-
Medical journals
- Career
CLINICAL TRIAL OF THE TEMPORARY BIOSYNTHETIC DERMAL SKIN SUBSTITUTE BASED ON A COLLAGEN AND HYALURONIC ACID NAMED COLADERM H/HM, FIRST PART
Authors: D. Potocká 1; D. Kevická 2; J. Koller 3
Authors‘ workplace: Department of Plastic Surgery, City Hospital of T. Baťa, Ltd., Zlín, Czech Republic 1; Department of Hand Surgery, University Hospital Bratislava, Ružinov Hospital, Bratislava Slovak Republic, and 2; Department of Burns and Reconstructive Surgery, University Hospital Bratislava, Ružinov Hospital, Bratislava, Slovak Republic 3
Published in: ACTA CHIRURGIAE PLASTICAE, 54, 2, 2012, pp. 31-38
INTRODUCTION
Coladerm H/HM is a term for a hybrid membrane with a specific bubble macrostructure, which is composed of natural components of skin, bovine atelocollagen I and hyaluronic acid, prepared by a biotechnological process. The material was developed at the Faculty of Chemical and Food Technology, Slovak University of Technology, Bratislava in cooperation with the Department of Burns and Reconstructive Surgery, University Hospital Bratislava, Ružinov Hospital Bratislava, Slovak Republic, in order to obtain biosynthetic, biodegradable dermal skin substitute (1). Development of the membrane was the subject of the research project of the Grant Agency for Science no. 96-03-13. Biocompatiblity of the membrane was studied by cytotoxicity tests on tissue cultures in vitro in the previous investigations, which have proven optimal properties and minimal cytotoxicity.
Subsequently, testing the membrane in experimental animals – rats was approached. Coladerm H/HM was applied as a subcutaneous implant and as a dressing of the surgically created full thickness skin defects. It has been proved that the membrane is well incorporated into the body, ingrows the autologous tissue over 2–3 weeks, resorbs in 4 weeks after implantation almost completely and completely within 12 weeks. Material does not produce negative side effects in the experimental animals after implantation (2, 3). After successful testing on experimental animals, with the authorization by the State Institute for Drug Control and with the Ethical Committee of the University Hospital Bratislava, Ružinov Hospital Bratislava approval, clinical trials have been initiated.
The purpose of the clinical testing of Colagen H/HM is to verify the properties of the membrane as a temporary biosynthetic cover for the split-thickness skin graft donor sites and for the treatment of burns; and as a dermal substitute for the treatment of deep burns, in combination with epidermal component. Subsequent verification in other clinical departments in the Slovak Republic or abroad is planned as well. This paper presents the results of the first phase of the clinical trial when Coladerm H/HM was used for covering the donor sites of split-thickness skin autografts in 20 patients. The donor site was covered partially by Coladerm H/HM, the rest by Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) used standardly for donor sites at the Department of Burns and Reconstructive Surgery, University Hospital Bratislava, Slovakia, which allowed one to compare the healing of both parts of the donor site.
MATERIAL AND METHODS
Coladerm H/HM was applied to the split-thickness skin graft donor sites after harvesting the dermoepidermal autografts of the thickness of 250 μm, according to the approved protocol about clinical testing. 20 patients requiring split-thickness skin grafting treated at the Department of Burns and Reconstructive Surgery of the University Hospital Bratislava were estimated. Donor site of the same patient was partially covered by a membrane Coladerm H/HM, the rest by the standard dressing used to cover the donor sites at our department, which is Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) and is set to be as a control area for comparison. Patients included in this part of the study were randomly selected and met the following criteria: age of 18–65 years, no severe accompanying diseases such as diabetes, renal failure, immune disorders, severe arteriosclerosis, severe allergic conditions. Each patient was given full information about the study and signed the informed agreement. Evaluation was done according to the protocol.
Primary assessment
The primary assessment of this clinical study was to investigate the rate of epithelization of the split-thickness skin graft donor site after application of Coladerm H/HM in comparison to standard coverage (Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter)) used in the same patients, which means, each patient served as its own control. Inspection intervals and assessments of the wounds were in 7, 10, 14 and 21 days. Percentage of epithelization in each time period was recorded.
Secondary assessment
Differences observable during the healing process of the split-thickness skin graft donor sites in the application of Coladerm H/HM in comparison to the part of the wound where Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) was applied, were recorded in the secondary assessment. Following parameters were observed:
- Secretion of the wound, which was assessed as no, mild and hard.
- Color of donor area was recorded as red, violet and pink.
- Surrounding of the wound was evaluated in terms of “inflamed, irritated and non-irritated.”
- Bacteriological findings if present.
Inspection intervals of above mentioned parameters were in 7, 10, 14 and 21 days after covering the split-thickness skin graft donor site.
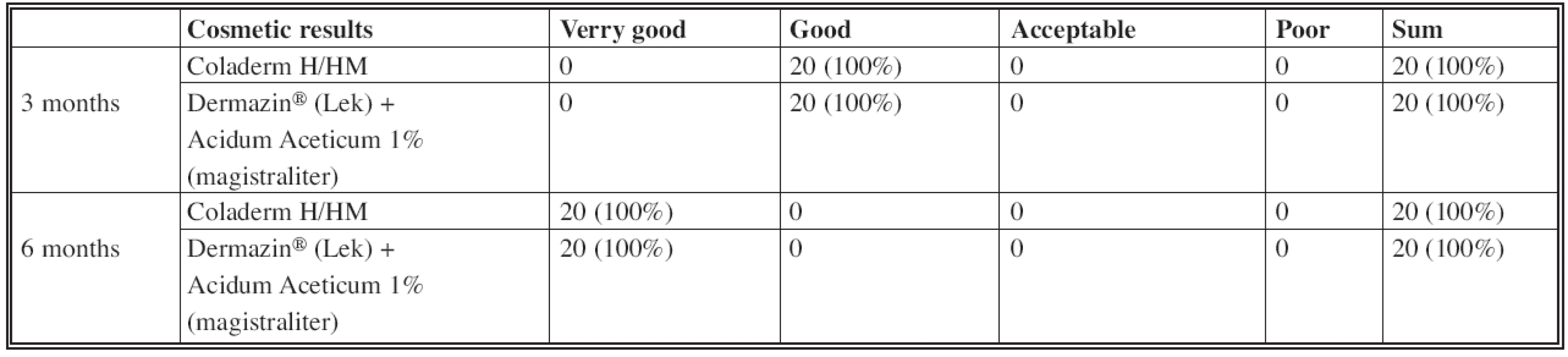
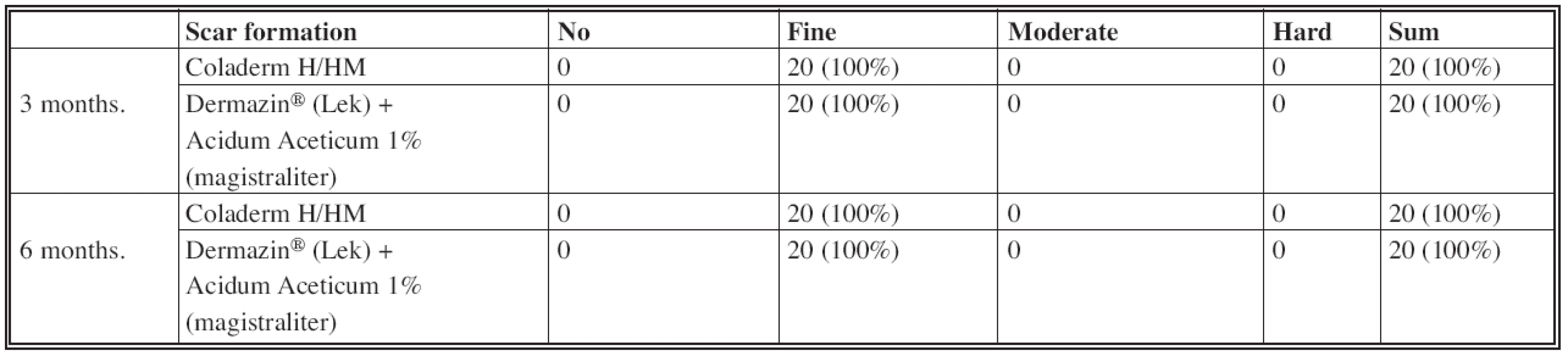
Three and six months after application of Coladerm H/HM were cosmetic results and scar formation evaluated. Cosmetic results were assessed as very good, good, acceptable and poor; scar formation as no, fine, moderate and hard.
The doctor, who manipulated with Coladerm H/HM evaluated its analgetic effect.
Other parameters assessed by a doctor were the following: available product information, which could be good, adequate, not adequate, distinct or too complicated to understand, the administration of the Coladerm H/HM, which was recorded as “good, acceptable and could be better” and preferred size was recorded as well. Next, the handling with fixation of the Coladerm H/HM were evaluated, both as easy, acceptable and problematic.
Efforts in nursing connected with application of Coladerm H/HM compared to other materials were evaluated as higher, comparable, lower.
Patients evaluated pain, if present, in split-thickness skin graft donor site after application of Coladerm H/HM in comparison to the applied Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter), which they named as no, mild, moderate and strong. They also evaluated the application of Coladerm H/HM in the sense of “comfortable, acceptable and not comfortable.” They also described the irritation like itching, burning, etc., if any occurred.
RESULTS
Primary assessment
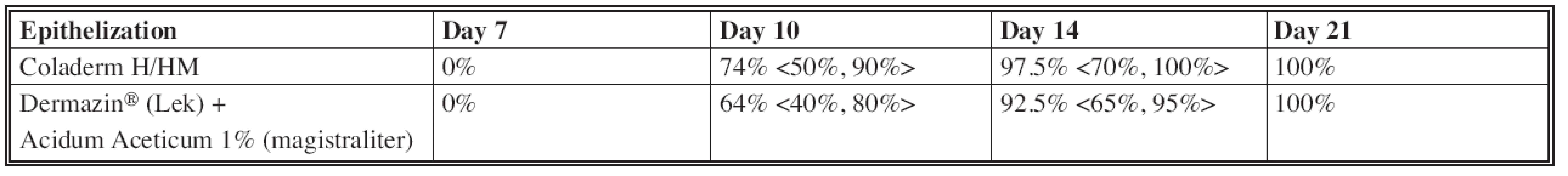
The most significant difference in the rate of epithelization of the donor site after application of Coladerm H/HM compared to Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) was in the interval of 10 days (Fig. 1), when the average of epithelized surface using Coladerm H /HM was 74% (lowest was 50%, highest 90%) while using the Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) was 64% (lowest 40%, highest 80%) in the same period. This difference was decreased in the next interval of 14 days, and completely lost in the last interval of 21 days (Fig. 2, Fig. 3), when the epithelization was 100% in both materials. Using chi-square test, p=0.807>0.05, the rate of epithelization compared to using Coladerm H/HM and Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) is not statistically significant in any period (Table 1).
Fig. 1. Inspection interval of 10 days CDH: Coladerm H/HM, D + AA: Dermazin<sup>®</sup> (Lek) + Acidum Aceticum 1% (magistraliter) 
Fig. 2. Inspection interval of 14 days CDH: Coladerm H/HM, D + AA: Dermazin<sup>®</sup> (Lek) + Acidum Aceticum 1% (magistraliter) 
Fig. 3. Inspection interval of 21 days CDH: Coladerm H/HM, D + AA: Dermazin<sup>®</sup> (Lek) + Acidum Aceticum 1% (magistraliter) 
Secondary assessment
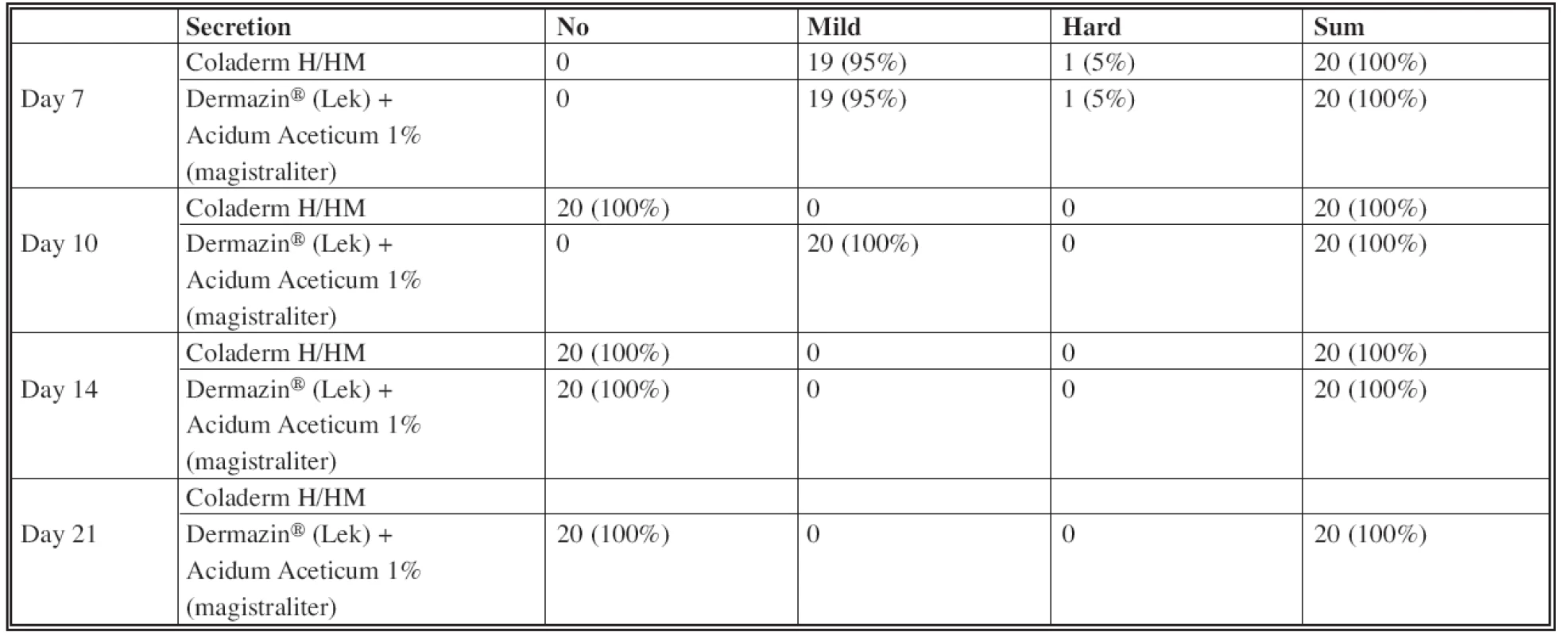
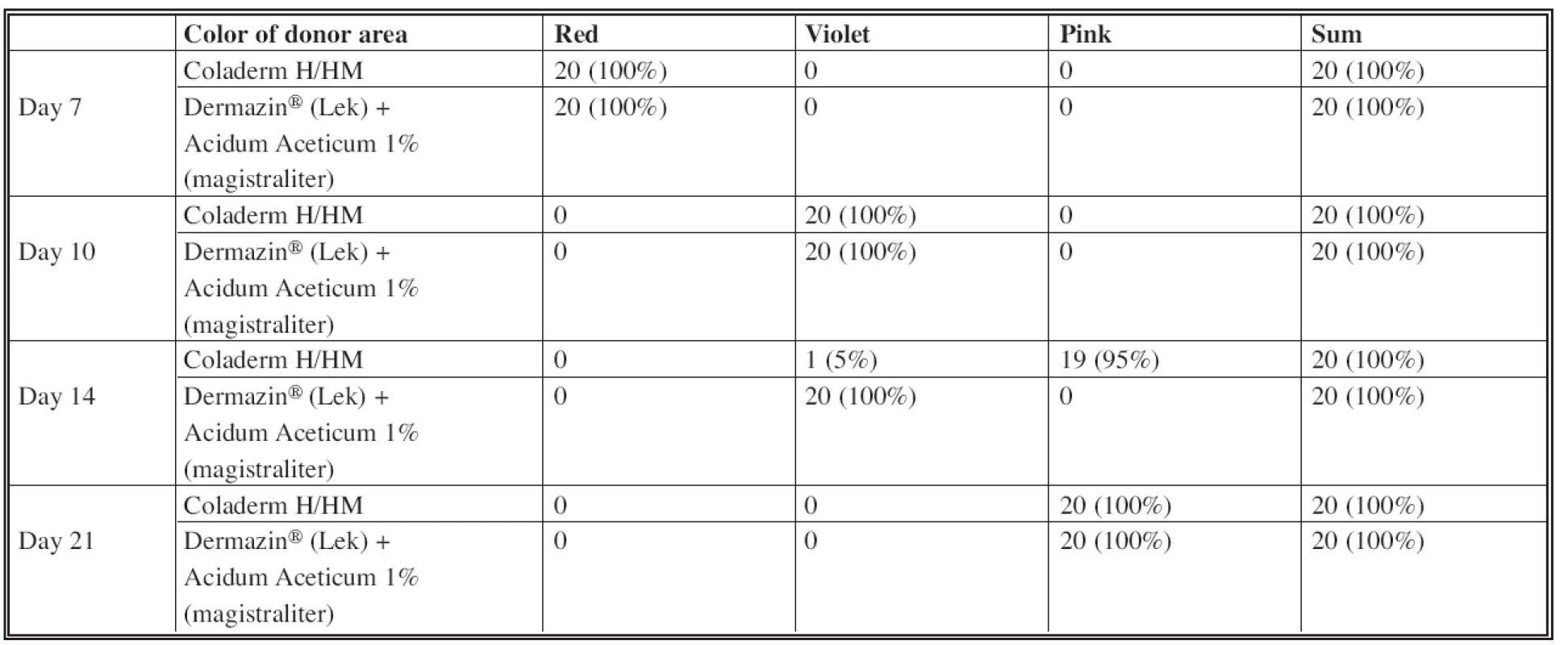
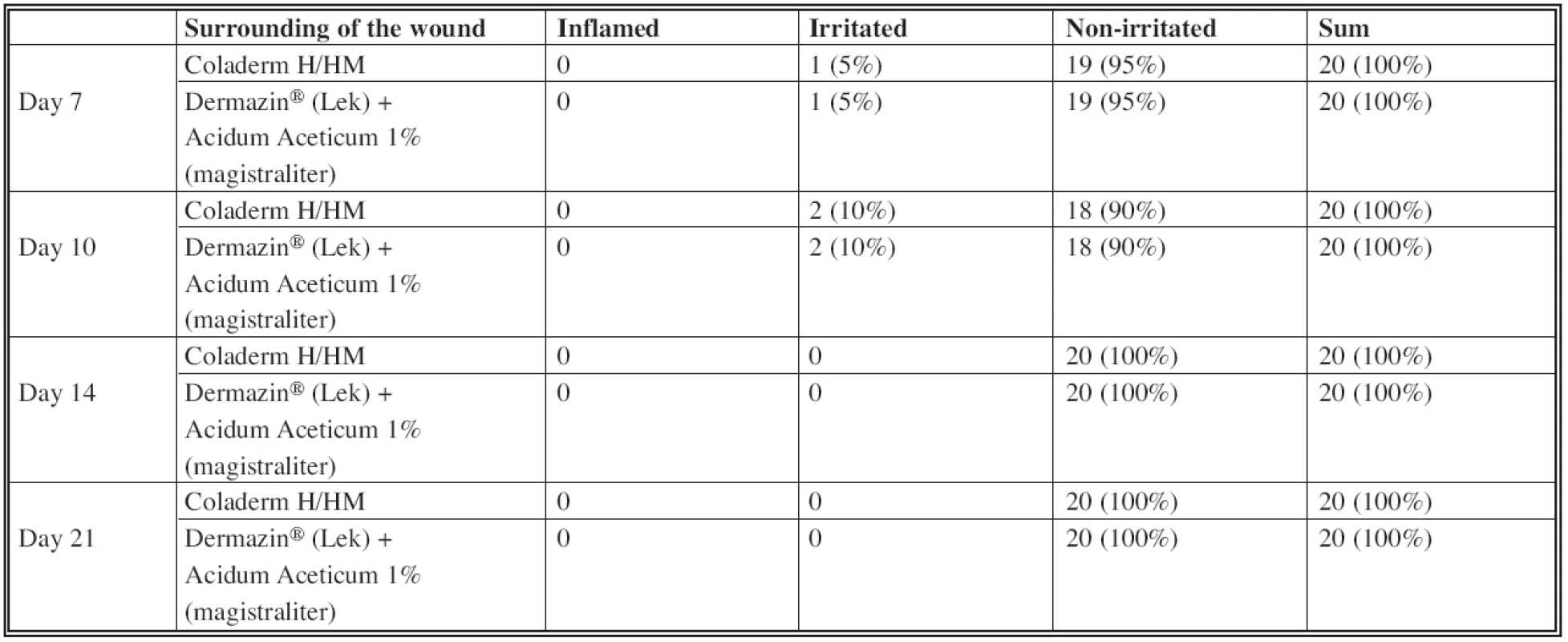
Assessing the wound secretion in sense of seroma formation (Table 2), the difference was observed in using Coladerm H/HM and Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) in the inspection interval of 10 days (see Fig. 1). There was no wound secretion in the part of the donor site covered by Coladerm H/HM, while part covered by Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) expressed mild secretion in all patients. This is statistically verified as significant with p=0.000 <0.05. No difference in terms of wound secretion has been demonstrated in periods of 7, 14 and 21 days. The difference between color of donor area was in the period of 14 days (Table 3). Only 5% were violet using the Coladerm H/HM and 95% pink, while using the Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) resulted in violet donor sites in all patients in the interval of 14 days. This is statistically significant (p=0.000<0.05). No difference was recorded in other periods. Surrounding of the wound was irritated in 5% of patients in the intervals of 7 days and 10% in the interval of 10 days, when using both Coladerm H/HM and Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter). Rest of the patients had no irritation; other periods were all without irritation or inflammation in the surrounding of the wound (Table 4). There was a positive bacteriological finding in one patient with Pseudomonas aeruginosa, Acinetobacter sp. and massive candida, in the interval of 14 days (Fig. 4). The sample for cultivation was obtained from the whole surface of the donor site. Before and after this period in this patient as well as in all other patients in all periods were bacteriological findings negative.
Fig. 4. Inspection interval of 14 days showing the patient, where positive bacteriological finding was cultivated from the whole surface of the donor site CDH: Coladerm H/HM, D + AA: Dermazin<sup>®</sup> (Lek) + Acidum Aceticum 1% (magistraliter) 
Evaluating the cosmetic results and scar formation (Fig. 5, Fig. 6), there was no difference in results when using Coladerm H/HM and Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) (Table 5, Table 6). Cosmetic results were good in the interval of 3 months and up to 6 months very good. Scar formation was evaluated in both intervals as fine.
Fig. 5. Inspection interval of 3 months CDH: Coladerm H/HM, D + AA: Dermazin<sup>®</sup> (Lek) + Acidum Aceticum 1% (magistraliter) 
Fig. 6. Inspection interval of 6 months CDH: Coladerm H/HM, D + AA: Dermazin<sup>®</sup> (Lek) + Acidum Aceticum 1% (magistraliter) 
Analgetic effect of both materials evaluated by a doctor who treated the patient was approved as good in 70% and mild in 30% of patients in the use of Coladerm H/HM. The use of Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) was rated to have mild analgetic effect in all patients. Good analgetic effect of Coladerm H/HM is statistically significant against Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) (p=0.000< 0.05) (Table 7). Information about Coladerm H/HM was for 95% of doctors involved good, for 5% distinct. Doctors evaluated administration of Coladerm H/HM as good in all patients. All the doctors preferred the size of Coladerm H/HM 10 x 10 cm. Handling and fixation of the Coladerm H/HM was easy for doctors in all the patients. Nurses evaluated the effort connected with application of Coladerm H/HM in comparison to other materials as comparable. Patients compared the pain in the donor site, if present, covered by Coladerm H/HM and Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter). While Coladerm H/HM caused no pain (5%) or the pain was mild (95%), Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) was assessed by all the patients as causing moderate pain. Mild pain after application of Coladerm H/HM is statistically significant if compared to the pain after application of Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) labelled by patients as moderate (p=0.000<0.05) (Table 8). Application of Coladerm H/HM was comfortable for 95% and acceptable for 5% of patients. One patient described irritation as itching in the site of application of the Coladerm H/HM in the 1st and 2nd inspection interval, which disappeared completely subsequently.
8. Pain in the donor site (patient) 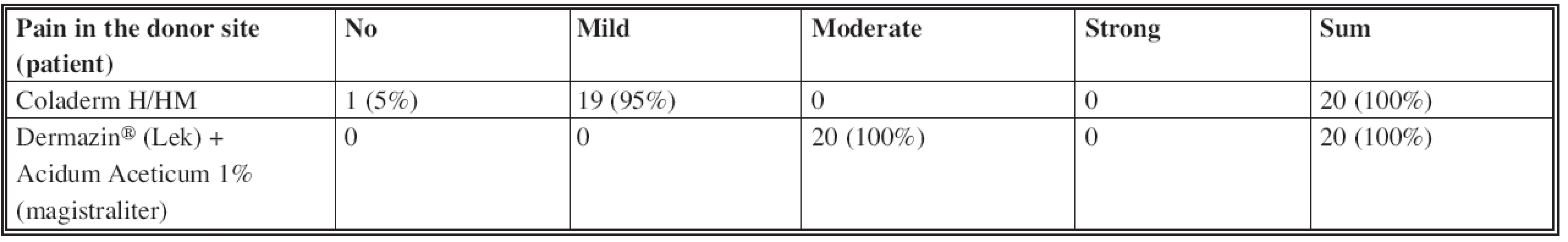
DISCUSSION
The aim of this study was to verify the clinical properties of temporary biosynthetic dermal skin substitute based on a collagen and hyaluronic acid, named Coladerm H/HM, which was applied to a part of the split-thickness skin graft donor site; its effects and properties were compared to Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter), applied to the rest of the donor site; some parameters were evaluated only in Coladerm H/HM. Twenty patients were included in this clinical study.
Primary assessment was determined for epithelization rate after application of Coladerm H/HM and Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter), which was evaluated in 4 inspection intervals. The wound covered by Coladerm H/HM epithelized faster during the first three inspection intervals, but in the last interval epithelization was 100% with using both materials. Coladerm H/HM does not influence the rate of epithelization from a long term point of view. On the other hand, the initial faster epithelization significantly reduces the surface of the open wound and thus contributes not only to the comfort of the patient (pain reduction), but also reduces the risk of complications, which may arise from a long term open wounds.
Secondary assessment monitored more items. Coladerm H/HM showed the effect on secretion of the wounds in the second inspection interval when the wound was dry in the whole group compared to mild secretion after application of Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) in the whole group as well. No difference in secretion of the wound was monitored in other periods; evaluation in the last period was recorded as “no secretion” in both materials, but this is natural in a completely epithelized wound. The effect of Coladerm H/HM on the color of donor area was observed statistically significant temporarily in the 3rd inspection interval, when the color was pink in most of the patients (95%), while using Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter) the color was violet in 100% of patients. However, Coladerm H/HM does not influence the color of donor area from a long term point of view if compared to classical dressing. No effect of Coladerm H/HM in reducing or increasing the irritation in the surrounding of the wound was recorded in comparison to Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter). No inflammation was observed. Temporary, minimal irritation (Fig. 7), which disappeared spontaneously, was observed around Coladerm H/HM as well as around Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter). Positive bacteriological finding occurred in one patient was not due to applied Coladerm H/HM, because it was found in the part of the donor site covered by standard coverage as well. After antibiotic treatment according to sensitivity of bacterial colonization the infection was not present. No differences in cosmetic results and scar formation were observed in a long term point of view. Assessing the analgetic effect of Coladerm H/HM by a doctor, this was expressed as good in 70% of patients compared to only mild using the standard dressing in 100% of patients. This can be explained by no need of repetitive changing of the Coladerm H/HM. Patient is prevented from painful manipulation in the donor site. In addition, the membrane exceeds the properties of the classical dressing by its adherence and ability to cover the wound by less irritating the nerve endings in the open wound. Coladerm H/HM itself was evaluated by doctors according to available information about the product, administration, handling and fixation. The results were positive in all of above mentioned parameters. The most suitable size of Coladerm H/HM is 10 x 10 cm. Nurses did not confirm a difference between the Coladerm H/HM and other materials in handling. Patients reported only mild pain after the application of the Coladerm H/HM (95%) compared to moderate pain in Dermazin® (Lek) + Acidum Aceticum 1% (magistraliter). This analgetic effect can be explained by a fact, that the membrane behaves as a temporary dermal substitute and patients are protected from repeated painful manipulation in the site of wound, as noted above. Application of Coladerm H/HM was comfortable for most of the patients (95%). There were no significant side effects recorded, only 1 patient stated itching, but it disappeared spontaneously.
Fig. 7. Temporary irritation observed around Coladerm H/HM as well as around Dermazin<sup>®</sup> (Lek) + Acidum Aceticum 1% (magistraliter) CDH: Coladerm H/HM, D + AA: Dermazin<sup>®</sup> (Lek) + Acidum Aceticum 1% (magistraliter) 
There is no ideal composite skin substitute for permanent wound closure currently commercially available. Engineering skin substitutes represent a prospective source of advanced therapy in combating acute and chronic skin wounds. Skin substitutes should have some essential characteristics which include: being easy to handle and apply to the wound site; provide vital barrier function with appropriate water flux; be readily adherent; have appropriate physical and mechanical properties; undergo controlled degradation; be sterile, non-toxic, non-antigenic; and evoke minimal inflammatory reactivity. Additionally, they should incorporate into the host with minimal scarring and pain and facilitate angiogenesis, while still being cost effective (4). There are two key challenges for developing the dermal substitute: first, the primary generation of degradable polymers have deficiencies in terms of mechanical and degradation properties (5). The second challenge is how to fabricate these polymers into scaffolds that have defined shapes and a complex porous internal architecture that can direct tissue growth (6). New technologies are emerging for accurate manufacture, creating materials of defined pore size using novel technologies using three-dimensional printing and electrospinning (7–10). The current focus of research is towards developing a tissue-engineered skin equivalent that combines living cells with natural or synthetic cellular components (11, 12).
CONCLUSION
With regards to favourable results of this clinical study, it is planned to proceed to the next parts of clinical trials testing the Coladerm H/HM, like verifying the properties of the membrane as a temporary biosynthetic dressing in the treatment of burns and as a temporary dermal substitute in surgically excised deep burns. This should be followed by verification at other departments in the Slovak Republic or abroad. The membrane could be used for other purposes in the future clinical practice, except of previously mentioned, like for the cell cultivation in vitro (mainly keratinocytes), as a carrier for cultured keratinocytes and fibroblasts, which, in the case of autologous cells, could represent a permanent skin substitute. It can also serve as a three-dimensional matrix for the cultivation of other autologous cells such as chondrocytes or osteoblasts to fill defects of cartilage or bone. The membrane is expected to be used as a temporary mucosal substitute for the mucosal defects after surgery in the oral cavity, as well as resorbable implantable carrier of growth factors, therapeutic agents – particularly antibiotics and for filling the cavities in septic surgery after removal the septic foci. After successful completion of all the parts of clinical trial, production of the Coladerm H/HM and its introduction into the clinical practice are planned.
Address for correspondence:
Darina Potocká, M.D.
Department of Plastic Surgery
City Hospital of T. Baťa, Ltd.
Havlíčkovo nábřeží 600
762 75 Zlín
Czech Republic
E-mail: darina.potocka@gmail.com
Sources
1. Bakoš D., Jorge-Herrero E., Koller J. Resorption and calcification of chemically modified collagen/hyaluronan hybrid membranes. Polim. Med., 2000, 30, p. 57-64.
2. Koller J., Bakoš D., Sadloňová I. Biocompatibility studies of a new biosynthetic dermal substitute based on collagen/hyaluronan conjugative. Cell Tissue Bank, 2000, 1, p. 75-80.
3. Koller J., Bakoš D., Sadloňová I. Biocompatibility studies of modified collagen/hyaluronan membranes after implantation. Cell Tissue Bank, 2001, 2, p. 135-142.
4. Griffith LG. Emerging design principles in biomaterials and scaffolds for tissue engineering. Ann. NY Acad. Sci., 2002, 961, p. 83–95.
5. Boyce ST., Warden GD. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am. J. Surg., 2002, 183, p. 445–456.
6. Griffith LG., Schwartz MA. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell. Biol., 2006, 7, p. 211–224.
7. Li M., Mondrinos MJ., Gandhi MR., Ko FK., Weiss AS., Lelkes PI. Electrospun protein fibers as matrices for tissue engineering. Biomaterials, 2005, 26, p. 5999–6008.
8. Luu YK., Kim K., Hsiao BS., Chu B., Hadjiargyrou, M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J. Control Release, 2003, 89, p. 341–353.
9. Mironov V., Boland T., Trusk T., Forgacs G., Markwald, RR. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol., 2003, 21, p.157–161.
10. Seitz H., Rieder W., Irsen S., Leukers B., Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater., 2005, 74, p. 782–788.
11. Auger FA., Brthod F., Moulin V., Pouliot R., Germain, L. Tissue-engineered skin substitutes: from in vitro constructs to in vivo applications. Biotechnol. Appl. Biochem., 2004, 39, p. 263–275.
12. Philips TJ. New skin for old: developments in biological skin substitutes. Arch. Dermatol., 1998, 134, p. 344–349.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2012 Issue 2-
All articles in this issue
- INDEX Acta Chir. Plast. Vol. 54, 2012
- CLINICAL TRIAL OF THE TEMPORARY BIOSYNTHETIC DERMAL SKIN SUBSTITUTE BASED ON A COLLAGEN AND HYALURONIC ACID NAMED COLADERM H/HM, FIRST PART
- EXTENSION OF OROFACIAL CLEFT SIZE AND GESTATIONAL BLEEDING IN EARLY PREGNANCY
- HETEROLOGOUS RECONSTRUCTION AND RADIOTHERAPY: THE ROLE OF LATISSIMUS DORSI FLAP AS A SALVAGE
- FASHIONING REVERSED AXIAL PATTERN FOREARM TISSUES IN DIFFERENT CHALLENGING CONDITIONS OF THE FOREARM TERRITORY AS A RELIABLE SUBSTITUTE FOR FREE TISSUE TRANSFER
- GIANT MALIGNANT MELANOMA: A CASE REPORT
- OUR EIGHT-YEAR EXPERIENCE WITH BREAST RECONSTRUCTION USING ABDOMINAL ADVANCEMENT FLAP (207 RECONSTRUCTIONS)
- CULTURED KERATINOCYTES AND THEIR POSSIBLE APPLICATIONS
- CONTENTS Acta Chir. Plast. Vol. 54, 2012
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- GIANT MALIGNANT MELANOMA: A CASE REPORT
- HETEROLOGOUS RECONSTRUCTION AND RADIOTHERAPY: THE ROLE OF LATISSIMUS DORSI FLAP AS A SALVAGE
- CLINICAL TRIAL OF THE TEMPORARY BIOSYNTHETIC DERMAL SKIN SUBSTITUTE BASED ON A COLLAGEN AND HYALURONIC ACID NAMED COLADERM H/HM, FIRST PART
- EXTENSION OF OROFACIAL CLEFT SIZE AND GESTATIONAL BLEEDING IN EARLY PREGNANCY
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career