-
Medical journals
- Career
Tailoring postoperative management through sentinel lymph node biopsy in low - and intermediate-risk endometrial cancer – the SENTRY clinical trial
Authors: P. Sorokin 1; M. Novozhilov 1; D. Utkin 1; Z. Abduragimova 1; I. Dudina 2; A. Nikiforchin 3; S. Kulikova 1
Authors‘ workplace: Department of Gynecologic Oncology, Moscow City Oncology Hospital No. 62, Moskovskaya Oblast, Russian Federation 1; Department of Medical Oncology, Moscow City Oncology Hospital No. 62, Moskovskaya Oblast, Russian Federation 2; Department of Surgical Oncology, Mercy Medical Center, Weinberg building, Baltimore, MD, USA 3
Published in: Klin Onkol 2024; 37(2): 126-133
Category: Original Articles
doi: https://doi.org/10.48095/ccko2024126Overview
Background: While total hysterectomy and bilateral salpingo-oophorectomy without lymph node staging are standard for low - and intermediate-risk endometrial cancer, certain histopathologic factors revealed after surgery can necessitate additional interventions. Our study assessed the influence of sentinel lymph node biopsy on postoperative decision-making. Materials and methods: In the SENTRY trial (July 2021 – February 2023), we enrolled patients with International Federation of Gynaecology and Obstetrics (FIGO) stage IA–IB low-grade endometrioid endometrial cancer. Laparoscopic sentinel lymph node mapping using indocyanine green was performed alongside total hysterectomy with bilateral salpingo-oophorectomy. Subsequent management changes based on sentinel lymph node biopsy results were evaluated. The trial was registered at ClinicalTrials.gov (NCT04972682). Results: Of the 100 enrolled participants, a bilateral detection rate of 91% was observed with a median detection time of 10 min (interquartile range 8–13 min). Sentinel lymph node metastases were found in 8% (N = 8) of participants. Postoperative FIGO staging increased in 15% (N = 15) and decreased in 5% (N = 5) of patients. Sentinel lymph node biopsy results altered the adjuvant treatment plan for 20% (N = 20): external beam radiotherapy was omitted in 12% (N = 12) while 6% (N = 6) had external beam radiotherapy +/ − systemic chemotherapy added due to sentinel lymph node metastases. In 2% (N = 2), the external beam radiotherapy field was expanded with the paraaortic region. No intraoperative complications were reported and no 30-day major morbidity and mortality occurred. Throughout a median follow-up of 14 (95% CI 12–15 months, neither patient-reported lymphedema nor pelvic recurrence surfaced in the cohort. Conclusions: Sentinel lymph node biopsy using indocyanine green is a safe procedure and allows tailoring adjuvant therapy in presumed low - and intermediate-risk endometrial cancer. It assists in avoiding external beam radiotherapy overtreatment and introducing additional modalities when necessary.
Keywords:
indocyanine green – gynecological cancer – Drug therapy – Hysterectomy – Uterine cancer – radiotherapy – sentinel lymph node – adjuvant treatment
Introduction
Endometrial cancer stands as the predominant gynecologic malignancy [1]. A significant proportion of patients are diagnosed with presumed stage I low--grade endometrioid carcinoma warranting the primary treatment of total hysterectomy accompanied by bilateral salpingo-oophorectomy (BSO) [2,3]. Decisions regarding the necessity for lymph node staging derive from various factors: tumor type, depth of myometrial invasion, and cervical stroma involvement. However, a prevalent challenge remains the inconsistency between preoperative risk estimations and the final pathology report. Notably, the status of lymphovascular space invasion (LVSI), a critical determinant of postoperative risk, remains predominantly elusive pre-surgery. Daix et al. documented a 37% underestimation rate in preoperative risk assessments accompanied by an overestimation in 10% of cases [4]. Historically, to bridge this knowledge gap and inform adjuvant therapy decisions, systematic lymph node dissection (LND) was employed. Yet, given the low likelihood of lymph node metastases and the potential for significant postoperative morbidity, there is a consensus against systematic LND for patients in the low - and intermediate-risk categories [2]. This has been further bolstered by two randomized controlled trials that demonstrated no therapeutic advantage of incorporating systematic pelvic LND alongside total hysterectomy [5,6].
Building on this, contemporary international guidelines advocate for the sentinel lymph node (SLN) biopsy, especially for cases with presumed uterine-confined disease that fall within the low - to intermediate-risk spectrum [3,7]. Several prospective studies have validated the superior accuracy of the SLN biopsy relative to systematic LND [8,9]. In addition, the SLN biopsy method has been shown to detect a greater number of metastases compared to traditional lymphadenectomy whilst simultaneously reducing postoperative complications [10,11]. Given these advantages, a pertinent question arises: how can insights from the SLN biopsy guide adjuvant treatment decisions? Determined to answer this question, we launched a prospective study to evaluate the influence of SLN biopsy on the postoperative management of patients with presumed low - and intermediate-risk endometrial cancer, who, based on prevailing local guidelines, would typically undergo only a total hysterectomy and BSO without any lymph node evaluation [12].
Materials and methods
Study design and settings
The SENTRY study was a prospective open-label single-arm clinical trial aimed at evaluating the influence of SLN biopsy on postoperative management in patients with presumed low - and intermediate-risk endometrial cancer. The study was conducted within the Departments of Gynecologic Oncology, Pathology, and Medical Oncology at the high-volume oncology center – Moscow City Oncology Hospital No. 62 (Istra, Moskovskaya Oblast, Russian Federation) between July 2021 and November 2023.
Ethics
The study secured ethical approval from the Institutional Review Board and was executed in adherence to the Helsinki Declaration of 1964 and its subsequent amendments [13]. All relevant details were disclosed to patients with each providing written informed consent before undergoing any procedure. Registration for the SENTRY trial occurred on ClinicalTrials.gov in July 2021, prior to recruitment, under the registration number NCT04972682.
1. Patient criteria. 
FIGO – International Federation of Gynaecology and Obstetrics, G – grade, LND – lymph node dissection Participants
Patients qualified as low-risk if they presented with histologically confirmed low - -grade (G1–G2) endometrioid adenocarcinoma of the endometrium, accompanied by less than half myometrial invasion as visualized on pelvic MRI (International Federation of Gynaecology and Obstetrics (FIGO) stage IA). The intermediate - -risk category encompassed those with a verified low-grade endometrioid adenocarcinoma of the endometrium that exhibited more than a half of myometrial invasion without any extension beyond the uterus (FIGO stage IB). While the European Society of Gynecologic Oncology (ESGO) guidelines classified patients with stage IA high-grade endometrioid adenocarcinoma of the endometrium as intermediate-risk, these individuals were excluded due to the considerable molecular heterogeneity within this group [3]. Table 1 offers a comprehensive breakdown of the inclusion and exclusion criteria. Preoperative staging predominantly utilized pelvic MRI and contrast-enhanced CT of the thoracic and abdominal regions. When MRI was contraindicated, transvaginal ultrasounds were employed. To ensure proficiency with a technique new to us, patient enrollment for the study commenced only after our institution had gained experience from conducting the first 30 cases of SLN mapping and biopsy, in line with recommendations for this procedure [11].
SLN mapping, biopsy, and pathology assessment
Eligible patients were subjected to a laparoscopic SLN biopsy, succeeded by a laparoscopic total hysterectomy and BSO. Both procedures were executed by one of five experienced gynecologic oncologists. SLN mapping utilized indocyanine green (ICG) at a standard concentration of 2.5 mg/mL. We administered 1 mL of this diluted ICG into the cervix at the 3 and 9 o‘clock positions (total dose – 5 mg) to a depth of 5–10 mm, initiated right after general anesthesia induction [14]. Diagnostic laparoscopy and a thorough examination of the abdomen and pelvis employed the Image 1S equipment (KARL STORZ©, Tuttlingen, Germany; the brand names are used for clarity, not endorsement). Upon examination, fluorescence in the near-infrared spectrum was observed (Fig. 1). Successful mapping was indicated by identifying a lymphatic vessel with at least one lymph node. Detected SLNs were then extracted and the total hysterectomy with BSO was completed. While we did not routinely resort to a frozen section of SLN, its application remained at the surgeon‘s discretion. If metastasis surfaced in SLN either during the frozen section or routine assessment, the option for systematic LND in a subsequent procedure existed although not mandatory.
All surgical specimens underwent rigorous analysis by our dedicated team of gynecologic oncology pathologists. Every SLN was dissected perpendicular to its longest axis, producing 2 mm slices after formalin fixation. Standard staining employed hematoxylin and eosin (Fig. 2). Immunohistochemical staining was exclusively used for ambiguous findings.
Adjuvant treatment and follow-up
Adjuvant care was aligned with ESGO and our national guidelines offering chemoradiotherapy, vaginal brachytherapy, and systemic chemotherapy as potential treatment options [3,12]. Each patient’s postoperative case underwent two rounds of discussion on the tumor board – the initial review occurred prior to receiving the SLN biopsy results with the subsequent review post-receipt of these results.
Follow-up
In congruence with local endometrial cancer management guidelines, follow-up appointments with gynecologic oncologist encompassed physical examinations, chest X-rays, and abdominal and pelvic ultrasound [12]. Regular intervals were every 3 months for the first two years transitioning to biannual check-ups until the fifth year. However, should symptoms emerge, patients were seen sooner.
1. SLNs with macro-metastases (A, C, D) and isolated tumor cells (B, encircled). Stained with conventional H&E (A–C) and pan-cytokeratin (D). Magnifi cation: (A) ×10, (B) ×40, (C) ×10, (D) ×10. Slides (С) and (D) made from one specimen. 
H&E – hematoxylin and eosin, SLNs – sentinel lymph nodes Endpoints
The primary endpoint of our study centered on the influence of SLN biopsy on postoperative treatment decisions. We defined a change in treatment strategy as any difference between treatment plans set by the tumor board before and after receiving the SLN biopsy information.
Secondary endpoints encompassed: 1) adjustments in FIGO staging; 2) bilateral SLN detection; 3) intraoperative complications associated with SLN mapping and biopsy; 4) major postoperative morbidity and mortality within 30 days following the procedure; 5) incidence of lymphedema, and 6) pelvic recurrence rate and associated time frame. To classify postoperative morbidity and mortality, we employed the Clavien-Dindo classification, with grades III–IV designated as major complications [15].
Data quality
Throughout the study, participating physicians meticulously documented all perioperative, pathology, and follow-up data in paper-based case report forms (CRFs). Subsequently, these details were digitized into our database. Before the trial commencement and prior to patient enrollment, all involved physicians underwent comprehensive training on completing the CRFs. To ensure patient confidentiality, no personally identifiable information was recorded. Data quality control was the responsibility of a principal investigator.
Statistical analysis
Sample size determination was facilitated using IBM SPSS SamplePower software (Version 3.0; Armonk, NY: IBM Corporation; for identification purposes only). Based on our null hypothesis, we postulated that the integration of SLN biopsy into the conventional care protocol (total hysterectomy with BSO) would result in treatment modifications for 10% of patients with presumed low - and intermediate-risk endometrial cancer. This estimation was influenced by existing data indicating a 9–11% prevalence of SLN metastases [16,17]. Further parameters incorporated into our calculations included an error margin of 6%, a type I error rate of 0.05, and a power of 0.8. Anticipating a potential 5% cohort attrition due to loss to follow-up, the derived sample size totaled 102 patients. Subsequent statistical evaluations were executed using IBM SPSS Statistics software for Windows (Version 23.0; Armonk, NY: IBM Corporation; brand names are used for clarity, not endorsement). The data were depicted with continuous variables being displayed as medians accompanied by the interquartile range (IQR) and categorical variables as proportions.
2. Left external iliac SLN identified by near-infrared fluorescence after ICG injection into the cervix. 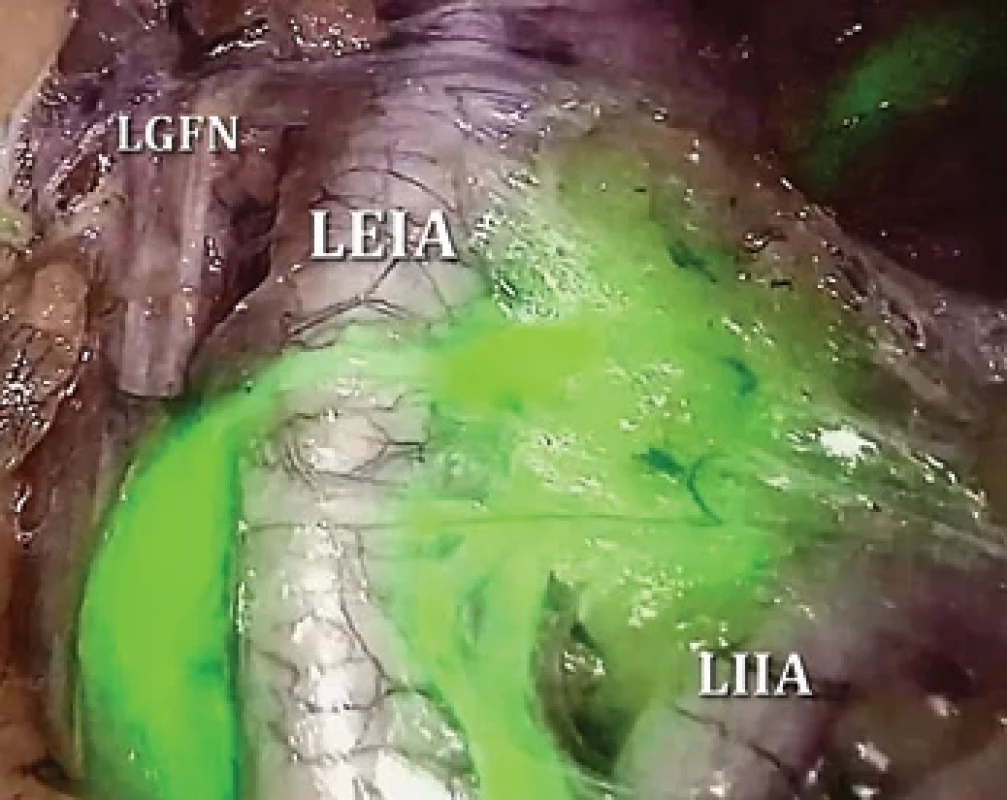
ICG – indocyanine green, LEIA – left external iliac artery, LGFN – left genitofemoral nerve, LIIA – left internal iliac artery, SLN – sentinel lymph node 2. Patient and procedure characteristics. 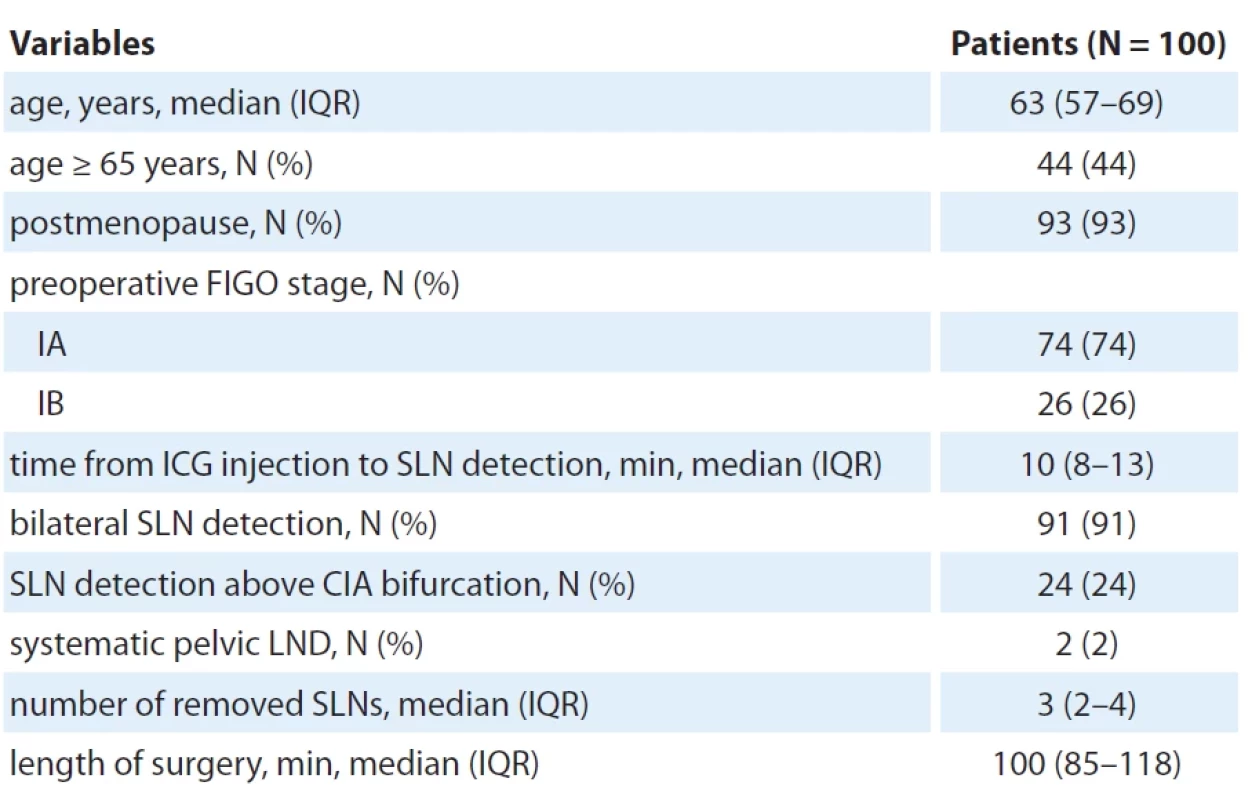
CIA – common iliac artery, FIGO – International Federation of Gynaecology and Obstetrics, ICG – indocyanine green, IQR– interquartile range, LND – lymph node dissection, min – minutes, SLN – sentinel lymph node Results
Participants
From the SENTRY trial, 102 consecutive patients were enrolled. Due to equipment malfunctions during surgery, 2 patients were excluded, leading to 100 participants in the final analysis. Enrollment spanned from July 2021 to February 2023. The participants‘ median age stood at 63 (IQR 57–69) years, with 44% (N = 44) aged 65 years or above (Tab. 2). A significant majority, 93% (N = 93), were postmenopausal. All patients had a preoperative confirmation of low-grade endometrioid adenocarcinoma categorized into presumed low risk (stage IA) at 74% (N = 74) and intermediate risk (stage IB) at 26% (N = 26).
SLN biopsy and surgery
The median time post-ICG injection to SLN detection was 10 (IQR 8–13) min (Tab. 2). SLNs were detected bilaterally in 91% (N = 91) of participants; 24% (N = 24) had their SLNs identified above the common iliac artery. For two patients under presumed intermediate risk, a pelvic LND was executed due to failed SLN detection even after re-administering ICG. Other patients were spared from systematic LND given the significant risk of postoperative complications of this procedure. Median number of removed SLNs was 3 (IQR 2–4) and the median surgery duration was 100 (IQR 85–118) min.
Patient outcomes
The process faced no intraoperative complications linked to the SLN mapping and biopsy (Tab. 3). Both 30-day major morbidity and mortality rates were zero. The SLN metastasis rate was 8% (N = 8), with macro-metastases in 7% (N = 7) and isolated tumor cells (ITC) in 1% (N = 1). Notably, every case with a positive SLN had the metastatic lymph node in either the obturator or external iliac region. Metastatic SLNs in atypical locations (mostly presacral and rarely parametrial) never presented as a sole metastasis. Our cohort lacked any skip metastases in the para - aortic zone. LVSI (+) was observed in 15% (N = 15) of cases. Throughout a median follow-up of 14 months (95% CI 12–15), neither lymphedema nor pelvic recurrence surfaced in the cohort. The last enrolled patient had a 9-month follow-up and no participants were lost to follow-up to date.
3. Patient outcomes. 
FIGO – International Federation of Gynaecology and Obstetrics, IQR – interquartile range, LVSI – lymphovascular space invasion, min – minutes, SLN – sentinel lymph node Change of adjuvant treatment
Post-pathology report, FIGO staging was adjusted for 20% (N = 20) of the patients: 15% (N = 15) underwent upstaging and 5% (N = 5) – downstaging (Tab. 3). SLN status altered the adjuvant treatment course for 20% (N = 20) of participants. SLN biopsy results enabled 12% (N = 12) of patients with LVSI (+) and cervical stroma involvement, but a negative SLN biopsy, to abstain from external beam radiotherapy (EBRT). On the other hand, 6% (N = 6) required adding adjuvant treatment due to SLN metastases resulting in an administration of chemoradiotherapy combined with systemic chemotherapy. Without the SLN biopsy results, these patients would have been limited to observation or vaginal brachytherapy. For two specific cases with pT2 and pT3a and positive pelvic SLN, the radiation field was expanded to encompass the paraaortic region.
Discussion
The SENTRY trial evaluated the role of SLN biopsy in tailoring postoperative management for patients with presumed low - and intermediate-risk endometrial cancer. Our data demonstrated that this approachable and safe procedure influenced the adjuvant treatment decisions in every fifth patient. The majority of these changes led to the prevention of unnecessary EBRT. However, it is notable that 8% of our cohort had SLN metastases without other evident high-intermediate - or high-risk features. This led to an escalation in postoperative treatment to encompass EBRT with or without systemic chemotherapy. Of these, six patients lacked tumor features that would conventionally indicate the need for EBRT. With a bilateral SLN detection rate of 91%, our outcomes are consistent with other studies and align with the ESGO quality benchmarks [11,14,18,19].
While the ESGO guidelines promote ultrastaging for SLN pathology assessment, we chose not to adopt this protocol [3]. Firstly, the financial and time constraints posed significant challenges for a high-volume oncology center in a middle-income country that relies on government-funded healthcare with an annual expenditure equivalent to 6.3% of gross domestic product in 2021 [20,21]. Secondly, existing prospective data on the implications of low-volume metastases, when considering adjuvant treatment decisions, are inconclusive. In their retrospective analysis Plante et al. found comparable recurrence-free and overall survival rates between SLN-negative patients, those with micro-metastases, and patients with ITC [22]. Another study by Backes et al. indicated no association of adjuvant therapy with long-term prognosis among 175 patients with ITC in stage I–II endometrioid endometrial cancer [23]. Ghoniem et al. highlighted micro-metastases as a standalone recurrence prognostic indicator in their multi-institutional retrospective analysis [24]. It is also worth noting that the prevailing evidence on adjuvant therapy efficacy, as demonstrated by the PORTEC III and GOG 258 trials, predominantly revolves around uterine and lymph node attributes [25,26]. In instances where lymph node evaluation was executed, it was predominantly via pelvic +/ – paraaortic LND, thus inadvertently uncovering low-volume metastases on occasion. Our decision to sidestep ultrastaging could have potentially accounted for the lower SLN metastases incidence observed in our study relative to previous reports [27].
It is well-documented that there can be a discrepancy between pre - and postoperative tumor type and grade with reports suggesting it in up to 40% of cases [28]. Yet in the SENTRY trial, such discrepancies were notably absent. The unwavering uniformity of histologic type and grade can possibly be attributed to the reviews conducted by pathologists specializing in gynecologic oncology. However, we must also consider the potential for confirmation bias to play a role. Our study predominantly encompassed patients diagnosed with low-grade endometrial cancer. While the intermediate-risk category does cover high-grade endometrioid tumors with limited myometrial invasion and non-endometrioid tumors without endometrial infiltration, we consciously excluded these patient groups. Given that neither ultrasound nor MRI offers absolute accuracy in ruling out deep myometrial invasion [29], the incorporation of these tumor types might have increased the upstaging rate as well as the cohort heterogeneity.
The ESGO and National Comprehensive Cancer Network (NCCN) guidelines emphasize that significant LVSI in cases of uterine-confined endometrioid cancer necessitates EBRT irrespective of lymph node metastases [3,7]. This advisory primarily derives from the PORTEC II findings, which presented superior patient outcomes with EBRT over vaginal brachytherapy [30]. However, it is essential to note that staging lymphadenectomy was an exclusion criterion and the observed diminished local recurrence in the EBRT cohort could be attributable to its efficacy against possible lymph node metastases. Given the high precision of SLN biopsy in discerning lymph node metastases, we sidestepped EBRT for patients exhibiting LVSI (+) but without lymph node involvement and other high-risk attributes mandating EBRT.
The consensus is that low - and intermediate-risk patient groups demonstrate a scant likelihood of lymph node metastases making the omission of EBRT feasible for unstaged individuals [3]. Periodically though, additional risk factors emerge post-uterus evaluation. We anticipated our trial would validate the redundancy of overtreatment. This was evident in 12% of patients, who post-operationally displayed heightened risk markers, predominantly cervical stroma invasion and LVSI (+). Nonetheless, SLN metastases were identified in 8% of our participants, and for 6% of them, it was the solitary determinant for adjuvant therapy. These findings do not correspond with the data of Burg et al., who observed a de-escalation in only 2% of their cases [17]. Their study accentuated that the primary impact of SLN biopsy, particularly in the assumed low - and intermediate-risk clusters, was the initiation of supplementary adjuvant treatment due to lymph node metastases.
While recent guidelines from ESGO and NCCN advocate for SLN biopsy even in patients perceived to be of low and intermediate risk, there are variations in local guidelines and clinical practices across different regions [12]. In countries and institutions where pelvic lymph node dissection remains a preference for these cohorts, SLN biopsy can serve as a valuable alternative to reduce the incidence of lower extremity lymphedema and other post-LND complications [31]. When total hysterectomy and BSO stand as the normative treatment, SLN biopsy offers the capability to calibrate postoperative treatments without escalating the risks of intra - and postoperative complications. Yet it is critical to acknowledge that the current understanding of the SLN biopsy’s long-term implications is largely anchored in retrospective analyses underscoring the pressing need for more prospective and randomized controlled trials.
The SENTRY trial has its share of limitations. To start with, our study is single-centered and does not encompass a parallel control arm. Introducing a control group with LND as a comparator intervention for the SLN biopsy arm was discounted given that lymphadenectomy is widely deemed excessive for patients with promising low-risk profiles. A cohort undergoing total hysterectomy and BSO without any lymph node evaluation would have illuminated the extent of overtreatment but remained opaque regarding undertreatment. Furthermore, certain factors like pT2 and LVSI (+) were not deemed decisive for EBRT, especially if N0 status was confirmed via SLN biopsy or pelvic LND. While this deviates from common practice, our approach was informed by the outcomes of PORTEC I and PORTEC II trials, which highlighted enhanced local control post-EBRT without any advancements in overall survival [30,32]. Another consideration is the relatively brief median follow-up period of 14 months in our trial constraining our ability to draw more conclusive remarks on lymphedema and pelvic recurrence rates – our assertions in this domain, hence, may lack finality. However, given that this is the inaugural prospective study on SLN biopsy within this patient subset in our country, we felt compelled to share our findings with our peers both domestically and internationally, particularly in institutions, where such an approach is not widely adopted. We remain committed to continued follow-up of our study participants and intend to release their long-term outcomes in subsequent publications.
Conclusions
The SENTRY trial demonstrates the potential of SLN biopsy as a valuable tool in the postoperative management of patients with presumed low - and intermediate-risk endometrial cancer. Our findings indicate that this safe procedure results in significant alteration of the adjuvant treatment plans for a substantial proportion of patients, primarily avoiding overtreatment with EBRT. The SENTRY trial also underscores an unmet need for additional randomized controlled trials to confirm the long-term impacts of SLN biopsy and serves as a foundation for further exploration in this context, especially in regions, where it is not yet standard practice.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The dataset used and analyzed during the current study is available from the corresponding author upon reasonable request
Sources
1. Siegel RL, Miller KD, Fuchs HE et al. Cancer statistics, 2022. CA Cancer J Clin 2022; 72 (1): 7–33. doi: 10.3322/caac.21708.
2. Colombo N, Creutzberg C, Amant F et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diag - nosis, treatment and follow-up. Radiother Oncol 2015; 117 (3): 559–581. doi: 10.1016/j.radonc.2015.11.013.
3. Concin N, Matias-Guiu X, Vergote I et al. ESGO/ ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 2021; 31 (1): 12–39. doi: 10.1136/ijgc-2020-002230.
4. Daix M, Angeles MA, Migliorelli F et al. Concordance between preoperative ESMO-ESGO-ESTRO risk classification and final histology in early-stage endometrial cancer. J Gynecol Oncol 2021; 32 (4): e48. doi: 10.3802/jgo.2021.32.e48.
5. Kitchener H, Swart AM, Qian Q et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet 2009; 373 (9658): 125–136. doi: 10.1016/S0140-6736 (08) 6 1766-3.
6. Benedetti Panici P, Basile S, Maneschi F et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst 2008; 100 (23): 1707–1716. doi: 10.1093/jnci/djn397.
7. Abu-Rustum N, Yashar C, Arend R et al. Uterine neoplasms, version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 2023; 21 (2): 181–209. doi: 10.6004/jnccn.2023.0006.
8. Ballester M, Dubernard G, Lécuru F et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO). Lancet Oncol 2011; 12 (5): 469–476. doi: 10.1016/S1470-2045 (11) 70070-5.
9. Rossi EC, Kowalski LD, Scalici J et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol 2017; 18 (3): 384–392. doi: 10.1016/S1470-2045 (17) 30 068-2.
10. Burg LC, Hengeveld EM, In’t Hout J et al. Ultrastaging methods of sentinel lymph nodes in endometrial cancer – a systematic review. Int J Gynecol Cancer 2021; 31 (5): 744–753. doi: 10.1136/ijgc-2020-001964.
11. Khoury-Collado F, Glaser GE, Zivanovic O et al. Improving sentinel lymph node detection rates in endometrial cancer: how many cases are needed? Gynecol Oncol 2009; 115 (3): 453–455. doi: 10.1016/j.ygyno.2009. 08.026.
12. Nechushkina VM, Kolomiets LA, Kravets OA et al. Practical recommendations for drug treatment of uterine cancer and uterine sarcomas. Malignant Tumors 2020; 10 (3s2–1): 242–256.
13. Shrestha B, Dunn L. The declaration of Helsinki on medical research involving human subjects: a review of seventh revision. J Nepal Health Res Counc 2020; 17 (4): 548–552. doi: 10.33314/jnhrc.v17i4.1042.
14. Persson J, Salehi S, Bollino M et al. Pelvic sentinel lymph node detection in high-risk endometrial cancer (SHREC-trial) – the final step towards a paradigm shift in surgical staging. Eur J Cancer 2019; 116 : 77–85. doi: 10.1016/j.ejca.2019.04.025.
15. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240 (2): 205–213. doi: 10.1097/01.sla.0000133083.54934.ae.
16. Chi DS, Barakat RR, Palayekar MJ et al. The incidence of pelvic lymph node metastasis by FIGO staging for patients with adequately surgically staged endometrial adenocarcinoma of endometrioid histology. Int J Gynecol Cancer 2008; 18 (2): 269–273. doi: 10.1111/j.1525-1438.2007.00996.x.
17. Burg LC, Kruitwagen R, de Jong A et al. Sentinel lymph node mapping in presumed low - and intermediate-risk endometrial cancer management (SLIM): a multicenter, prospective cohort study in the netherlands. Cancers 2022; 15 (1): 271. doi: 10.3390/cancers150 10271.
18. Concin N, Planchamp F, Abu-Rustum NR et al. European Society of Gynaecological Oncology quality indicators for the surgical treatment of endometrial carcinoma. Int J Gynecol Cancer 2021; 31 (12): 1508–1529. doi: 10.1136/ijgc-2021-003178.
19. Abu-Rustum NR. Update on sentinel node mapping in uterine cancer: 10-year experience at Memorial Sloan-Kettering Cancer Center. J Obstet Gynaecol Res 2014; 40 (2): 327–334. doi: 10.1111/jog.12227.
20. The World Bank Open Data. [online]. Available from: https: //data.worldbank.org/country/russian-federation? view=chart. Accessed 15.12.2023.
21. Klepach AN, Luk‘yanenko RF. Healthcare in Russia: macroeconomic parameters and structural issues. Stud Russ Econ Dev 2023; 34 (2): 207–220. doi: 10.1134/S10 75700723020065.
22. Plante M, Stanleigh J, Renaud MC et al. Isolated tumor cells identified by sentinel lymph node mapping in endometrial cancer: does adjuvant treatment matter? Gynecol Oncol 2017; 146 (2): 240–246. doi: 10.1016/ j.ygyno.2017.05.024.
23. Backes FJ, Felix AS, Plante M et al. Sentinel lymph node (SLN) isolated tumor cells (ITCs) in otherwise stage I/II endometrioid endometrial cancer: to treat or not to treat? Gynecol Oncol 2021; 161 (2): 347–352. doi: 10.1016/ j.ygyno.2021.02.017.
24. Ghoniem K, Larish AM, Dinoi G et al. Oncologic outcomes of endometrial cancer in patients with low-volume metastasis in the sentinel lymph nodes: an international multi-institutional study. Gynecol Oncol 2021; 162 (3): 590–598. doi: 10.1016/j.ygyno.2021.06.031.
25. de Boer SM, Powell ME, Mileshkin L et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol 2019; 20 (9): 1273–1285. doi: 10.1016/S1470-2045 (19) 30395-X.
26. Matei D, Filiaci V, Randall ME et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med 2019; 380 (24): 2317–2326. doi: 10.1056/NEJMoa1813181.
27. Holloway RW, Gupta S, Stavitzski NM et al. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecol Oncol 2016; 141 (2): 206–210. doi: 10.1016/j.ygyno.2016.02.018.
28. Karateke A, Tug N, Cam C et al. Discrepancy of pre - and postoperative grades of patients with endometrial carcinoma. Eur J Gynaecol Oncol 2011; 32 (3): 283–285.
29. Alcázar JL, Gastón B, Navarro B et al. Transvaginal ultrasound versus magnetic resonance imaging for preoperative assessment of myometrial infiltration in patients with endometrial cancer: a systematic review and meta-analysis. J Gynecol Oncol 2017; 28 (6): e86. doi: 10.3802/jgo.2017.28.e86.
30. Wortman BG, Creutzberg CL, Putter H et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br J Cancer 2018; 119 (9): 1067–1074. doi: 10.1038/s41416-018-0310-8.
31. Glaser G, Dinoi G, Multinu F et al. Reduced lymphedema after sentinel lymph node biopsy versus lymphadenectomy for endometrial cancer. Int J Gynecol Cancer 2021; 31 (1): 85–91. doi: 10.1136/ijgc-2020-001924.
32. Creutzberg CL, Nout RA, Lybeert ML et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys 2011; 81 (4): e631–e638. doi: 10.1016/j.ijrobp.2011.04.013.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2024 Issue 2-
All articles in this issue
- Editorial
- Significance of aberrant DNA methylation for cancer diagnostics and therapy
- Prostate cancer invasion is promoted by the miR-96-5p-induced NDRG1 deficiency through NF-κB regulation
- Potential application of body fluids autofluorescence in the non-invasive diagnosis of endometrial cancer
- Feasibility of implementation of the early tumor shrinkage as a potential predictive marker to daily clinical practice in patients with RAS wild type metastatic colorectal cancer, treated with cetuximab – a non-interventional observational study
- Factors influencing overall survival and GvHD development after allogeneic hematopoietic stem cell transplantation – single centre experience
- Tailoring postoperative management through sentinel lymph node biopsy in low- and intermediate-risk endometrial cancer – the SENTRY clinical trial
- Tebentafusp in the treatment of metastatic uveal melanoma – the first patient treated in the Czech Republic
- Carcinoid syndrome with right-sided valve involvement – a case report and review of the literature
- REPORTS FROM THE LITERATURE
- STUDY REPORT
- VARIOUS
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Significance of aberrant DNA methylation for cancer diagnostics and therapy
- Factors influencing overall survival and GvHD development after allogeneic hematopoietic stem cell transplantation – single centre experience
- Carcinoid syndrome with right-sided valve involvement – a case report and review of the literature
- Tailoring postoperative management through sentinel lymph node biopsy in low- and intermediate-risk endometrial cancer – the SENTRY clinical trial
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

