-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Immunotherapy in the Precision Medicine Era: Melanoma and Beyond
In a Perspective, Mack Su and David Fisher discuss the development of immunotherapies for treatment of melanoma and other cancer types.
Published in the journal: . PLoS Med 13(12): e32767. doi:10.1371/journal.pmed.1002196
Category: Perspective
doi: https://doi.org/10.1371/journal.pmed.1002196Summary
In a Perspective, Mack Su and David Fisher discuss the development of immunotherapies for treatment of melanoma and other cancer types.
Innovations in cancer immunotherapy over the past decade have reinvigorated the field, which currently stands poised to transform treatment of certain important cancer types. While recent discoveries in immunotherapy have occurred in an era of intense focus on precision medicine—spurred in large part by advances in sequencing technologies allowing for unprecedented insights into individual and tumor genomes—the field of cancer immunotherapy is rooted in a history that is anything but precise. The idea that the immune system could be used to target cancer has existed for over a century. In the 1890s, William Coley provided a spark by noting a connection between spontaneous tumor regressions and concurrent bacterial infections [1]. Suspecting an immune mechanism, Coley and many others in the decades that followed experimented with live bacteria, toxins, vaccines, and countless other agents to coax the immune system to attack cancer, yielding only a tiny number of positive responses and little understanding of the underlying immune mechanisms. As recently as 2010, immunotherapy remained a strikingly imprecise endeavor, with the only United States Food and Drug Administration (FDA)-approved cancer immunotherapies being a pair of systemic cytokines—interferon-α and interleukin-2—and intravesical bacillus Calmette-Guérin (BCG). However, as recent successes reshape the field, efforts to comprehend and harness precise mechanisms in immunotherapy will be required.
Breakthroughs in Melanoma
Perhaps the most profound impacts of both immunotherapy and precision medicine, in the form of targeted therapy, can be appreciated in the transformation of treatment for metastatic melanoma during the past decade. The discovery of the oncogenic V600E driver mutation in BRAF, which is present in ~50% of melanomas, led to the development of vemurafenib and dabrafenib, which are inhibitors of the mutant BRAF kinase [2]. Although targeting mutant BRAF kinase and inhibiting the downstream mitogen-activated protein kinase (MAPK) pathway produces high response rates in melanoma patients, the duration of responses is brief [3], highlighting the need for additional therapies. Meanwhile, preclinical evidence suggested that blocking negative regulators, or checkpoints, of T cell responses could improve antitumor immune responses (Fig 1). The first such negative regulator tested was cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), a T cell surface receptor that binds with high affinity to the costimulatory ligands B7-1 and B7-2 found on the surface of antigen-presenting cells. The interaction with CTLA-4 prevents binding of the costimulatory ligands to cluster of differentiation 28 (CD28), a major costimulatory receptor on the T cell necessary for a robust T cell response. Despite concerns about uncontrollable autoimmunity resulting from systemic CTLA-4 blockade, ipilimumab, a monoclonal antibody against CTLA-4, improved overall survival in patients with metastatic melanoma [4].
Fig. 1. Central and peripheral immune checkpoints. 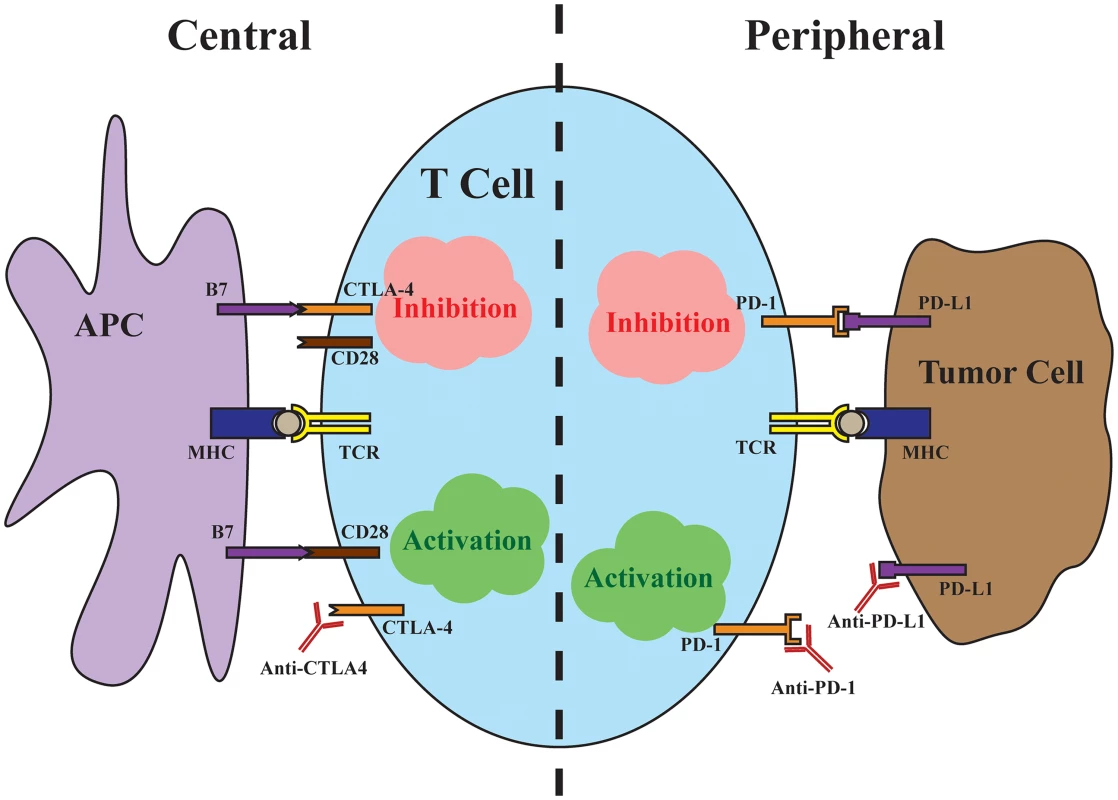
Central immune inhibition occurs as a result of CTLA-4 binding to the costimulatory B7 ligands found on antigen-presenting cells (APCs) with high affinity, preventing signaling through CD28. Antibodies targeting CTLA-4 release this checkpoint to allow T cell activation. Peripheral immune inhibition occurs through binding of the PD-L1 found on tumor cells to the PD-1 receptor on T cells. Antibodies targeting either the ligand or the receptor release this checkpoint. The successes of ipilimumab were quickly followed by trials targeting the immune inhibitory interaction between programmed cell death protein 1 (PD-1), found on T cells, and its ligand, PD-L1, found on tumor cells. In contrast to the central immune inhibitory effects of CTLA-4, inhibition through PD-1 predominantly occurs at the periphery, with tumor cells up-regulating PD-L1 in response to local immune signals such as interferon-γ. In a major head-to-head clinical trial, pembrolizumab, a monoclonal antibody against PD-1, demonstrated improved progression-free and overall survival with fewer high-grade adverse events compared to ipilimumab [5]. Importantly, in a minority of cases, checkpoint inhibition produces strikingly durable responses, with up to a third of advanced melanoma patients alive at five years of follow-up [6]. The majority, unfortunately, fail to respond to immunotherapy, with objective response rates to checkpoint inhibitor monotherapy ranging from 10%–40%, with PD-1/PD-L1 targeted antibodies benefiting larger proportions of patients [4,5,7]. Because the mechanisms of CTLA-4 and PD-1 blockade are distinct and potentially complementary, dual checkpoint blockade increases response rates to around 60%, albeit with significantly more adverse events [8,9]. In a matter of a few years, immune checkpoint blockade has become first-line therapy for advanced melanoma and transformed the outlook for patients. Looking ahead, uncovering predictive markers of response to immunotherapy and ultimately understanding the mechanisms of response will be necessary to extend the promise of immunotherapy to broader groups of cancer patients.
Efforts to uncover the precise mechanisms of immune checkpoint inhibition and identify patients likely to benefit from treatment are well under way. Pathologists have long recognized the prognostic significance of tumor-infiltrating lymphocytes [10], and, unsurprisingly, pretreatment CD8+ T cell density is associated with favorable response to immune checkpoint blockade [11]. However, preclinical data suggest that CD8+ T cells in the tumor microenvironment can promote immune inhibition as a result of interferon-γ-mediated induction of PD-L1 on tumor cells [12]. Response to anti-PD1 therapy in melanoma as well as other cancers has been suggested to positively correlate with tumor expression of PD-L1 [13,14], highlighting the central role of this ligand in tumor immune evasion, although PD-L1 expression thus far appears to be an imperfect predictive biomarker of response. A comprehensive understanding of immunotherapy requires a deeper analysis of the interaction between immune system and tumor.
Genomics-Guided Advances
The rapid progress in cancer genomics in recent years has enabled a close examination of the genetic makeup of thousands of individual cancers across the entire spectrum of major cancer types. Amidst the search for mutations responsible for driving carcinogenesis, it has become evident that overall mutation rates vary dramatically both within and between cancer types [15]. While the vast majority of mutations contribute little, if at all, to intrinsic biological function in tumor development and survival, they represent novel, “non-self” epitopes that, if processed and presented, carry the potential to elicit a tumor-specific immune response [16]. By mining exome sequencing data to predict neoantigens present in a tumor, several groups have demonstrated a strong correlation between total neoantigen burden and positive response to immune checkpoint blockade [17,18]. These insights provide a mechanistic rationale to expand checkpoint inhibition to mismatch repair-deficient cancers of any type, because the dramatically elevated mutation rates in these cancers produce significantly more neoantigens and the potential for a more robust immune response [19].
Although the importance of neoantigens is now firmly established, substantial overlap in neoantigen burden exists between nonresponders and responders, highlighting the need to more precisely identify the tumor-specific antigens required for a successful immune response. Emerging evidence suggests that clonal neoantigens—that is, neoantigens present in all cancer cells—rather than total neoantigen load are critical for T cell recognition and clinical benefit from checkpoint blockade [20]. The critical neoantigens also need not be shared between patients. One targeted approach with the potential to improve and increase responses to checkpoint inhibition is to produce personalized vaccines specific to the neoantigens present in a patient’s tumor [16,21].
However, selecting and manufacturing the optimal neoantigen vaccines within a clinically meaningful time frame remains challenging, and successful targeting of tumor neoantigens requires antigen-specific T cells, which may not be present. Indeed, the efficacy of CTLA-4 blockade likely stems in part from an expansion of the peripheral T cell receptor (TCR) repertoire [22], which presumably includes neoantigen-specific TCRs. Nascent experimental and computational methods may help to resolve the critical TCR–antigen interactions in each tumor [23,24], providing the critical blueprints for delivering precision immunotherapy.
As the precise mechanisms of immune checkpoint inhibition continue to be unraveled, great interest has emerged in combining checkpoint blockade with current targeted therapies. Substantial research on BRAF mutant melanoma indicates that BRAF inhibition synergizes with immune checkpoint blockade by facilitating T cell recognition of melanoma antigens in a more favorable tumor microenvironment [25,26]. Although one early trial combining immune checkpoint blockade with BRAF inhibition stalled because of hepatotoxicity [27], many additional trials are ongoing to optimize dosing and sequencing of the combination. An attractive feature of combining immune checkpoint blockade with targeted therapy is the potential to leverage current advances in precision medicine to impact many cancer types. For example, in BRCA1-deficient ovarian cancer, inhibition of poly(ADP-ribose) polymerases (PARPs), a family of enzymes involved in DNA repair, markedly increases mutational load in tumor cells and has demonstrated synergistic potential with CTLA-4 blockade [28]. Targeting driver mutations such as those in the genes encoding c-KIT or epidermal growth factor receptor (EGFR) with specific inhibitors appears to foster antitumor immunity [29,30]. In many cancers, disrupting tumor angiogenesis by inhibiting vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor (VEGFR) promotes immune cell recruitment and infiltration, which may derive additional benefit from checkpoint inhibition [31]. In all of these cases and others, clinical trials are ongoing to determine the ability of targeted therapies to potentiate response to immune checkpoint inhibition.
Future of Precision Immunotherapy
Propelled by recent successes, enthusiasm for immunotherapy has surged beyond targeting CTLA-4 and PD-1 pathways. Many additional immune regulators, both stimulatory and inhibitory, are being explored as potential targets for cancer immunotherapy, each with a unique set of advantages and potential risks. Novel insights into neoantigens and combination with immune checkpoint inhibition have breathed new life into cancer vaccines, which had been tested for decades with little success. In addition, cell-based therapies may be required in some patients to elicit a robust antitumor response. Adoptive cell transfer of autologous T cells selected for tumor reactivity [32] and genetically engineered T cells with chimeric antigen receptors targeting tumor antigens [33] have demonstrated promise in melanoma and other cancer types. As new immunotherapies expand the arsenal of cancer therapies, deeper mechanistic insight will be required to inform clinical decisions. The exciting advances toward understanding and delivering precision immunotherapy are poised to change the way cancer is treated.
Zdroje
1. Coley W. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am J Med Sci. 1893;105 : 487–511.
2. Holderfield M, Deuker MM, McCormick F, McMahon M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer. 2014;14(7):455–67. doi: 10.1038/nrc3760 24957944
3. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. doi: 10.1056/NEJMoa1103782 21639808
4. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466 20525992
5. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–32. doi: 10.1056/NEJMoa1503093 25891173
6. Hodi FS, Kluger H, Sznol M, Carvajal R, Lawrence D, Atkins M, et al. Abstract CT001: Durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a phase I trial. Cancer Research. 2016;76(14 Supplement):CT001–CT.
7. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082 25399552
8. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17. doi: 10.1056/NEJMoa1414428 25891304
9. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030 26027431
10. Larsen TE, Grude TH. A retrospective histological study of 669 cases of primary cutaneous malignant melanoma in clinical stage I. 3. The relation between the tumour-associated lymphocyte infiltration and age and sex, tumour cell type, pigmentation, cellular atypia, mitotic count, depth of invasion, ulceration, tumour type and prognosis. Acta Pathol Microbiol Scand A. 1978;86A(6):523–30. 716913
11. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954 25428505
12. Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504 23986400
13. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74. doi: 10.1158/1078-0432.CCR-13-3271 24714771
14. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. doi: 10.1056/NEJMoa1501824 25891174
15. Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8. doi: 10.1038/nature12213 23770567
16. Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–81. doi: 10.1038/nature13988 25428507
17. Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–11. doi: 10.1126/science.aad0095 26359337
18. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–99. doi: 10.1056/NEJMoa1406498 25409260
19. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–20. doi: 10.1056/NEJMoa1500596 26028255
20. McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–9. doi: 10.1126/science.aaf1490 26940869
21. Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348(6236):803–8. doi: 10.1126/science.aaa3828 25837513
22. Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res. 2014;20(9):2424–32. doi: 10.1158/1078-0432.CCR-13-2648 24583799
23. Li B, Li T, Pignon JC, Wang B, Wang J, Shukla SA, et al. Landscape of tumor - infiltrating T cell repertoire of human cancers. Nat Genet. 2016;48(7):725–32. doi: 10.1038/ng.3581 27240091
24. Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–6. doi: 10.1038/nature14001 25428506
25. Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19(5):1225–31. doi: 10.1158/1078-0432.CCR-12-1630 23307859
26. Cooper ZA, Juneja VR, Sage PT, Frederick DT, Piris A, Mitra D, et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol Res. 2014;2(7):643–54. doi: 10.1158/2326-6066.CIR-13-0215 24903021
27. Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368(14):1365–6. doi: 10.1056/NEJMc1302338 23550685
28. Higuchi T, Flies DB, Marjon NA, Mantia-Smaldone G, Ronner L, Gimotty PA, et al. CTLA-4 Blockade Synergizes Therapeutically with PARP Inhibition in BRCA1-Deficient Ovarian Cancer. Cancer Immunol Res. 2015;3(11):1257–68. doi: 10.1158/2326-6066.CIR-15-0044 26138335
29. Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–63. doi: 10.1158/2159-8290.CD-13-0310 24078774
30. Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17(9):1094–100. doi: 10.1038/nm.2438 21873989
31. Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109(43):17561–6. doi: 10.1073/pnas.1215397109 23045683
32. Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–7. doi: 10.1158/1078-0432.CCR-11-0116 21498393
33. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222 25317870
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 12- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Tumor Evolution in Two Patients with Basal-like Breast Cancer: A Retrospective Genomics Study of Multiple Metastases
- Expression of lncRNAs in Low-Grade Gliomas and Glioblastoma Multiforme: An In Silico Analysis
- Exploratory Analysis of Mutations in Circulating Tumour DNA as Biomarkers of Treatment Response for Patients with Relapsed High-Grade Serous Ovarian Carcinoma: A Retrospective Study
- The Subclonal Architecture of Metastatic Breast Cancer: Results from a Prospective Community-Based Rapid Autopsy Program “CASCADE”
- Predictors of Chemosensitivity in Triple Negative Breast Cancer: An Integrated Genomic Analysis
- Histological Transformation and Progression in Follicular Lymphoma: A Clonal Evolution Study
- Somatic Genomics and Clinical Features of Lung Adenocarcinoma: A Retrospective Study
- Patterns of Immune Infiltration in Breast Cancer and Their Clinical Implications: A Gene-Expression-Based Retrospective Study
- IL-7 Receptor Mutations and Steroid Resistance in Pediatric T cell Acute Lymphoblastic Leukemia: A Genome Sequencing Study
- Cancer Genomics: Large-Scale Projects Translate into Therapeutic Advances
- Overcoming Steroid Resistance in T Cell Acute Lymphoblastic Leukemia
- Precision Cancer Diagnostics: Tracking Genomic Evolution in Clinical Trials
- Immunotherapy in the Precision Medicine Era: Melanoma and Beyond
- Sequencing Strategies to Guide Decision Making in Cancer Treatment
- Blood-Based Analysis of Circulating Cell-Free DNA and Tumor Cells for Early Cancer Detection
- Base-Position Error Rate Analysis of Next-Generation Sequencing Applied to Circulating Tumor DNA in Non-Small Cell Lung Cancer: A Prospective Study
- Clonal Evolutionary Analysis during HER2 Blockade in HER2-Positive Inflammatory Breast Cancer: A Phase II Open-Label Clinical Trial of Afatinib +/- Vinorelbine
- Mutational Profile of Metastatic Breast Cancers: A Retrospective Analysis
- Genomic Analysis of Uterine Lavage Fluid Detects Early Endometrial Cancers and Reveals a Prevalent Landscape of Driver Mutations in Women without Histopathologic Evidence of Cancer: A Prospective Cross-Sectional Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genomic Analysis of Uterine Lavage Fluid Detects Early Endometrial Cancers and Reveals a Prevalent Landscape of Driver Mutations in Women without Histopathologic Evidence of Cancer: A Prospective Cross-Sectional Study
- Overcoming Steroid Resistance in T Cell Acute Lymphoblastic Leukemia
- Clonal Evolutionary Analysis during HER2 Blockade in HER2-Positive Inflammatory Breast Cancer: A Phase II Open-Label Clinical Trial of Afatinib +/- Vinorelbine
- Predictors of Chemosensitivity in Triple Negative Breast Cancer: An Integrated Genomic Analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



