authors: D. Krahulík 1; M. Nevrlý 2; P. Otruba 2; P. Kaňovský 2
Authors‘ workplace: Palacky University Medical School, Olomouc Department of Neurosurgery 1; Palacky University Medical School, Olomouc Department of Neurology 2
Published in: Cesk Slov Neurol N 2014; 77/110(1): 54-58
Category: Original Paper
Overview
Aim:
Deep Brain Stimulation (DBS) is a very useful procedure for the treatment of idiopathic Parkinson disease (PD), essential tremor and dystonia. The authors describe their experience, technical approach and results for placing electrodes into the STN nucleus, GPI nucleus and ViM nucleus, including the methodology for electrophysiological mapping of the mentioned nuclei, clinical outcomes and complications.Method:
Forty ‑ five adult patients with PD, tremor or various forms of dystonia were operated from January 2009 to January 2012. The baseline neurological status and DBS‑related improvement in motor function were measured using patients diaries, Burke ‑ Fahn ‑ Marsden Dystonia Rating Scale (BFMDRS) and Clinical Global Improvement (CGI) tests. The implantation of the DBS leads was performed using Magnetic Resonance Imaging (MRI) for planning, fusion with CT with a head frame, multiple ‑ cell microelectrode recording, and intraoperative test stimulation to determine the thresholds for stimulation‑induced adverse effects.Conclusions:
The implantation of DBS electrodes in patients with PD, tremor and dystonia is a very useful and technically feasible procedure with a very low morbidity rate. The statistically evaluated position of the electrodes showed excellent accuracy of electrode placement associated with excellent outcome.Key words:
Parkinson’s disease – dystonia – tremor – deep brain stimulationIntroduction
Parkinson’s disease
Parkinson’s disease (PD) is the second most common neurodegenerative disease. Although new drugs have been effective in the treatment of PD in the last twenty ‑ five years, the effects of pharmacotherapy are insufficient during the late stages of the disease. Stereotactic surgery is useful in some of these patients.
Stereotactic lesional surgery developed before the era of levodopa (L ‑ DOPA) in 1950s. Especially pallidotomy and later, due to its better effect on tremor, thalamotomy were widely used. Since the late 1960s, pharmacotherapy has become a more preferred option again because of L ‑ DOPA, while stereotactic surgery remained in the background.
Benabid et al. revived deep brain stimulation (DBS) in 1987. They published the first reports on the effects of electric high‑frequency stimulation by electrodes implanted to the subthalamic nucleus Luysi on parkinsonian symptoms. The robust development of DBS at the turn of the millennium led to the DBS being established as a standard therapeutic method during the late stages of PD. Unlike lesional surgery, the DBS has the advantage of reversibility of its effects. However, the exact pathophysiological process induced by high‑frequency electric stimulation is not yet known.
There are four possible target sites for the placement of the stimulating electrodes: although the stimulation of the ventrointermediate thalamic nucleus (ViM) has a clear effect on tremor, DBS of the STN or globus pallidus internus (GPi) has a broader influence on all parkinsonian symptoms and currently represents the treatment of choice in the majority of PD patients. The pedunculopontine nucleus (PPN) has recently become, be it still an experimental, target that may be appropriate for patients with gait freezing [1,2]. Since the majority of patients undergoing the DBS procedure have bilateral symptoms, both right and left STN or GPi are usually implanted for the most significant benefit.
Three recent randomized controlled studies in patients with PD reported that STN DBS plus the best medical therapy was more effective than the best medical therapy alone in improving motor function and quality of life but was also associated with an increased risk of serious adverse events [3 – 6]. In addition, reduction of dopaminergic therapy after STN DBS may help to reduce some psychiatric symptoms, such as visual hallucinations and impulse control abnormalities, i.e. frequent behavioral complications of the treatment with dopamine agonists [7].
Complications related to surgery are primarily intracerebral hemorrhage (less than 2% in the majority of centers) and infections (in about 4% of cases) [8]. STN DBS can worsen speech and gait in some patients, requiring an adjustment of stimulation parameters. A recent study reported that depression worsened with STN DBS but was improved with GPi DBS[9]. There are several reports describing neuropsychiatric symptoms following STN DBS in PD patients. However, such symptoms were generally transient and mild if managed appropriately [10]. Medium ‑ and long‑term studies have provided evidence that stimulation‑induced motor improvement was still evident at 5 – 8‑year follow‑up [11,12]. However, DBS does not modify progression of the underlying PD pathology and, therefore, patients can still develop disabling levodopa‑resistant symptoms, such as gait disturbances and cognitive impairment.
Dystonia
Dystonia is a movement disorder that presents with sustained, uncontrolled, often painful muscle contractions causing repetitive movements and abnormal postures. Patients with symptoms that cause significant disability, despite well‑tolerated pharmacotherapy, should be candidates for DBS treatment. Factors that influence selection of patients with various types of dystonia for treatment with DBS have recently been reviewed by Bronte ‑ Stewart et al. [13].
Neuronal models of dystonia have postulated hyperactivity of the direct putamen ‑ pallidal pathway with reduced inhibitory output of the GPi, with subsequently increased thalamic input to the (pre‑) motor cortex, resulting in excessive motor cortex excitation [14].
GPi DBS is currently the most promising technique for the treatment of patients with severe drug‑resistant dystonia. Three randomized controlled trials investigated this procedure in primary generalized dystonia and found significant clinical improvement on the Burke ‑ Fahn ‑ Marsden Dystonia Rating Scale (BFMDRS) after six and 12 months [15 – 17], sustained after a 3‑year follow‑up period [18].
Essential tremor
Essential tremor (ET) is one of the most common movement disorders. Only 50% of the treated patients show a good response to therapy [19]. In the mid 20th century, the ventrolateral thalamus became the main surgical target for parkinsonian and various other types of tremor, including ET. After the introduction, in the early 1960s, of micro recording during stereotactic surgery, it became apparent that small lesions of the ViM could suppress tremor. Afterwards, unilateral stereotactic ViM lesioning was a procedure frequently used in many clinical centers worldwide and resulted in permanent significant contralateral improvement of the most common types of tremor. In fact, almost 30% of the patients who underwent the ablative procedure bilaterally experienced permanent speech and cognitive deficits [20]. Introduction of DBS of the thalamic ViM nucleus in ET treatment helped to reduce complication rate while maintaining high efficacy. Therefore, ViM DBS is viewed as the target therapy for patients with debilitating ET. Although the exact etiology and pathophysiology of ET is still unknown, it is believed that high‑frequency stimulation of the ViM nucleus may block the abnormal oscillatory activity within the interconnected regions, including the cerebellum and the motor cortex [21].
DBS should be managed by a team of different specialists (neurosurgeon, neurologist, psychologist, internist and psychiatrist) [22]. It is essential that the procedure as well as the expected outcome of DBS is explained to the patient.
Clinical material and methods
Inclusion criteria and clinical evaluation
All patients fulfilled the UK ‑ Brain Bank criteria for the diagnosis of idiopathic Parkinson’s disease. Patients treated by DBS for dystonia showed an insufficient effect of pharmacological treatment, including botulinum toxine ‑ A. All DBS subjects with the diagnosis of tremor suffered from drug‑resistant essential tremor.
All patients were fully informed about the procedure and the procedure was performed by a single surgeon (K. D.) and neurologists (N. M., O. P.).
Patient group
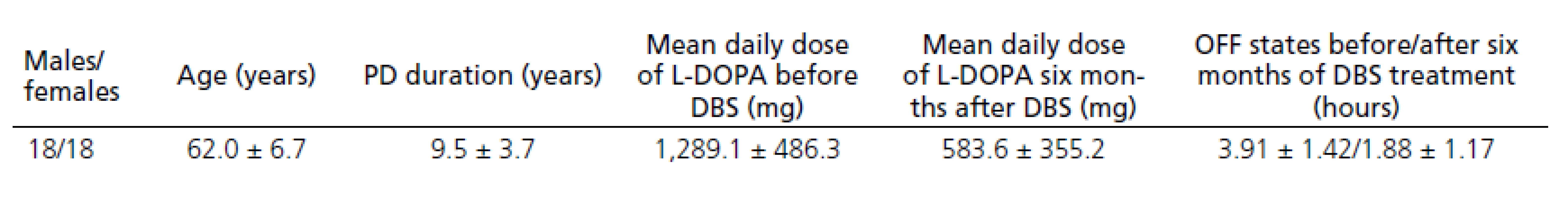
Thirty ‑ six patients who fulfilled the UK ‑ Brain Bank criteria for the diagnosis of idiopathic Parkinson’s disease from December 2008 to January 2011 were implanted DBS. Demographic data of the PD patients are provided in Tab. 1. Data on only thirty ‑ four patients were evaluated after six months of DBS treatment as two patients discontinued treatment due to surgical complications.
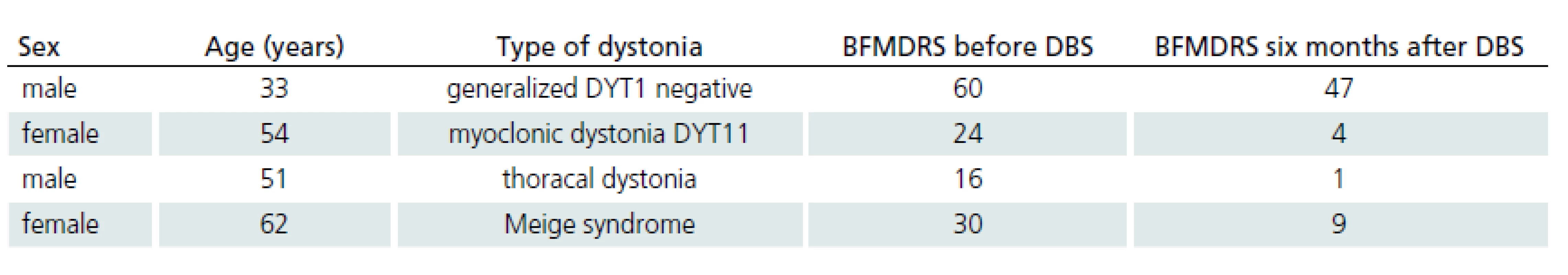
Five patients with different types of dystonia were treated by GPi DBS. Demographic data of four of these patients and the results of the BFMDRS [23] are shown in Tab. 2. The fifth patient is not currently treated due to surgical complications.
Four patients with ET were treated by ViM DBS. Demographic data of ET patients and the results of the clinical global improvement scale are provided in Tab. 3.
Surgical procedure
All patients underwent bilateral implantation of electrodes. Total of 41 patients with IPD and tremor were implanted in two stages and all patients were awake. Five patients with dystonia were implanted in one stage using general anesthesia.
Stereotactic targeting
Three MR image sets were obtained several days prior to the surgery:
- a volumetric 3D Gd ‑ enhanced gradient echo MR imaging sequence covering the whole brain in 1 - mm axial slices, mainly for trajectory planning,
- T2 images turbo spin echo in 2 - mm slices,
- IR ‑ FSE image set covering only the basal ganglia region, in 2 - mm axial slices, mainly for direct visualization of the borders of the GPI and surrounding structures.
Images were obtained using the Magnetom Avanto 1.5 - Tesla unit (Siemens). After a stereotactic Leksell frame was placed, a whole ‑ brain CT scan with contrast was performed in 1 - mm slices. Both the MRI and CT image sets were imported into a stereotactic surgical planning software package (Framelink, Medtronic®), computationally fused, and reformatted to produce images orthogonal to the AC ‑ PC line and midsagittal plane.
The target points for the tip of the electrodes were selected using a combination of direct (visualized) and indirect targeting in IPD and dystonia and only with indirect targeting in tremor. The STN nucleus and the GPi nucleus are well visualized using MRI and we combined the MRI images with stereotactic coordinates of each nucleus. We targeted the ViM nucleus using its stereotactic coordinates only. Trajectories were visualized on the volumetric MR images using “navigation” views. Small adjustments in the arc and ring angles were then made to avoid traversing the sulci, cortical veins, and dural venous lakes (easily seen on Gd ‑ enhanced images) and lateral ventricles.
Intraoperative MicroElectrode Registration (MER)
In order to perform MER in STN ‑ DBS, four MER/ macrostimulation needles were placed in an array with a central, lateral, anterior and posterior, and an anterior position placed 2 mm apart, to delineate the borders of the nucleus. Depending on the preoperative MRI, it was decided in some cases to record with three or five microelectrodes rather than four. In GPi ‑ DBS, based on the pre‑operative MRI and the better visibility of the GP structures and internal capsule, usually three to four channel recordings were performed in the central, medial, posterior, and lateral channel to define the distance of the calculated target to the border between GPi and the internal capsule. Starting for STN and GPi, respectively, 10 mm above the MRI‑based target, microelectrodes were advanced in steps of 500 µm towards the target by an electric microdrive. When the needles were inside the STN, GPe (globus pallidus externus) and GPi at each depth, the spiking activity of the neurons lying close to the needle was recorded. Depending on the neuronal density not more than 3 – 5 units were recorded simultaneously. The more distant units could not be distinguished from the background level.
Macro‑test stimulation
After MER, the tip of the microelectrode was retracted. The channels that showed significant multi‑unit activity over a length of more than 3 mm were selected for intraoperative test stimulation (60 – µs pulse ‑ duration; 130 – Hz pulse frequency). The complete electrode with the macro‑tip was then advanced to be used for macro‑test stimulation performed by an experienced neurologist at two or three depths with a 2 - mm interval, all within the boundaries of the target nucleus as determined by MER. After the evaluation of the selected channels by macro‑test stimulation, the one with the largest therapeutic window, i.e. the lowest current threshold for improvement of symptoms and the highest threshold for adverse effects, was selected for permanent electrode implantation. For dystonic patients, the threshold for capsular side effects was used to select the best electrode. In addition, improvement of mobile dystonia was sought when present. With respect to the depth of the implantation of the electrodes in STN ‑ DBS, it is our practice to implant the contact number 1 at the point with the best stimulation parameters. For GPi ‑ DBS, we position the deepest contact point at the inferior border of the nucleus as determined by MER.
Lead anchoring and implantable pulse generator placement
The leads were anchored to the skull with a lead anchoring device (Stimlock, Medtronic®). After the scalp was closed and head frame removed in dystonia patients in continuing general anesthesia, the lead extenders and pulse generators were placed. In dystonia patients, the pulse generators were placed during the same operative session as the leads. The duration of surgery (from initial skin incision until the pulse generators were placed) was 4 to 5 hours for the same ‑ session bilateral implantation. IPD and tremor patients are awake during the two‑stage surgery. The second session in general anesthesia is performed within 3 – 4 days after the first session. Postoperative CT imaging was performed the same day as the surgery.
Stimulator programming
Devices in the IPD patients (stimulation of STN) were programmed within the first month after the surgery. Typical initial configuration was as follows: pulse frequency was 130 Hz and the pulse duration was 60 µs. Voltage was individual in each case according to the clinical effect, usually about 3.0 V.
Devices in the patients with tremor (stimulation of ViM thalamic nucleus) were programmed within 10 days after the surgery. Typical initial configuration was as follows: pulse frequency was 145 Hz and pulse duration was 60 µs. Voltage was individual in each case according to the clinical effect, usually about 2.5 V.
Devices in dystonia patients (stimulation of GPi) were programmed within 10 days after the surgery. Because of the small group of patients with different types of dystonia, the initial configuration was individualized.
Voltage was gradually increased over the initial two to six months on the basis of clinical and adverse effects of the stimulation. We started the stimulation with monopolar stimulation. In some cases, we also used bipolar stimulation, depending on clinical and adverse effects.
Results
Patient population
The patients treated with DBS for PD reported significant improvement in motor fluctuation, documented in patient diaries before and six months after DBS STN. Diaries where patients mark one of three states (OFF state, ON without dyskinesias, ON with dyskinesias) every hour were used to evaluate the clinical effect. Each patient completed the diary for three consecutive days before the DBS procedure and for three consecutive days six months after the DBS. The mean OFF time (in hours) before the DBS was compared to the mean OFF time after the DBS. The mean OFF time after six months of DBS was reduced by 52%; dopaminergic medication used was reduced by about 54.3%.
Five patients with different types of dystonia were treated with GPi DBS. Demographic data of four of these patients and the results of the BFMDRS [23] are shown in Tab. 2. The fifth patient was not treated due to surgical complication.
Four patients with ET were treated with ViM DBS. Demographic data of the ET patients and the results of the clinical global improvement or change scale (CGI ‑ C) are shown in Tab. 3. CGI ‑ C was scored as follows: +3 = very much improved, +2 = much improved, +1 = mildly improved, 0 = no change, – 1 = mildly worse, – 2 = much worse, and – 3 = very much worse [24].
Surgical outcomes and complications
Three patients undergoing STN ‑ DBS postoperatively had an infection that led to explantation of the entire system; two of these patients underwent reimplantation with no further complications. Postoperative CT scans showed bilateral subdural air with no clinical symptoms. No other complications were noted in the clinical records of the rest of the patients.
Discussion
DBS has provided a substantial clinical improvement in patients with several different diseases and disorders. DBS is a well‑established treatment option for patients with PD, ET, and dystonia. Although other effective methods (e. g. continuous subcutaneous infusions of apomorphine or continuous intestinal infusions of L ‑ DOPA) are also used in the therapy of advanced PD, DBS has an irreplaceable role in the treatment of advanced PD [25,26]. DBS is used as a safe treatment modality in all these three indications.
Our understanding of how DBS exerts its action has advanced over the past twenty years but there still is much to be learned. Our experience with DBS is very good. For all three indications, the accuracy of the surgical procedure as well as correct programming and treatment optimization lead to a very good clinical effect in our patients. Our results as well as the number of complications are comparable with published results from larger centers with a higher number of implanted patients and longer experience with DBS.
Conclusion
Deep brain stimulation is a safe and highly effective procedure for the three indications discussed in this paper and it is well tolerated by the patients. We believe that, in the future, DBS will be used in many more indications.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Accepted for review: 15. 1. 2013
Acdepted for print: 15. 10. 2013
MUDr. David Krahulík, Ph.D.
Department of Neurosurgery
Palacky University Medical School
I. P. Pavlova 6
775 20 Olomouc
e-mail: david.krahulik@fnol.cz
Sources
1. Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 2007; 130(6): 1596 – 1607.
2. Wilcox RA, Cole H, Wong D, Coyne T, Silburn P, Kerr G. Pedunculopontine nukleus deep brain stimulation produces sustained improvement in primary progressive freezing of gait. J Neurol Neurosurg Psychiatr 2011; 82(11): 1256 – 1259.
3. Deuschl G, Schade ‑ Brittinger C, Krack P, Volkmann J,Schäfer H, Bötzel K et al. A randomized trial of deep ‑ brain stimulation for Parkinson’s disease. N Engl J Med 2006; 355(9): 896 – 908.
4. Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ jr et al. Bilateral deep brain stimulation vs best medical therapy for patiens with advanced Parkinson disease: a randomized controlled trial. JAMA 2009; 301(1): 63 – 73.
5. Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open ‑ label trial. Lancet Neurol 2010; 9(6): 581 – 591.
6. Baláž M, Bočková M, Bareš M, Rektorová I, Dírerová V, Rektor I. Kvalita života po hluboké mozkové stimulaci u pacientů s pokročilou Parkinsonovu nemocí. Cesk Slov Neurol N 2011; 74/ 107(5): 564 – 568.
7. Lulé D, Heimrath J, Pinkhardt EH, Ludolph AC, Uttner I, Kassubek J. Deep brain stimulation and behavioural changes: is comedication the most important factor? Neurodegener Dis 2012; 9(1): 18 – 24.
8. Kleiner ‑ Fisman G, Herzog J, Fisman D, Tamma F, Lyons K, Pahwa R et al. Subthalamic nucleus deep brain stimulation: summary and metaanalysis of outcomes. Mov Disord 2006; 21 (Suppl 14): S290 – S304.
9. Follett K, Weaver F, Stern M, Hur K, Harris CL, Luo P et al. Pallidal versus subthalamic deep ‑ brain stimulation for Parkinson’s disease. N Engl J Med 2010; 362(22): 2077 – 2091.
10. Volkmann J, Daniels C, Witt K. Neuropsychiatric effects of subthalamic neurostimulation in Parkinson disease. Nat Rev Neurol 2010; 6(9): 487 – 498.
11. Fasano A, Romito LM, Daniele A, Piano C, Zinno M,Bentivoglio AR et al. Motor and cognitive outcome in patients with Parkinson’s disease 8 years after subthalamic implants. Brain 2010; 133(9): 2664 – 2676.
12. Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S,Volkmann J et al. Long‑term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord 2010; 25(5): 578 – 586.
13. Bronte ‑ Stewart H, Taira T, Valldeoriola F, Merello M, Marks WJ Jr, Albanese A et al. Inclusion and exclusion criteria for DBS in dystonia. Mov Disord 2001; 26 (Suppl 1): S5 – S16.
14. Vitek JL. Pathophysiology of dystonia: a neuronal model. Mov Disord 2002; 17 (Suppl 3): S49 – S62.
15. Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P,Benabid AL, Cornu P et al. Bilateral deep ‑ brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005; 352(5): 459 – 467.
16. Kupsch A, Benecke R, Müller J, Trottenberg T, Schneider GH, Poewe W et al. Pallidal deep ‑ brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355(19): 1978 – 1990.
17. Valldeoriola F, Regidor I, Mínguez ‑ Castellanos A, Lezcano E, García ‑ Ruiz P, Rojo A et al. Efficacy and safety of pallidal stimulation in primary dystonia: results of the Spanish multicentric study. J Neurol Neurosurg Psychiatry 2010; 81(1): 65 – 69.
18. Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P,Lagrange C, Yelnik J et al. Bilateral, pallidal, deep ‑ brain stimulation in primary generalised dystonia: a prospective 3 year follow‑up study. Lancet Neurol 2007; 6(3): 223 – 229.
19. Lyons KE, Pahwa R, Comella CL, Eisa M, Elble RJ,Fahn S et al. Benefits and risks of pharmacological treatments for essential tremor. Drug Saf 2003; 26(7): 461 – 481.
20. Pizzolato G, Mandat T. Deep brain stimulation for movement disorders. Front Integr Neurosci 2012; 6 : 2.
21. Dostrovsky O, Lozano AM. Mechanisms of deep brain stimulation. Mov Disord 2002; 17 (Suppl 3): S63 – S68.
22. Urgošík D, Jech R, Růžička E. Hluboká mozková stimulace u nemocných s extrapyramidovými poruchami pohybu – stereotaktická procedura a intraoperační nálezy. Cesk Slov Neurol N 2011; 74/ 107(2): 175 – 186.
23. Burke RE, Fahn S, Marsden CD, Bressman P, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1995; 35(1): 73 – 77.
24. US Department of Health. ECDEU Assessment Manual for Psychopharmacology – revised. Washington, DC 1976 : 218 – 222.
25. Kanovský P, Kubová D, Bares M, Hortová H, Streitová H, Rektor I et al. Levodopa‑induced dyskinesias and continuous subcutaneous infusions of apomorphine: results of a two‑year, prospective follow‑up. Mov Disord 2002; 17(1): 188 – 191.
26. Kianička B, Žák J, Bareš M. Využití perkutánní endoskopické gastrostomie – přehled indikací, popis techniky a současné trendy v neurologii. Cesk Slov Neurol N 2012; 75/ 108(2): 165 – 169.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2014 Issue 1-
All articles in this issue
- Surgical Treatment of Hydrocephalus
- Movement Activities in Patients with Multiple Sclerosis
- A Notice on Classification, Terminology and Contents Innovations to the Primary Headaches Section of the International Classification of Headache Disorders (ICHD-3 Beta)
- Is Long-term Disability in Multiple Sclerosis Associated with Diffuse Cerebral Pathology Independent of Relapses?
- Prediction of Postoperative Clinical Outcome in Cervical Spondylotic Myelopathy
- Validity of the Montreal Cognitive Assessment in the Detection of Mild Cognitive Impairment in Parkinson’s Disease
- Quality Assessment of a Clinical Guidelines Czech Neurological Society
- Quantitative Flowmetry of Parent and Branching Arteries during Surgical Treatment of Cerebral Aneurysma
- International Standards for Neurological Classification of Spinal Cord Injury – Revision 2013
- Intraspinal Juxtaarticular Cysts of the Lumbar Spine
- Microsurgical Resection of Symptomatic Pineal Cysts
- Czech Version of the Autonomic Scale for Outcomes in Parkinson’s Disease (SCOPA-AUT) – Questionnaire to Assess the Presence and Severity of Autonomic Dysfunction in Patients with Parkinson’s Disease
- Importance of Electromyography in the Reconstructive Surgery of the Upper Extremity
- Cranial Nerve Palsies and Necrotizing External Otitis – Two Case Reports
- Neuropathic Pain Relief through Distraction Technique – a Case Report
- Local Thrombolysis in Severe Cerebral Venous and Sinus Thrombosis – Two Case Reports
- Stiff‑ person Syndrome Associated with Myotonic Dystrophy Type 2 – a Case Report
- Deep Brain Stimulation in Olomouc – Techniques, Electrode Locations, and Outcomes
- Underuse of Oral Anticoagulation in Primary Prevention of Cardioembolic Stroke – Results of a Descriptive Prevalence Study
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Microsurgical Resection of Symptomatic Pineal Cysts
- Surgical Treatment of Hydrocephalus
- Stiff‑ person Syndrome Associated with Myotonic Dystrophy Type 2 – a Case Report
- International Standards for Neurological Classification of Spinal Cord Injury – Revision 2013
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career