-
Medical journals
- Career
Microsurgical replantation after forehead avulsion – success or failure? A case report
Authors: M. Kubík 1; M. Molitor 2; B. Zálešák 1
Authors‘ workplace: Department of Plastic Surgery, 1st Faculty of Medicine, Charles University and University Hospital Bulovka, Prague, Czech Republic 1; Department of Plastic and Aesthetic Surgery, Faculty of Medicine and Dentistry, Palacký University Olomouc and Olomouc University Hospital, Czech Republic 2
Published in: ACTA CHIRURGIAE PLASTICAE, 66, 1, 2024, pp. 16-21
doi: https://doi.org/10.48095/ccachp202416Introduction
Replantation is a surgical procedure intended to reattach a body part that has been completely separated – amputated from a person’s body during trauma. The goal is to restore structures and their original position, shape, look and function. In general, the possibility of using the original body part during replantation gives the best possible solution and outcome for the patient.
The first replantation was performed by Ronald A. Malt (Boston, USA) in 1962. The patient was a 12-year-old child with an amputated proximal humerus, and no magnification in terms of a microscope was available at that time. In 1963, the first reported replantation with a microscope was performed on a patient – a mechanist presented with an amputated arm at the level of the distal forearm and was operated on by Chen Zhongwei in Shanghai 6th People’s Hospital in China [1].
Some important milestones of replantation in the facial region are listed below. The first microvascular replantation of an avulsed scalp was described by Miller et al. in 1976 when both superficial temporal arteries along with five veins were successfully re-anastomosed [2].
Generally, forehead avulsion injuries with microanastomosis reconstruction have been published so far mainly as a part of complex facial avulsions with periorbital or hemifacial region degloving, and nose, lip, scalp and ear avulsions [3–6].
One of the first published accounts of nasal reattachment was the case of Fioraventi (1570). A gentleman regrettably argued with a Spanish soldier, who subsequently cut off the man’s nose in a duel. Fioravanti cleansed the nose and bound it in place for 10 days. The replant survived (no microanastomosis was used so it was no true replantation) [7]. The first successful microvascular nasal replantation was published by Norman J. James in 1976 (USA) [8]. The first successful ear replantation was performed by Pennigton in 1980 in Sydney [9].
Case presentation
A 57-year-old male patient suffered an amputation injury of the left half of his forehead after a dog bite (Fig. 1, 2). The owner of the dog is a friend of the patient’s son, who commanded the dog to attack the man during a quarrel.
The patient takes no medication, has no significant medical history apart from nicotine abuse – smoking 15 cigarettes a day.
The patient was admitted to the university hospital emergency unit and after basic examination was transferred to the plastic surgery department for replantation surgery. Initial unfavorable conditions, such as dog bite injuries with a higher risk of infection and avulsion/contusion mechanisms of the injury, are rather challenging for a good outcome.
1. Forehead avulsion defect. Source: archive of authors. 
2. Forehead avulsion fl ap. Source: archive of authors. 
3. Microanastomosis of supratrochlear vessels. Source: archive of authors. 
4. Vital fl ap after replantation. Source: archive of authors. 
Surgical procedure
The dog bite caused avulsion of a full-thickness skin flap of the left forehead, sized 7 × 6 cm with partial loss of the left eyebrow. The patient brought in the flap packed and surrounded by ice cubes. Other minor facial wounds along with an additional significant one on the right forehead that had a triangular shape 2 × 1 × 2 cm was treated with simple sutures.
Under general anesthesia, after debridement, the very thin and fragile supratrochlear artery and vein were identified. Both vessels were sutured with 10-0 threads (Fig. 3), revascularization was successful, arterial and venous blood circulation was reestablished, followed by insetting the flap onto the original position. In the eyebrow region, there was a residual defect 5 × 5 mm in size. During skin suturing, there was a short episode of flap ischemia, most probably due to spasms with a good response to massage and papaverine treatment of the vessels after which the flap was fully vital again with good capillary refill (Fig. 4). Cefazolin, gentamicin, heparin, hydrocortisone, and Voluven were administered during the procedure.
Medication
Perioperatively, he was given intravenous antibiotics, anticoagulation and antiedemic medication: heparin continuously 10,000 IU/24 hrs for 7 days, low molecular weight heparin – clexane subcutaneously 0.4 mL/24 hrs, magnesium sulfate 200 mg/mL continuously 4,000 mg/24 hrs for 7 days, and Dexamed 8 mg intramuscular every 12 hrs for 3 days, then every 24 hrs for 3 days.
5. Fifth post-op day during dressing change. Source: archive of authors. 
6. Flap ischemia 2 hrs after the dressing change. Source: archive of authors. 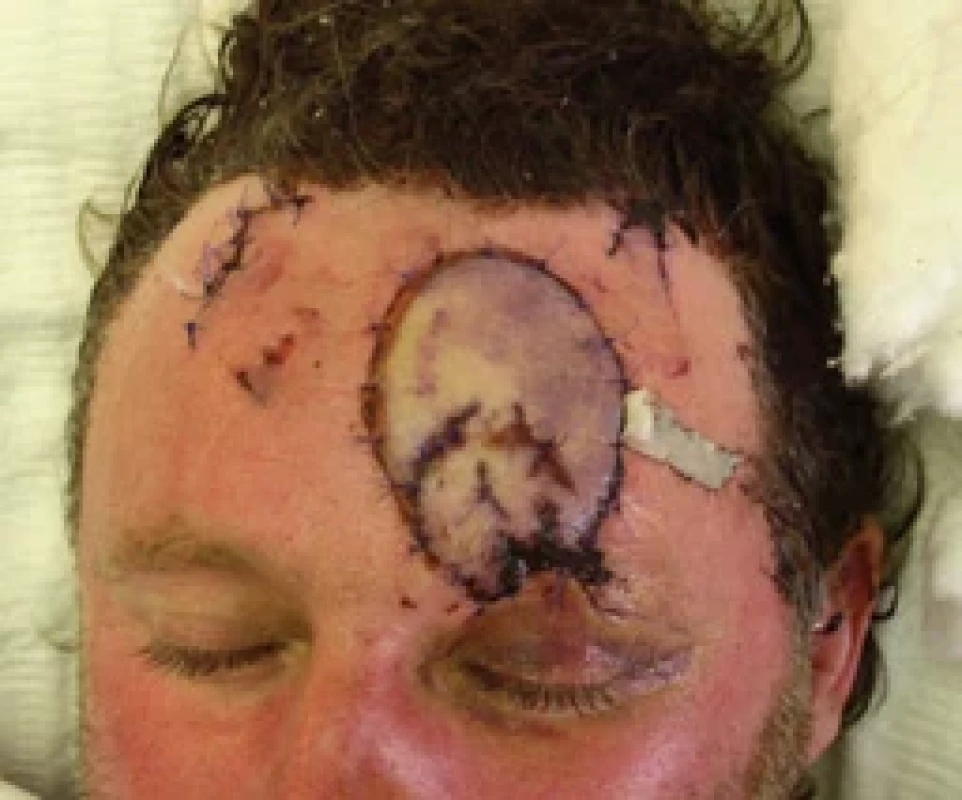
7. Flap reperfusion after revision surgery. Source: archive of authors. 
8. Flap ischemia reoccurs the day after revision. Source: archive of authors. 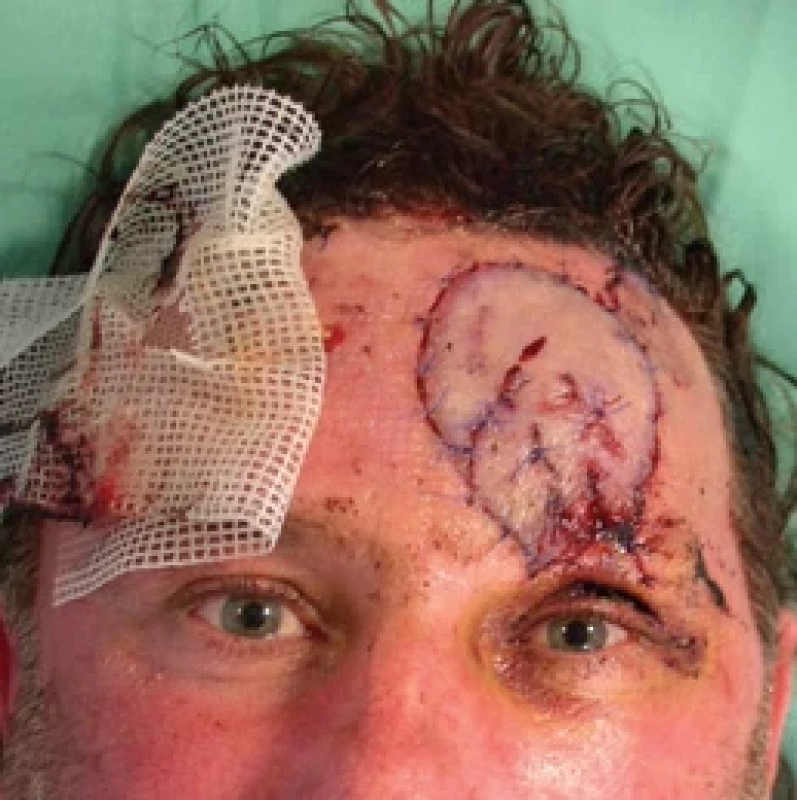
Course of the hospital stay
After the successful primary surgery with restoration of blood circulation in the flap, there was no significant complication (Fig. 5).
On the 5th post-operative day, 2 hrs after a regular change of the wound dressing, an acute ischemia of the flap occurred (Fig. 6).
After failure of conservative therapy – heparin bolus, spasmolytics, and corticoids, the patient was indicated for acute revision surgery to find an arterial thrombosis in the very fragile and easy-to-spasm vessels. The flap reperfusion was restored once again (Fig. 7).
Unfortunately, the very following day (6th post-operative day), the same ischemic complications recurred (Fig. 8, 9), and due to the time passed from the injury and primary surgery together with unfavorable conditions of the wound and sutured vessels (avulsion injury mechanism, fragility, spasmic vessels), no further revision surgery was indicated. The flap was to be observed and treated with intravenous and local medication with plans for possible necrosis of the flap to be handled after demarcation.
Partial necrosis of the flap occurred, approximately 50% (Fig. 10), which was subsequently solved mainly with superficial necrectomy (Fig. 11) and a full-thickness skin graft (FTSG) with a very good result (Fig. 12) leading to patient satisfaction (Fig. 13). Following the patient’s decision, he chose to harvest the FTSG from the groin region, especially because of the small donor site morbidity despite possible pigmentation dissimilarity.
9. Flap ischemia 3rd day after revision. Source: archive of authors. 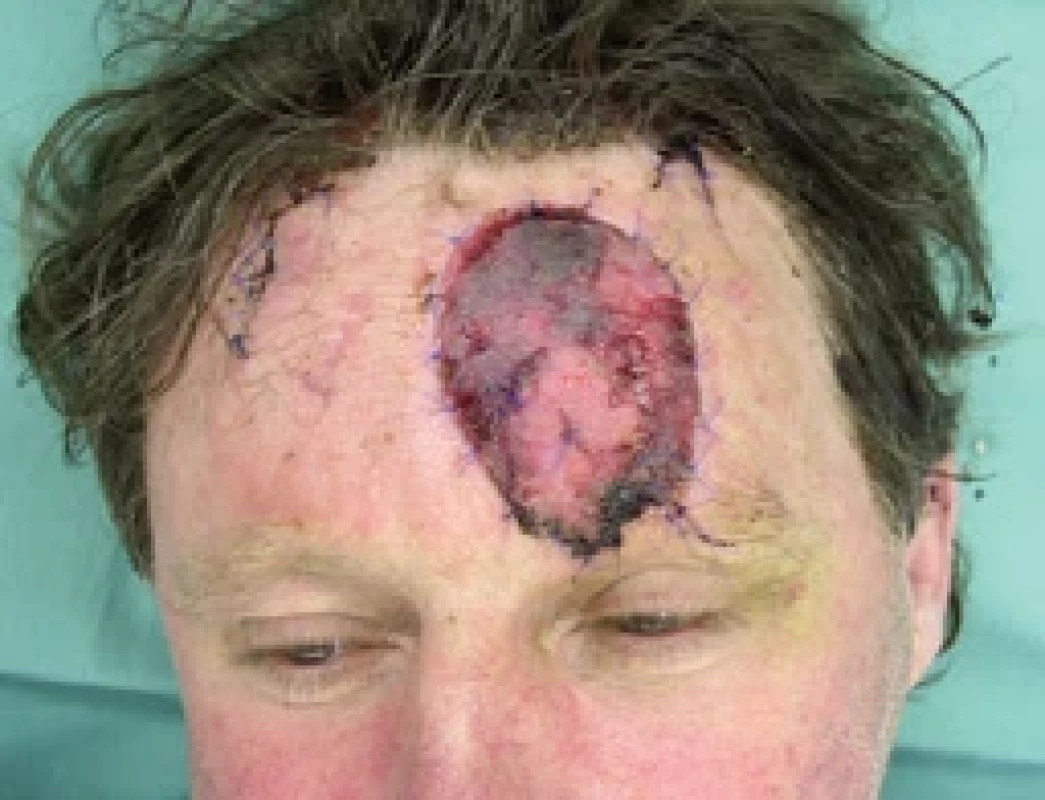
10. Flap necrosis 2 weeks after fl ap ischemia. Source: archive of authors. 
11. Necrectomy. Source: archive of authors. 
12. FTSG defect covering. Source: archive of authors. 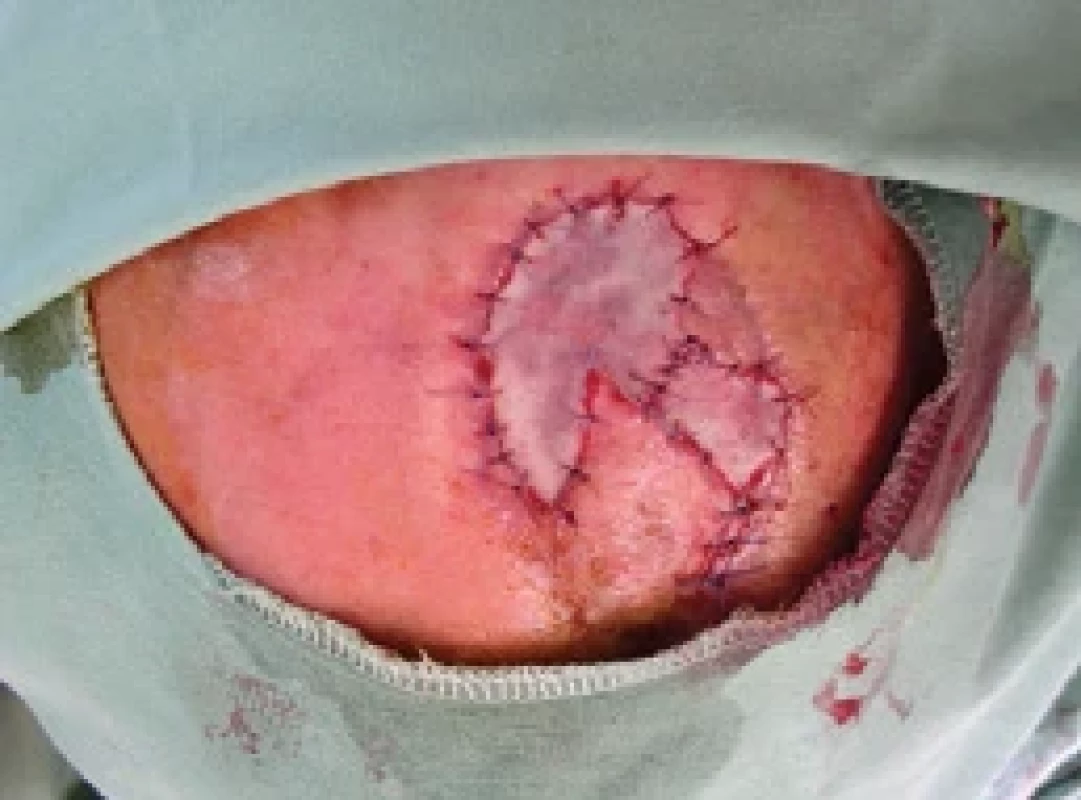
Discussion
Vulnera morsa are wounds considered to be infected and possess the contusion and avulsion mechanism influencing the quality of the vessels. Mitchell (Melbourne, Australia) described the possible extent of arterial damage of the intima and media up to 2–3 cm from the point of rupture during avulsion forces, which are more present on the proximal segments in 30–50% of cases; on the other hand, there were no observable, significant lesions to the veins [10]. During Glover’s experiments with rabbits, arteries were damaged even up to 6 cm of their length and again with no damage to veins [11].
Nicotine abuse is a common risk factor for wound healing in general, with microanastomosis in particular. The nutrition and gas exchange at the microvascular level is diminished, which could possibly cause ischemic complications. According to Mosely, in cases of extremity injury or surgery, smoking may adversely affect wound healing, and on a rabbit model – nicotine retarded wound healing especially from the 6th to the 10th day. However, there were equal rates of healing from the 12th to the 20th day of the observation period [12].
Smoking may especially affect small caliber vessels such as suptratrochlear artery and vein. In this particular case, the diameter of vessels was not measured directly, but we estimated the diameter to be between 0.6–0.8 mm. Supratrochlear artery is one of the terminal branches of ophthalmic artery (a. carotis interna). After exiting the orbit, it passes superficially to the m. corrugator supercilii and deep to the orbicularis oculi and frontalis muscles. Then, it ascends the forehead in a paramedian position, in a proximate distance of 2 cm from the midline. More specifically, proximally to the corrugator supercilii muscle, it branches off to give rise to a superficial and deep periosteal branch in a position 1.18 ± 0.36 cm distal to the supraorbital ridge and 1.35 ± 0.34 cm lateral to the midline. Average depth of the superficial branch from the epidermal surface was found to be 1.5 mm, where the deep branch penetrates the corrugator supercilii muscle before it reaches the pericranium. However, there have been some reports where the deep branch was actually not present at all.
According to the anatomy review of Agorgianitis L., the supratrochlear artery’s average diameter is reported to be approximately 1 mm with possible differences (0.8 mm) in the contralateral artery.
In replantation surgery, any appropriate vessels can be used. In our case, we used the supratrochlear vascular bundle, but there were more options and several arterial variations of the facial blood supply with a rich anastomotic network on the contralateral side as well [13].
The possible causes of ischemic complications may originate in arterial spasm with subsequent thrombosis, because of manipulation during dressing change or the occasional patient’s noncompliance in terms of smoking. Due to the previously mentioned reasons, we decided not to perform revision surgery again, but partially to rely on a possible scar neovascularization of the flap after almost 6 days since the replantation, which may be a matter of further discussion. If we consider scar tissue healing similar to skin grafting healing, the first signs of proper revascularization of a skin graft during neoangiogenesis are recognizable after 72 hrs with a full vascular supply around the 7th–10th day [14–17].
Even though moist wound healing was applied for the whole time to prevent desiccation together with vasodilatation medications, about 50% of the flap underwent necrosis. It occurred only on the peripheral part of the flap – distant from the anastomosed vessels, and only superficially – epidermis and partially the dermis. Despite the fact that spontaneous healing could be taken into account during a few weeks, we decided to use FTSG to accelerate the healing process. The overall length of the treatment was 3 weeks leading to the patient’s satisfaction at the end.
13. Healed, stitches removed. Source: archive of authors. 
14. Six months after trauma. Source: archive of authors. 
Conclusion
Forehead avulsion is mostly associated with avulsions of other parts of the head and face as well. Most of the time, it is a complex injury of the scalp, hemi-face, nose and ear. We could not find any publication referring to forehead microvascular reconstruction as a single unit. To our best knowledge, this particular case report is a first of its kind describing the replantation of a part of the forehead alone.
The surgical procedure is performed under unfavorable conditions (dog bite, avulsion, nicotine abuse) with a good outcome after all. Aesthetic and functional results with respect to negative circumstances together with the patient’s subjective opinion was all satisfactory and could be considered as a successful reconstruction (Fig. 14).
Roles of the authors
Marek Kubík, MD – author, data collection, manuscript preparation, study of the literature, translation;
Bohumil Zálešák, MD, PhD – manuscript correction, chief of the department;
Assoc. Prof. Martin Molitor, PhD, MBA – co-author, manuscript preparation and correction, treating surgeon.
Conflict of interest: Hereby we state, that in relation with the theme, creation, and publication of this manuscript, we are not in conflict of interest. The creation of the manuscript and its publication was not financially supported by any pharmaceutical or other company or other subject and none of the authors was influenced during the creation of the paper in any way.
Note: all of the images included are created by the authors and in their exclusive possession.
Sources
1. Wing-Yee CW. Evolution and clinical application of microsurgery. BMC Proc. 2015, 9 (Suppl 3): A53.
2. Miller GDH., Anstee EJ., Snell JA. Successful replatnation of anf avulsed scalp by microvascular anastomoses. Plast Reconstr Surg. 1976, 58 (2): 133–136.
3. Dadaci M., Yildirim MEC., Ince B. Experience of replantation and reconstruction in total scalp, partial forehead, and ear avulsions. J Craniofac Surg. 2019, 30 (7): 2268–2270.
4. Eren S., Hess J., Larkin GC. Total scalp replantation based on one artery and one vein. Microsurgery. 1993, 14 (4): 266–271.
5. Nasir S., Karaaltin M., Erdem A. Total scalp replantation. J Craniofac Surg. 2015, 26 (4): 1192–1195.
6. Hu W., Henry AS., Lucas C., et al. Microsurgical replantation of a two-segment total scalp avulsion. J Craniofac Surg. 2016, 27 (4): 1068–1069.
7. Miller PJ., Hertler C., Alexiades G., et al. Replantation of the amputated nose. Arch Otolaryngol Head Neck Surg. 1998, 124 (8): 907–910.
8. James NJ. Survival of large replanted segment of upper lip and nose. Plast Reconstr Surg. 1976, 58 (5): 623–625.
9. Pennington DG., Lai MF., Pelly AD., et al. Successful replantation of a completely avulsed ear by microvascular anastomosis. Plast Reconstr Surg. 1980, 65 (6): 820–823.
10. Mitchell GM., Frykman GK., Morrison WA., et al. The nature and extent of histopathologic injury in human avulsed arteries and veins and in experimentally avulsed monkey arteries. Plast Reconstr Surg. 1986, 78 (6): 801–810.
11. Glover M., Seaber A., Urbaniak J. Arterial intimal damage in avulsion and crush injuries in rat limbs. J Reconstr Microsurg. 1985, 1 (4): 247–251.
12. Mosely LH., Finseth F., Goody M. Nicotine and its effect on wound healing. Plast Reconstr Surg. 1978, 61 (4): 570–575.
13. Agorgianitis L., Panagouli E., Tsakotos G., et al. The supratrochlear artery revisited: an anatomic review in favor of modern cosmetic applications in the area. Cureus. 2020, 12 (2): e7141.
14. Young CMA. The revascularization of pedicle skin flaps in pigs. Plast Reconstr Surg. 1982, 70 (4): 455–464.
15. Calcagni M., Althaus MK., Knapik AD., et al. In vivo visualization of the origination of skin graft vasculature in a wild-type/GFP crossover model. Microvasc Res. 2011, 82 (3): 237–245.
16. Capla JM., Ceradini DJ., Tepper OM., et al. Skin graft vascularization involves precisely regulated regression and replacement of endothelial cells through both angiogenesis and vasculogenesis. Plast Reconstr Surg. 2006, 117 (3): 836–844.
17. Abdelhakim M., Dohi T., Yamato M., et al. A new model for specific visualization of skin graft neoangiogenesis using Flt1-tdsRed BAC transgenic mice. Plast Reconstr Surg. 2021, 148 (1): 89–99.
Marek Kubík, MD
Department of Plastic Surgery
University Hospital Bulovka
Budínova 67/2
180 81 Praha 8-Libeň
Czech Republic
e-mail: marek.kubik1@gmail.com
Submitted: 25. 9. 2023
Accepted: 29. 2. 2024
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2024 Issue 1-
All articles in this issue
- No drains in reduction mammaplasty – a systematic review
- Inset techniques for the DIEP flap – what improves aesthetic outcomes?
- Microsurgical replantation after forehead avulsion – success or failure? A case report
- Nail bed trauma reconstruction and artificial nail replacement – a case report
- Double right triangular shape full-thickness skin grafts technique for short rectangular or square shape donor site defect – original method with a case report
- Congenital isolated juvenile xanthogranuloma of the sole – a unique case report in a newborn
- Reports on vascular catheter-associated thromboembolic events in a burn unit – a gap in the literature?
- The top 10 AI tools for academic surgeons right now
- Editorial
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- No drains in reduction mammaplasty – a systematic review
- Nail bed trauma reconstruction and artificial nail replacement – a case report
- Inset techniques for the DIEP flap – what improves aesthetic outcomes?
- The top 10 AI tools for academic surgeons right now
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

