-
Medical journals
- Career
EXTENSION OF OROFACIAL CLEFT SIZE AND GESTATIONAL BLEEDING IN EARLY PREGNANCY
Authors: M. Peterka 1,3; J. Hrudka 1; M. Tvrdek 3; J. Velemínská 2; A. Panczak 3; J. Borský 3; Z. Likovský 1; R. And Peterkova 1
Authors‘ workplace: Department of Teratology, Institute of Experimental Medicine, Academy of Sciences of the Czech Republic, Prague 1; Department of Anthropology and Human Genetics, Faculty of Science, Charles University Prague, and 2; Department of Plastic Surgery, University Hospital Kralovske Vinohrady, Charles University Prague, Czech Republic 3
Published in: ACTA CHIRURGIAE PLASTICAE, 54, 2, 2012, pp. 39-44
INTRODUCTION
During early human development, a common oro-nasal cavity with continuous upper lip and jaw arch develops. Then the oro-nasal cavity divides into nasal and oral cavities as a consequence of palatal shelf fusion at midline and secondary palate formation. These developmental events take place from embryonic day 30 to 60. During this time the morphogenesis of the orofacial area can be affected, leading to the development of an orofacial cleft (1). The orofacial cleft is a very frequent malformation in newborns. Its incidence in the Czech Republic is 1 : 530 of living newborns (2).
There are three basic types of orofacial clefts ranked according to the increasing extension of cleft size: the cleft lip (CL) is caused by failing fusion of medial nasal with maxillary facial process between embryonic days 30–40; the cleft palate (CP) results from non-fusion of palatal shelves between embryonic days 40–60; finally, the CLP is the most extensive and severe cleft type in terms of its clinical and aesthetic impact on the patient. Its origin implies that the formation of the lip, jaw and palate was affected during the approximate period of embryonic days 30–60 (Fig. 1) (3–8). It is generally known that proportions of orofacial cleft types are different according to gender, CL and being more frequent in boys and CP in girls (9, 10, 11).
Fig. 1. Critical periods for the development an orofacial clefts in man. CL – cleft lip; CP – cleft palate; CLP – total (combined) cleft lip + cleft palate. These cleft types originate during three critical periods of orofacial development – I., II.a, II.b. x axis – embryonic day of origin of particular cleft type; y axis – theoretic proportion of cleft types in embryos 
The orofacial cleft is believed to be a multifactorial disorder caused by external and/or genetic factors. Gestational bleeding is a common complication of early pregnancy. According to published data, the incidence of gestational bleeding in the first trimester varies between 9–22% of all pregnancies (12–18). Approximately 66–75% of all gestational bleeding during pregnancy occurs in the first trimester (17, 19). There are several causes of gestational bleeding – e.g. disturbance or malfunction of placenta, threatened or ongoing spontaneous abortion (20). Literary data document that vaginal bleeding from the placenta threatens an embryo/fetus, depending on its intensity and duration, and may lead to spontaneous abortion in about 10% of women with gestational bleeding. If the embryo or fetus survives, the presence of an inborn abnormality is more probable than in a child of a non-bleeding mother (11, 12, 16, 17, 21–29).
We hypothesized that vaginal bleeding during the critical period of upper lip and palate development (see Fig. 1) might act as to produce non-specific worsening of embryo status resulting in extension of the basic cleft type (CL or CP) into the more serious CLP. Therefore we compared cleft type distributions in children with an orofacial cleft ranked in two groups according to the presence/absence of maternal gestational bleeding during the first trimester of pregnancy: 1. Offspring of bleeding mothers and 2. Comparison (control) group of offspring of non-bleeding mothers. We also took into account the sex of the child, hereditary factors and occurrence of additional malformations.
MATERIAL AND METHODS
In total we analyzed medical history records on 2524 children with orofacial clefts born in the Bohemia region of the Czech Republic during 1983–2009. These data were collected at the Department of Plastic Surgery, University Hospital Kralovske Vinohrady. This department specializes in the surgical treatment of cleft patients from the whole of Bohemia and provides surgery for the majority (more than 95%) of afflicted children. A normalized questionnaire was used to record data obtained by physicians during the first examination of a baby with orofacial cleft and interview its parents. In addition to the data about cleft type and additional malformations in a child, the case records also included data about maternal vaginal bleeding during the first trimester of pregnancy (critical period of orofacial cleft development) and orofacial cleft occurrence in the family.
In this study we took into account cleft type (CL, CP, CLP) in the child, presence or absence of an orofacial cleft in the family (positive family history suggesting a genetic predisposition), presence or absence of additional congenital malformations in the baby (of the heart, external ear, hypospadia, microphtalmia, etc.) and information about the presence or absence of bleeding during pregnancy of the mother. The general critical period for the origin of various cleft types is between embryonic days 30–60 (28) (see Fig. 1). Since the exact time of conception is usually not known in a retrospective study, we took into account maternal bleeding during the whole first trimester of gravidity to be sure we were covering the interval of the cleft origin period. Accordingly, the cleft patients were ranked into two groups: 1. Offspring of bleeding mothers and 2. Comparison (control) group of offspring of non-bleeding mothers. Both groups of cleft patients were collected from the same geographical region during the same time period.
As the next step, the probands were distributed according to presence or absence of clefts among relatives (positive or negative family history). We focused on comparing the percentage distribution of the three basic types of clefts (CL, CP, CLP), and compared the offspring of mothers with and without bleeding during the first trimester of pregnancy. Then we analyzed the group with negative family history in more detail, separately for boys and girls. As the last step, we compared the proportion of patients with additional congenital malformation between the mothers with and without vaginal bleeding. The statistical significance of results was tested using contingency table (chi-square), calculating p-value.
This study was performed in the line with Institutional Review Board (IRB) of the Institute of Experimental Medicine of the Academy of Sciences of the Czech Republic. IRB approved the implementation of the study according to the protocols.
RESULTS
The present group of 2524 children comprised 1436 boys and 1088 girls. Bleeding in the first trimester of pregnancy occurred in 253 (10.0%) of interviewed mothers. A cleft among relatives was reported in 497 (19.7%) and an additional malformation in 297 (11.8%) of probands. In the group of all patients, there were 656 (26.0%) cases of CL, 898 (35.6%) of CLP, and 970 (38.4%) of CP.
First we analyzed the distribution of cleft types in the children with negative family history (Graph 1A). The mothers without bleeding had 26.5% of CL, 33.0% of CLP, and 40.5% of CP. Interestingly, the mothers with bleeding in the first trimester had a different cleft distribution in their offspring: 23.9% of CL, 45.5% of CLP, and 30.6% of CP (Table 1, see Graph 1A). In the mothers with bleeding, the proportion of CLP in their offspring was significantly higher (p < 0.001) while the proportion of CP was significantly lower (p = 0.007) than in the mothers without bleeding.
Graph 1. Distribution of the 3 basic cleft types in children of both sexes without clefts in the family (A) and with clefts in the family (B) whose mothers had vaginal bleeding, compared with children whose mothers did not have gestational bleeding in the first trimester of pregnancy. ** indicates a statistically significant difference p < 0.01 
1. Number and percent of three basic types of cleft compared between non-bleeding and bleeding mothers, divided according cleft among relatives of child, the childern without cleft among relatives then sorted according to gender 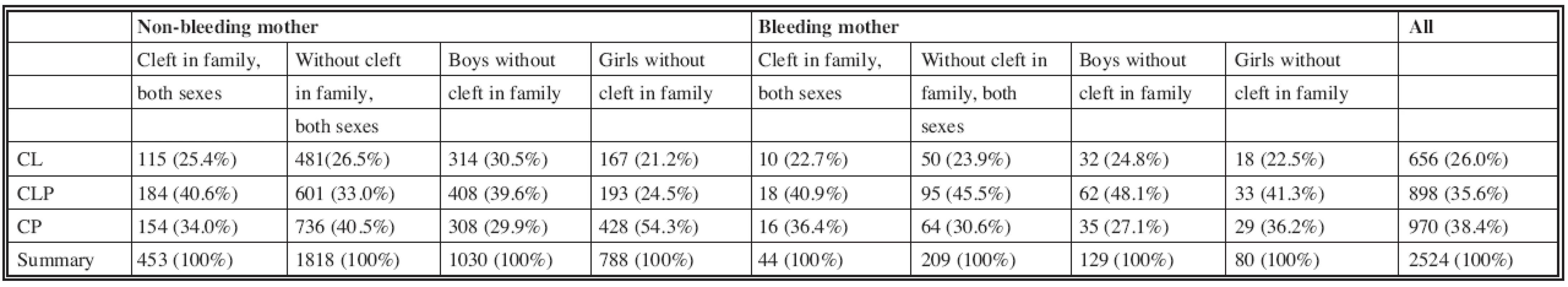
Then we established whether maternal bleeding is also associated with a change in cleft distribution in a group of children with presence of an orofacial cleft among relatives (children with positive family history) (Graph 1B). Distinctively different results were found: the percentage of CL, CLP and CP were almost the same in children of non-bleeding and bleeding mothers (see Table 1, see Graph 1B).
In order to reveal whether change in cleft distribution concerns more boys or girls in a group of probands without clefts in the family (see Graph 1A), a comparison of cleft distributions was then performed separately in boys and girls (see Graph 2). The distribution of cleft types in the sons of mothers without bleeding was 30.5% of CL, 39.6% of CLP and 29.9% of CP, while the cleft distribution in the sons of mothers with bleeding was 24.8% of CL, 48.1% of CLP and 27.1% of CP (see Table 1, Graph 2A). Compared to the mothers without bleeding, there was a lower percentage of sons with CL (not statistically significant), almost the same percentage of CP and a significantly higher (p = 0.030) percentage of sons with CLP in the mothers with bleeding.
Graph 2. Distribution of the 3 basic cleft types in boys (A) and girls (B) without cleft in the family whose mothers had gestational bleeding, compared with children whose mothers did not have gestational bleeding in the first trimester of pregnancy. ** indicates a statistically significant difference p < 0.01, * indicates p < 0.05 
Interestingly, we found higher statistically significant differences between cleft distribution in the group of girls with negative family history (Graph 2B). Among the daughters of mothers without gestational bleeding, there were 21.2% of CL, 24.5% of CLP and 54.3% of CP; among the daughters of mothers with bleeding, there were 22.5% of CL, 41.3% of CLP and 36.2% of CP (see Table 1, see Graph 2B). Compared to the mothers without bleeding, there was a statistically significant increase in the proportion of daughters with CLP (p = 0.002), and a statistically significant decrease in the proportion of daughters with CP (p = 0.003).
The incidence of maternal vaginal bleeding in the first trimester of pregnancy was compared between the groups of children with and without an additional malformation. In our set, there were 297 cleft-children with an additional malformation, and 38 mothers (12.8%) of those children reported bleeding. Among 2227 children without any additional malformation, 215 mothers (9.7%) reported bleeding. Although vaginal bleeding was slightly more frequent in the mothers of children with additional malformations, this difference was not statistically significant (p = 0.112).
DISCUSSION
Among children with orofacial clefts, self-reported history of first trimester maternal bleeding was correlated with a higher probability of CLP compared with other cleft subtypes. In the offspring of the mothers with vaginal bleeding, there was increased proportion of children with CLP at the expense of CP in the group of non-hereditary clefts. In the group of hereditary clefts we did not find any change in cleft type proportions.
As far as cleft extent is concerned, CL or CP are considered less serious affections, while the combined CLP represents a major malformation. Compared to the mothers without gestational bleeding, mothers with bleeding gave birth to statistically significantly more children with CLP. In general, the increase of CLP correlated with a decrease in the proportion of girls with CP and of boys with CL.
There is a different predisposition to a particular cleft type between the genders – boys are more likely to have CL and CLP, while CP is more common among girls (9, 10, 11). This rule was also confirmed by the present results (see Graph 2). A possible interpretation of the change in cleft distribution in offspring of the mothers with bleeding (see Graph 1A, see Graph 2) may be that the extension of the cleft increased, and the simple cleft type (CP in female embryos or CL in male embryos) was transformed into the more severe combined CLP. The critical period of origin of CL (cca ED 30–40) anticipates that of the CP (cca ED 40–60). The combined CLP originates during the summarized critical periods for CL+CP (see Fig. 1). Accordingly, the increase in the number of CLP might be explained by an extension of exposure time to a harmful factor to an earlier period (of CL origin) in female embryos with prospective CP, or to a later period (of CP origin) in the male embryos with CL (compare to Fig. 1). We can conclude that gestational bleeding in the first trimester of pregnancy is associated with cleft-size increase, i.e. the origin of the most severe cleft type – CLP. Felix-Schollaart et al. (30) have compared the reproductive histories of a group of 87 children with orofacial clefts (55 males and 32 females) to evaluate if CL, CLP, and CP are homogeneous entities. They found gestational bleeding and spontaneous abortions in the first trimester more often in the mothers of children with CLP.
In the present sample we found a positive family history indicating a genetic predisposition to the development of an orofacial cleft in 19.7% of patients. It has been reported that genetic factors may well determine cleft type (its extent) rather than external factors (9). Indeed, in the present group of cleft-patients with a positive family history no difference was observed in cleft type distribution in association with maternal bleeding. We suppose that the cleft size-increasing influence of the vaginal bleeding is negligible compared to the effect of genetic factors – positive family history.
We found a higher relationship between first trimester bleeding and the incidence of additional malformations, but this was not statistically significant (p = 0.112). In the offspring of mothers with first trimester bleeding, the increased number of both CLP and additional anomalies support the results by Calzorali et al. (31).
It is generally known that orofacial clefts have a multifactorial origin: both genes and environmental factors contribute to their etiology (32). The genetic component is assumed in 25–30% of orofacial clefts (33). The remaining cases are explained by an effect of environmental or unknown factors. Potential environmental risk factors include maternal smoking and alcohol consumption, infection agents, ionizing radiation, antimetabolites, nutritional deficiencies, hypervitaminosis A, anticonvulsant drugs, organic solvent and sex hormones (34). Some publications have reported an important relationship between first trimester bleeding and congenital malformations (14, 35, 36). This phenomenon has been interpreted as an effect of hypoxia (36). Other authors ascribe only slight importance to gestational bleeding (22) or no effect at all (37). Ornoy et al. (36) have analyzed data from a large group of 6223 women. The incidence of major malformations (malformations of the central nervous system, orofacial clefts, heart disorders, Down’s syndrome) was similar in the newborns of bleeding and non-bleeding mothers. However, there were more frequent minor malformations (strabismus, congenital deafness, inguinal hernia) in the babies of mothers with vaginal bleeding during the first trimester of pregnancy.
In our sample of cleft patients, gestational bleeding occurred in 10% of pregnancies. This incidence is about as frequent as in the normal population (13, 38, 39). Studies by various authors have suggested harmful external factors cause placental damage with subsequent bleeding; the disturbed perfusion of the placenta, resulting in hypoxia for example, induces a malformation in the embryo (13, 37, 36, 30). Hypoxia is a well-known embryotoxic factor; its effect during development was first described in chick embryos by Grabowski (40) and Chernoff and Rogers (41). A relationship has been proposed between first trimester bleeding and congenital malformations, and their origin has been explained as an effect of hypoxia (36).
Importantly, maternal bleeding may also be accompanied by psychological stress. It is well known that psychological stress can worsen pregnancy outcome (42). Stress is associated with an elevated production of corticoids (43). Carmichael et al. (44) have suggested a moderately increased risk of CLP among women who used corticosteroids during pregnancy. Myers (45) has experimentally demonstrated in rhesus monkeys that stress in the mother may lead to hypoxia in the fetus resulting from the maternal secretion of catecholamine and the subsequent vasoconstriction of the uterine arteries.
The above data suggest that gestational bleeding from the placenta may cause embryonic hypoxia. We hypothesize that hypoxia might act as an injurious factor that worsens the state of the embryo during the critical period of orofacial development and increases the probability of the origin of the most serious CLP in offspring of mothers with gestational bleeding.
CONCLUSIONS
In the group of children with an orofacial cleft, bleeding in the first trimester of pregnancy occurred in 253 (10.0%) of interviewed mothers. An orofacial cleft among relatives was reported in 497 (19.7%) and an additional malformation in 297 (11.8%) of cleft patients. The proportion of the most serious CLP was significantly higher (p < 0.001) while the proportion of CP was significantly lower (p = 0.007) in the offspring of mothers with vaginal bleeding than in the offspring of mothers without bleeding. Although vaginal bleeding was slightly more frequent in the mothers of children with additional malformations, this difference was not statistically significant (p = 0.112).
Acknowledgement
The authors thank Mr J. Dutt for English revision of the manuscript. This research has been supported by the research grants MSM 0021620843 and AV0Z50390703.
Address for correspondence:
Miroslav Peterka, M.D., DSc.
Department of Teratology, Institute of Experimental Medicine,
Academy of Sciences of the Czech Republic
Videnska 1083
142 20 Prague 4
Czech Republic
E-mail: peterka@biomed.cas.cz
Sources
1. Moore, KL., Persaud, TVN. The developing human: clinically oriented embryology. W.B. Saunders Company, 1993.
2. Peterka, M., Peterkova, R., Tvrdek, M., Kuderova, J., Likovsky, Z. Significant differences in the incidence of orofacial clefts in fifty-two Czech districts between 1983 and 1997. Acta Chir. Plast., 42, 2000, p. 124–129.
3. Peterka, M., Dostal, M. Influence of cleft palate on growth of the maxilla in mouse embryos. Cleft Palate J., 14, 1977, p. 206–210.
4. Jelínek, R., Peterka, M. The role of mandible in mouse palatal development revisited. Cleft Palate J., 14, 1977, p. 211–221.
5. Peterka, M., Jelínek, R. Differences in the size of palatal processes in mouse embryos with cleft palate induced in two critical periods. Cleft Palate J., 15, 1978, p. 13–19.
6. Jelínek, R., Pavlík, A., Peterka, M. Glucocorticoid receptor-mediated teratogenesis in the chick embryo. Teratogen. Carcin. Mut., 3, 1983, p. 1–7.
7. Peterka, M., Jelínek, R. Origin of hydrocortisone induced orofacial clefts in the chick embryo. Cleft Palate J., 20, 1983, p. 35–46.
8. Peterka, M., Peterkova, R., Likovsky, Z., Tvrdek, M., Fara, M. Incidence of orofacial clefts in Bohemia (Czech Republic) in 1964–1992. Acta Chir. Plast., 37, 1995, p. 122–126.
9. Fogh-Andersen, P. Inheritance of harelip and palate. Copenhagen: Nordisk Forlag. Arnold
10. Peterka, M., Peterkova, R., Halaskova, M., Tvrdek, M., Fara, M., Likovsky, Z. Sex differences in the incidence of orofacial clefts and the question of primary prevention in families with genetic risk. Acta Chir. Plast., 38, 1996, p. 57–60.
11. Lisi, A., Botto, LD., Rittler, M., Castilla, E., Bianca, S., Bianchi, F., Botting, B., De Walleh Erickson, JD., Gatt, M., DeVigan, C., Irgens, L., Johnson, W., Lancaster, P., Merlob, P., Mutchinick, Om., Ritvanen, A., Robert, E., Scarano, G., Stoll, C., Mastroiacovo, P. Sex and congenital malformations: an international perspective. Am. J. Medical Genetics, Part A, 134A, 2005, p. 49–57.
12. Thompson, JF., Lein, JN. Foetal survival following threatened abortion. Obstet. Gynecol., 18, 1961, p. 40–43.
13. Peckham, CH. Uterine bleeding during pregnancy. I. When not followed by immediate termination of pregnancy. Obstet. Gynecol., 35, 1970, p. 937–941.
14. South, J., Naldrett, J. The effect of vaginal bleeding in early pregnancy on the infant born after the 28th week of pregnancy. Br. J. Obstet. Gynaecol., 80, 1973, 236–241.
15. Batzofin, JH., Fielding, WL., Friedman, EA. Effect of vaginal bleeding in early pregnancy on outcome. ., 63, 1984, p. 515–518.
16. Strobino, B., Pantel-Silverman, J. Gestational vaginal bleeding and pregnancy outcome. Am. J. Epidemiol., 129, 1989, p. 806–815.
17. Sipilä, P., Hartikainen-Sorri, AL., Oja, H., Von Wendt, L. Perinatal outcome of pregnancies complicated by vaginal bleeding. BJOG, 99, 1992, p. 959–963.
18. Rempen, A. The incidence of abortions of viable pregnancies in the first trimester Zentralbl. Gynäkol., 115, 1993, p. 249–257.
19. Arafa, M., Abdel-Fataah, M., Zeid, HA., el-Khouly, A. Outcomes of pregnancies complicated by early vaginal bleeding. East Mediterr. Health J., 6, 2000, p. 457–464.
20. Promes, SB., Nobay, F. Pitfalls in the first-trimester bleeding. Emerg. Med. Clin. North Am., 28, 2010, p. 219–234.
21. Funderburk, SJ., Guthrie, D., Meldrum, D. Outcome of pregnancies complicated by early vaginal bleeding. BJOG, 87, 1980, p. 100–105.
22. Berkowitz, GS., Harlap, S., Beck, GJ., Freeman, DH., Baras, M. Early gestational bleeding and pregnancy outcome: a multivariable analysis. Int. J. Epidemiol., 12, 1983, p. 165–173.
23. Williams, MA., Hickok, DE., Zingheim, RW., Mittendorf, R., Kimelman, J., Mahony, BS. Low birth weight and preterm delivery in relation to early-gestation vaginal bleeding and elevated maternal serum alpha-fetoprotein. Obstet. Gynecol., 80, 1992, p. 745–749.
24. Heffner, LJ., Sherman, CB., Speizer, FE., Weiss, ST. Clinical and environmental predictors of preterm labor. Obstet. Gynecol., 81, 1993, p. 750–757.
25. al-Eissa, YA., Ba‘Aqeel, HS. Risk factors for spontaneous preterm birth in a Saudi population. ., 57, 1994, p. 19–24.
26. Escoffery, C., Greenwood, R., Ashley, D., Coard, K., Keeling, J., Golding, J. Deaths associated with intrapartum asphyxia in Jamaica. Paediatr. Perinat. Epidemiol., 8 Suppl 1, 1994, p. 119–142.
27. Greenwood, R., Golding, J., McCaw-Binns, A., Keeling, J., Ashley, D. The epidemiology of perinatal death in Jamaica. Paediatr. Perinat. Epidemiol., 8 Suppl 1, 1994, p. 143–157.
28. Spinillo, A., Nicola, S., Piazzi, G., Ghazal, K., Colonna, L., Baltaro, F. Epidemiological correlates of preterm premature rupture of membranes. Int. J. Gynecol. Obstet., 47, 1994, p. 7–15.
29. Jehan, I., McClure, EM., Salat, S., Rizvi, S., Pasha, O., Harris, H., Moss, N., Goldenberg, RL. Stillbirths in an urban community in Pakistan. Am. J. Obstet. Gynaecol., 197, 2007, p. 257. e1–8.
30. Felix-Schollaart, B., Hoeksma, JB., Van de Velde, JP., Puyenbroek, JI., Prahl-Andersen, B. Reproductive history of mothers of children with solitary, nonsyndromic cleft lip and/or palate. Cleft Palate-Craniofac. J., 29, 1992, p. 470–474.
31. Calzolari, E., Pierini, A., Astolfi, G., Bianchi, F., Seville, AJ., Rivieri, F. Associated anomalies in multi-malformed infants with cleft lip and palate: An epidemiologic study of nearly 6 million births in 23 EUROCAT registries. Am. J. Medic. Genet. Part A., 143, 2007, p. 528–537.
32. Mitchell, LE., Beaty, TH., Podral, AC., Munger, RG., Murray, JC., Saal, HM., Wyszynski, DF. International Consortium for Oral Clefts Genetics. Guidelines for the design and analysis of studies on nonsyndromic cleft lip and cleft palate in humans: summary report from a Workshop of the International Consortium for Oral Clefts Genetics. Cleft Palate-Craniofac. J., 39, 2002, p. 93–100.
33. Tolarova, M., Cervenka, J. Classification and birth prevalence of orofacial clefts. Am. J. Medic. Genet., 75, 1998, p. 126–137.
34. Leite, ICG., Paumgarten, FJR., Koifman, S. Chemical exposure during pregnancy and oral clefts in newborrns. Cadernos de Saude Publica, 18, 2002, p. 17–31.
35. Asanti, R., Vesanto, T. Effect of threatened abortion on foetal prognosis. Acta Obstet. Gynecol. Scand., 42, 1963, p. 107–116.
36. Ornoy, A., Benady, S., Kohen-Raz, R., Russell, A. Association between maternal bleeding during gestation and congenital anomalies in the offspring. Am. J. Obstet. Gynaecol., 124, 1976, p. 474–478.
37. Mau, G., Netter, P. Haemorrhage in early pregnancy: an indication of malformations in the infant? Zentralbl. Kinderheilk., 117, 1974, p. 79–88.
38. Yang, J., Savitz, DA., Dole, N., Hartmann, KE., Herring, AH., Olshan, AF., Thorp, JM. Jr. Predictors of vaginal bleeding during the first two trimesters of pregnancy. Paediatr. Perinat. Epidemiol.,19, 2005, p. 276–283.
39. Heinig, J., Steinhard, J., Schmitz, R., Nofer, JR., Witteler, R., Mosel, A., Ahrens, A., Kiesel, L., Klockenbusch, W. Ahrens, A., Kiesel, L., Klockenbusch, W. Does vaginal bleeding influence the first-trimester markers for Down syndrome? Prenat. Diagn., 27, 2007, p. 312–316.
40. Grabowski, CT. Physiological changes in the bloodstream of chick embryos exposed to teratogenic doses of hypoxia. Dev. Biol., 13, 1966, p. 199–213.
41. Chernoff, N., Rogers, JM. Hypoxia and the edema syndrome: elucidation of a mechanism of teratogenesis. Birth Defects Res. Part B: Dev. Reprod. Toxicol., 89, 2010, p. 300–303.
42. Wisborg, K., Barklin, A., Hedegaard, M., Henriksen, TB. Psychological stress during pregnancy and stillbirth: prospective study. Int. J. Obstet. Gynaecol., 115, 2008, p. 882–885.
43. Obel, C., Hedegaard, M., Henriksen, TB., Secher, NJ., Olsen, J., Levine, S. Stress and salivary cortisol during pregnancy. 30, 2005, p. 647–656.
44. Carmichael, SL., Shaw, GM., Ma, C., Werler, MM., Rasmussen, SA., Lammer, EJ. Maternal corticosteroid use and orofacial clefts. A. J. Obstet. Gynecol., 197, 2007, 585.e1–7; discussion 683–684, e1–7.
45. Myers, RE. Production of fetal asphyxia by maternal psychological stress. Pavlov J. Biol. Sci., 12, 1977, p. 51–62.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2012 Issue 2-
All articles in this issue
- INDEX Acta Chir. Plast. Vol. 54, 2012
- CLINICAL TRIAL OF THE TEMPORARY BIOSYNTHETIC DERMAL SKIN SUBSTITUTE BASED ON A COLLAGEN AND HYALURONIC ACID NAMED COLADERM H/HM, FIRST PART
- EXTENSION OF OROFACIAL CLEFT SIZE AND GESTATIONAL BLEEDING IN EARLY PREGNANCY
- HETEROLOGOUS RECONSTRUCTION AND RADIOTHERAPY: THE ROLE OF LATISSIMUS DORSI FLAP AS A SALVAGE
- FASHIONING REVERSED AXIAL PATTERN FOREARM TISSUES IN DIFFERENT CHALLENGING CONDITIONS OF THE FOREARM TERRITORY AS A RELIABLE SUBSTITUTE FOR FREE TISSUE TRANSFER
- GIANT MALIGNANT MELANOMA: A CASE REPORT
- OUR EIGHT-YEAR EXPERIENCE WITH BREAST RECONSTRUCTION USING ABDOMINAL ADVANCEMENT FLAP (207 RECONSTRUCTIONS)
- CULTURED KERATINOCYTES AND THEIR POSSIBLE APPLICATIONS
- CONTENTS Acta Chir. Plast. Vol. 54, 2012
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- GIANT MALIGNANT MELANOMA: A CASE REPORT
- HETEROLOGOUS RECONSTRUCTION AND RADIOTHERAPY: THE ROLE OF LATISSIMUS DORSI FLAP AS A SALVAGE
- CLINICAL TRIAL OF THE TEMPORARY BIOSYNTHETIC DERMAL SKIN SUBSTITUTE BASED ON A COLLAGEN AND HYALURONIC ACID NAMED COLADERM H/HM, FIRST PART
- EXTENSION OF OROFACIAL CLEFT SIZE AND GESTATIONAL BLEEDING IN EARLY PREGNANCY
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

