-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Sublingual Misoprostol versus Intramuscular Oxytocin for Prevention of Postpartum Hemorrhage in Uganda: A Double-Blind Randomized Non-Inferiority Trial
Background:
Postpartum hemorrhage (PPH) is a leading cause of maternal death in sub-Saharan Africa. Although the World Health Organization recommends use of oxytocin for prevention of PPH, misoprostol use is increasingly common owing to advantages in shelf life and potential for sublingual administration. There is a lack of data about the comparative efficacy of oxytocin and sublingual misoprostol, particularly at the recommended dose of 600 µg, for prevention of PPH during active management of labor.Methods and Findings:
We performed a double-blind, double-dummy randomized controlled non-inferiority trial between 23 September 2012 and 9 September 2013 at Mbarara Regional Referral Hospital in Uganda. We randomized 1,140 women to receive 600 µg of misoprostol sublingually or 10 IU of oxytocin intramuscularly, along with matching placebos for the treatment they did not receive. Our primary outcome of interest was PPH, defined as measured blood loss ≥500 ml within 24 h of delivery. Secondary outcomes included measured blood loss ≥1,000 ml; mean measured blood loss at 1, 2, and 24 h after delivery; death; requirement for blood transfusion; hemoglobin changes; and use of additional uterotonics.
At 24 h postpartum, primary PPH occurred in 163 (28.6%) participants in the misoprostol group and 99 (17.4%) participants in the oxytocin group (relative risk [RR] 1.64, 95% CI 1.32 to 2.05, p<0.001; absolute risk difference 11.2%, 95% CI 6.44 to 16.1). Severe PPH occurred in 20 (3.6%) and 15 (2.7%) participants in the misoprostol and oxytocin groups, respectively (RR 1.33, 95% CI 0.69 to 2.58, p = 0.391; absolute risk difference 0.9%, 95% CI −1.12 to 2.88). Mean measured blood loss was 341.5 ml (standard deviation [SD] 206.2) and 304.2 ml (SD 190.8, p = 0.002) at 2 h and 484.7 ml (SD 213.3) and 432.8 ml (SD 203.5, p<0.001) at 24 h in the misoprostol and oxytocin groups, respectively. There were no significant differences between the two groups in any other secondary outcomes. Women in the misoprostol group more commonly experienced shivering (RR 1.91, 95% CI 1.65 to 2.21, p<0.001) and fevers (RR 5.20, 95% CI 3.15 to 7.21, p = 0.005).
This study was conducted at a regional referral hospital with capacity for emergency surgery and blood transfusion. High-risk women were excluded from participation.Conclusions:
Misoprostol 600 µg is inferior to oxytocin 10 IU for prevention of primary PPH in active management of labor. These data support use of oxytocin in settings where it is available. While not powered to do so, the study found no significant differences in rate of severe PPH, need for blood transfusion, postpartum hemoglobin, change in hemoglobin, or use of additional uterotonics between study groups. Further research should focus on clarifying whether and in which sub-populations use of oxytocin would be preferred over sublingual misoprostol.Trial registration:
ClinicalTrials.gov NCT01866241
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(11): e32767. doi:10.1371/journal.pmed.1001752
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001752Summary
Background:
Postpartum hemorrhage (PPH) is a leading cause of maternal death in sub-Saharan Africa. Although the World Health Organization recommends use of oxytocin for prevention of PPH, misoprostol use is increasingly common owing to advantages in shelf life and potential for sublingual administration. There is a lack of data about the comparative efficacy of oxytocin and sublingual misoprostol, particularly at the recommended dose of 600 µg, for prevention of PPH during active management of labor.Methods and Findings:
We performed a double-blind, double-dummy randomized controlled non-inferiority trial between 23 September 2012 and 9 September 2013 at Mbarara Regional Referral Hospital in Uganda. We randomized 1,140 women to receive 600 µg of misoprostol sublingually or 10 IU of oxytocin intramuscularly, along with matching placebos for the treatment they did not receive. Our primary outcome of interest was PPH, defined as measured blood loss ≥500 ml within 24 h of delivery. Secondary outcomes included measured blood loss ≥1,000 ml; mean measured blood loss at 1, 2, and 24 h after delivery; death; requirement for blood transfusion; hemoglobin changes; and use of additional uterotonics.
At 24 h postpartum, primary PPH occurred in 163 (28.6%) participants in the misoprostol group and 99 (17.4%) participants in the oxytocin group (relative risk [RR] 1.64, 95% CI 1.32 to 2.05, p<0.001; absolute risk difference 11.2%, 95% CI 6.44 to 16.1). Severe PPH occurred in 20 (3.6%) and 15 (2.7%) participants in the misoprostol and oxytocin groups, respectively (RR 1.33, 95% CI 0.69 to 2.58, p = 0.391; absolute risk difference 0.9%, 95% CI −1.12 to 2.88). Mean measured blood loss was 341.5 ml (standard deviation [SD] 206.2) and 304.2 ml (SD 190.8, p = 0.002) at 2 h and 484.7 ml (SD 213.3) and 432.8 ml (SD 203.5, p<0.001) at 24 h in the misoprostol and oxytocin groups, respectively. There were no significant differences between the two groups in any other secondary outcomes. Women in the misoprostol group more commonly experienced shivering (RR 1.91, 95% CI 1.65 to 2.21, p<0.001) and fevers (RR 5.20, 95% CI 3.15 to 7.21, p = 0.005).
This study was conducted at a regional referral hospital with capacity for emergency surgery and blood transfusion. High-risk women were excluded from participation.Conclusions:
Misoprostol 600 µg is inferior to oxytocin 10 IU for prevention of primary PPH in active management of labor. These data support use of oxytocin in settings where it is available. While not powered to do so, the study found no significant differences in rate of severe PPH, need for blood transfusion, postpartum hemoglobin, change in hemoglobin, or use of additional uterotonics between study groups. Further research should focus on clarifying whether and in which sub-populations use of oxytocin would be preferred over sublingual misoprostol.Trial registration:
ClinicalTrials.gov NCT01866241
Please see later in the article for the Editors' SummaryIntroduction
Of the estimated 287,000 maternal deaths worldwide, 85% occur in low - and middle-income countries [1]. In Uganda, the maternal mortality ratio is among the highest in the world, estimated at over 360 for every 100,000 women [2] and claiming the lives of over 5,500 mothers annually. Twenty-five percent of these deaths occur because of postpartum hemorrhage (PPH) within 24 h of delivery [3].
Oxytocin, a hormone that stimulates uterine contractions and limits uterine bleeding after birth, is the standard of care for prevention of PPH during the third stage of labor [4]. The use of oxytocin in low-income countries, however, has historically been limited by a number of factors including a perceived requirement for administration by skilled personnel, cold chain storage, and a requirement for sterile syringes and needles [5],[6]. Recent work has begun to challenge these limitations, as exemplified by effective administration of oxytocin by lay community health officers during home births [7].
Misoprostol, a synthetic prostaglandin with uterotonic properties, has been proposed as an alternative strategy for prevention of PPH in settings where oxytocin use is not feasible. It has important advantages over oxytocin, including the potential for oral administration and a long shelf life at room temperature [8]. Moreover, misoprostol can be administered sublingually, enabling a more rapid onset of action and greater bioavailability by avoiding first-pass metabolism [9]. These characteristics have led civil society organizations in Uganda to champion increased accessibility and use of misoprostol as a complementary drug to oxytocin in prevention of PPH [6]. Yet despite these advantages, sublingual misoprostol remains a second-line option to injectable uterotonics according to most recommending agencies [4],[10] because of insufficient [11] or conflicting [12] evidence about its efficacy in the active management of the third stage of labor. Although prior studies have compared injectable oxytocin with misoprostol [11], the comparative efficacy of sublingual misoprostol versus oxytocin remains largely unknown because prior studies have focused on oral administration of misoprostol by less skilled birth attendants [13],[14], evaluated oral as opposed to sublingual administration of misoprostol [15], or evaluated suboptimal doses of either oxytocin [16], other injectable uterotonics, or misoprostol [17]–[19].
We performed a double-blind, double-dummy randomized controlled non-inferiority trial comparing sublingual misoprostol versus oxytocin at a publically funded regional referral hospital in rural, southwestern Uganda. We aimed to elucidate the comparative benefit of oxytocin versus sublingual misoprostol, at the World Health Organization recommended dose of 600 µg [4], for prevention of PPH during active management of uncomplicated labor at a large referral hospital in a resource-limited setting. We hypothesized that sublingual misoprostol would be non-inferior to oxytocin for prevention of primary PPH.
Methods
Ethics Statement
This study was approved by the Institutional Review Committee of Mbarara University of Science and Technology and the Uganda National Council for Science and Technology. Trial registration at ClinicalTrials.gov (NCT01866241) was completed approximately halfway through the study. The delayed registration was due to the prolonged leave of the single ClinicalTrials.gov administrator at the principal investigator's institution.
Study Design and Setting
We conducted a double-blind, double-dummy randomized controlled non-inferiority trial at Mbarara Regional Referral Hospital, a publically funded teaching hospital in southwestern Uganda serving ten districts with a population of over 5 million people. The hospital employs seven obstetricians and 22 midwives. Hospital staff perform over 10,000 deliveries annually. Prior to the study, we performed a retrospective review of hospital records to estimate rates of attended births, PPH, and maternal mortality. During that review, we counted 9,027 births over a 10-mo period. During that period, 2,979 mothers (33%) were recorded to have had PPH, and 11 mothers died during admission because of complications of PPH (0.12% of mothers in the review period).
Participants and Recruitment
Midwife research assistants (MRAs) screened laboring mothers in early active labor on arrival to the prenatal ward. Eligibility criteria were (1) age above 18 y, (2) 38–41 wk of amenorrhea, and (3) anticipated uncomplicated vaginal delivery as assessed by hospital staff. The exclusion criteria were (1) confirmed intrauterine fetal death, (2) self-reported maternal heart disease, (3) current diagnosis of severe malaria or acute bacterial infection, (4) multiple pregnancy, (5) induced or augmented labor, (6) elective cesarean section, (7) antepartum hemorrhage, (8) reported hypersensitivity to prostaglandins, and (9) altered cognitive status as assessed by MRA. MRAs obtained informed consent from all eligible participants after the birth was predicted to be an uncomplicated vaginal delivery. An MRA trained in human participant research conducted informed consent procedures with eligible mothers in the local language in a private area of the hospital. Only mothers in the early stages of labor (less than 6 cm dilation) were approached. All consenting participants gave written informed consent, or for those who could not write, a thumbprint was made on the consent form.
Randomization, Blinding, and Medicine Preparation
A study biostatistician generated a randomization list with a block size of ten, totaling 570 participants in each group. The list was shared only with the study clinical pharmacist, who prepared the study drugs and placebos. Each participant received a treatment (600 µg of misoprostol or 10 IU of oxytocin) and placebo (injection of 1 ml of sterile water or three pills containing maize starch, methyl hydroxybenzoate, and magnesium stearate) within 1 min of birth. An independent clinical pharmacist at Mbarara University of Science and Technology prepared the corresponding treatments and placebos. Misoprostol 200-µg pills (Cytotec Searle, United Kingdom) were procured from Laborex Uganda. Oxytocin (10 IU/1 ml) was procured from Joint Medical Store (Kampala, Uganda). Before the use of the medications, we performed bioequivalence testing for both active interventions at the Ugandan National Chemotherapeutics Research Laboratory. Bioequivalence for misoprostol ranged from 95.8% to 99.8%, and for oxytocin ranged from 94.7% to 103.5%. To achieve blinding of the participants and assessors, both inactive agents were manufactured and packaged to resemble actual study medicines in terms of shape, size, and color by Kampala Pharmaceuticals Industries (Uganda).
Study Procedures
MRAs received opaque envelopes with affixed study codes, containing both an injection (1 ml of oxytocin 10 IU or its placebo) and three pills (misoprostol 600 µg or its placebo), which were given intramuscularly and sublingually, respectively, within 1 min of delivery. Delayed cord clamping was preferred, and the placenta was delivered by controlled cord traction or manually if not delivered within 30 min postpartum, as per Ugandan national clinical guidelines [20]. Further care was provided by the hospital clinical care team in collaboration with MRAs in accordance with national guidelines, which recommend administration of a repeat dose of parenteral oxytocics along with bladder emptying, management of lacerations, and uterine massage if bleeding persists. All mothers were monitored for a minimum of 24 h postpartum.
Study Measures
A blood sample for complete blood count was drawn immediately after admission and again prior to hospital discharge, or before blood transfusion. MRAs recorded vital signs, duration of second and third stages of labor, secondary use of open-label uterotonics, placental retention, requirement for blood transfusion, and side effects, using a standardized data collection form. After the baby was born, the amniotic fluid was drained immediately. A clean plastic sheet specifically designed and piloted to collect blood for this trial was placed under the mother's buttocks during and after the third stage of labor. Blood was drained into a calibrated container to improve accuracy in blood loss measurement [21],[22]. All mothers were given preweighed standard sanitary pads to place in the perineum at all times. These pads were changed and weighed hourly for the first 6 h, and then every 6 h until 24 h postpartum. Blood loss was estimated as 1 ml per gram of weight of the pad after subtracting the dry pad weight, as previously described [22]. This estimated blood loss was added to the volume of blood from the plastic sheet. To improve consistency in estimation of blood loss, standardized electronic scales were used to weigh soiled sanitary pads.
Study Outcomes
Our primary outcome was primary PPH, defined as maternal loss of blood ≥500 ml within 24 h of birth, as conventionally defined [4]. Secondary outcomes included the following: (1) death; (2) severe PPH, defined as maternal blood loss ≥1,000 ml within 24 h of birth; (3) changes in red cell indices during hospitalization, defined as (a) postpartum hemoglobin <100 g/l, (b) >10% decrease between pre - and postpartum hemoglobin, (c) mean postpartum hemoglobin, and (d) mean postpartum hematocrit; (4) mean measured blood loss at 1, 2, and 24 h postpartum; (5) placental retention; (6) requirement for blood transfusion (which is indicated per clinical protocol at the study site for mothers with a hemoglobin <100 g/l and/or severe pallor); (7) requirement for additional management of PPH, including therapeutic uterotonic drugs or surgical or radiological procedures; and (8) duration of the third stage of labor. The outcomes of blood loss at 1 and 2 h postpartum were post hoc analyses added to enable direct comparisons with other studies, which have often used those end points. All patients were assessed for continued blood loss at 2 h postpartum, when a second blood sample was drawn from participants for complete blood count, blood type, and cross-matching. To avoid measurement bias, we used the values from this measurement to calculate changes in hemoglobin and hematocrit levels for women who received a subsequent blood transfusion. We also compared the safety profile of both treatment groups, including observed rates of shivering, nausea and vomiting, fever >37.5°C within 24 h of delivery, self-reported headache, diarrhea, abdominal afterpains, and the use of analgesics in the postpartum period.
Sample Size and Statistical Analysis
We followed CONSORT guidelines for conducting and reporting a non-inferiority study [23]. We designed the non-inferiority study with a 6% absolute risk difference as our non-inferiority margin (ΔNI). We selected the non-inferiority margin of 6% based on prior data comparing oxytocin with placebo for active management of labor that demonstrated a 50% relative reduction in the rate of PPH with oxytocin versus placebo [24]. Assuming a predicted incidence of PPH of 14% in mothers treated with prophylactic oxytocin, as reported previously by a well-powered clinical trial [15], a non-inferiority margin of 6% would correspond to an upper bound of PPH incidence of 20% among mothers treated with misoprostol. We chose this upper bound of non-inferiority so that a 20% PPH rate in the misoprostol arm would be similar to the rate for women treated with oxytocin and likely superior to predicted rates of PPH in women not receiving treatment [25]. Allowing for a two-sided type I error of 5%, we planned enrollment of 1,140 mothers to enable 90% power to demonstrate non-inferiority between groups. We compared dichotomous outcomes between study groups by estimating crude relative risks (RRs) with 95% confidence intervals, and testing for differences between treatment groups. We estimated p-values with chi-squared tests using a level of significance of 0.05. We also tested for the significance of absolute risk differences using the Z-test of proportions. For continuous outcomes, we estimated p-values using Student's t tests. All primary and secondary outcomes were analyzed using intention-to-treat analyses (although no participants were misallocated treatment) [23]. As per the revised CONSORT guidelines for reporting randomized trials [26], we assessed for sub-group effects for the following characteristics by testing the significance of interaction terms in a multivariable logistic regression model: (1) age (18–35 y and >35 y), (2) parity (1, 2–4, and ≥5), (3) birth weight (<2,500 g, 2,500–3,449 g, and ≥3,500 g), (4) placental weight (<0.8 and ≥0.8 kg), (5) any perinatal surgical procedure (episiotomy and/or perineal tear and no episiotomy or perineal tear), (6) admission hemoglobin (<120 g/l and ≥120 g/l), and (7) body mass index (<25 and ≥25 kg/m2). Finally, although our study was fully randomized, we noted differences between treatment groups in the proportion of women with the following characteristics: presence of perineal tears, requirement for episiotomy, and parity. As such, we performed post hoc analyses to assess for confounding by fitting multivariable logistic regression models to assess for differences in our by-treatment estimates after adjustment for these characteristics. All statistical analyses were performed using STATA version 12.0 (StataCorp, College Station, Texas, US). An independent data safety monitoring board composed of members at the Mbarara University of Science and Technology, Makerere University, and Mbarara Regional Referral Hospital reviewed preliminary results at 50% (570) and 75% (855) of projected enrollment, as specified in the protocol, and recommended continuing study procedures.
Results
Of 8,867 mothers screened for eligibility from 23 September 2012 to 9 September 2013, 4,314 were eligible. A total of 2,369 (55%) declined participation in the study (Figure 1), and 1,140 were enrolled, received a randomized treatment, and completed study procedures. Demographic and clinical characteristics were similar between the two treatment groups (Table 1). Primary PPH occurred in 163 (28.6%) participants in the misoprostol group and 99 (17.4%) participants in the oxytocin group (RR 1.64, 95% CI 1.32 to 2.05, p<0.001; absolute risk difference 11.2%, 95% CI 6.44 to 16.1; Table 2), corresponding to a number needed to treat of nine (meaning that nine women would need to be treated with oxytocin instead of misoprostol to prevent one case of PPH). The absolute risk difference between the two groups failed to meet the pre-specified non-inferiority margin of 6%. In stratified analyses to assess for differences in our primary outcome within sub-groups, none of the sub-group-by-treatment interaction terms was significant (Table 3). With the exception of women with parity ≥5, all of the point estimates favored oxytocin. Thus, while the study was not powered to estimate effects within sub-groups, our results do not suggest differential effects of treatment within specific sub-groups of mothers.
Fig. 1. Trial profile. 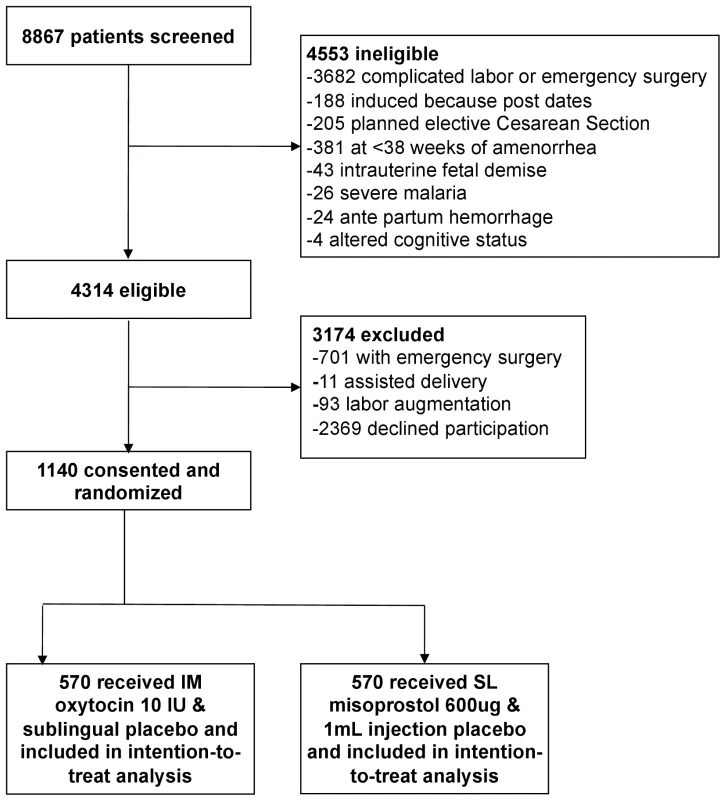
IM, intramuscular; SL, sublingual. Tab. 1. Baseline demographic and clinical characteristics by treatment group. 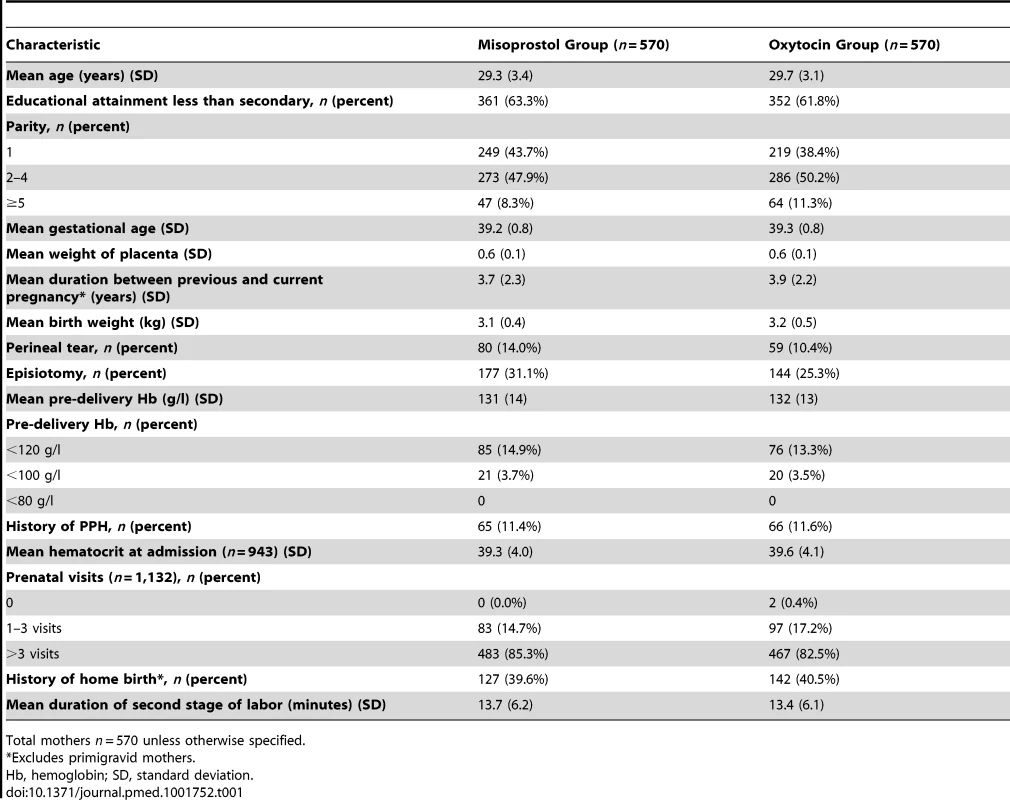
Total mothers n = 570 unless otherwise specified. Tab. 2. Primary and secondary outcomes by treatment group. 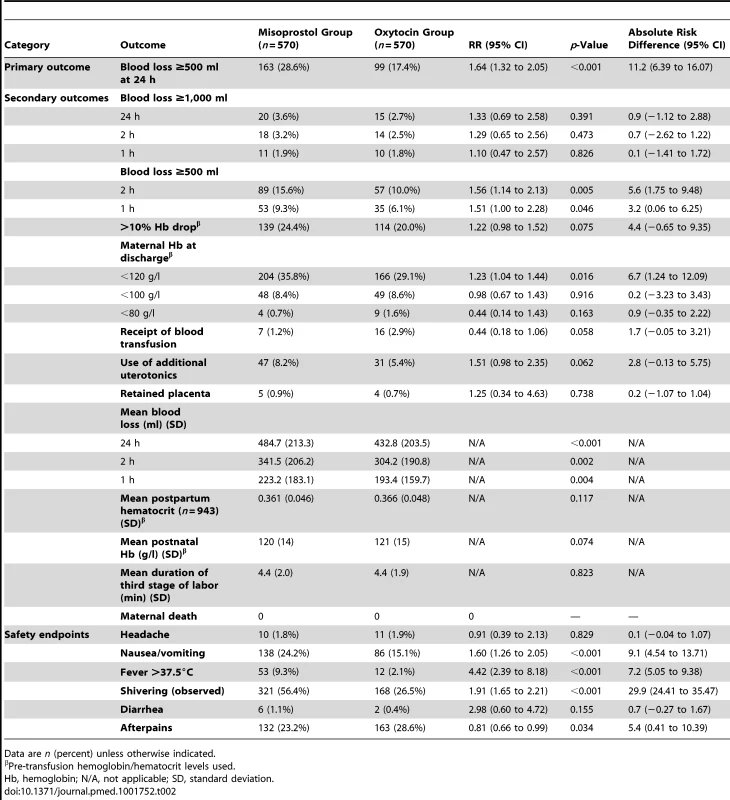
Data are n (percent) unless otherwise indicated. Tab. 3. Maternal baseline sub-groups by treatment group with PPH. 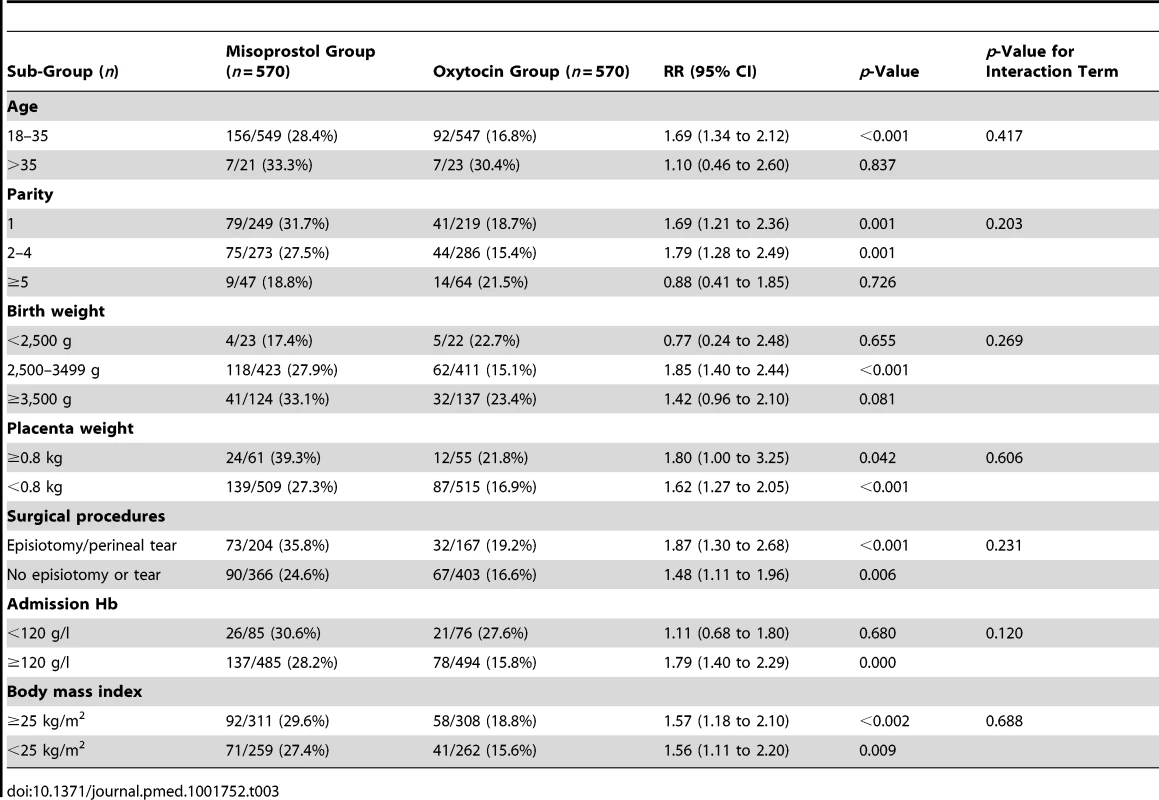
We found a benefit for oxytocin versus misoprostol in terms of measured blood loss at 1 and 2 h postpartum (Table 2). The measured blood loss distribution was skewed to the right for both those receiving misoprostol (range 46.7–1,557.4 ml; median 457.6 ml) and those receiving oxytocin (range 28.1–1,617.8 ml; median 410.4 ml) (Figure 2). Importantly, there were no deaths in either group, and we found no statistically significant difference in the incidence of severe PPH at 24 h postpartum, which occurred in 20 (3.6%) participants in the misoprostol group and 15 (2.7%) in the oxytocin group (RR 1.33, 95% CI 0.69 to 2.58, p = 0.391; absolute risk difference 0.9%, 95% CI −1.12 to 2.88). There was also no difference in the rate of severe PPH between groups as estimated at 1 and 2 h postpartum. More mothers in the misoprostol group than in the oxytocin group received additional open-label oxytocin (p = 0.062). The number of mothers requiring and receiving a blood transfusion was higher in the oxytocin group than in the misoprostol group, but the difference did not reach statistical significance (2.9% versus 1.2%, p = 0.058). There were also no significant differences in hemoglobin change (p = 0.075), mean postpartum hemoglobin (p = 0.074), rate of retained placenta (p = 0.378), or duration of the third stage of labor (p = 0.823) (Table 2).
Fig. 2. Distribution of blood loss and hemoglobin change by treatment arm. 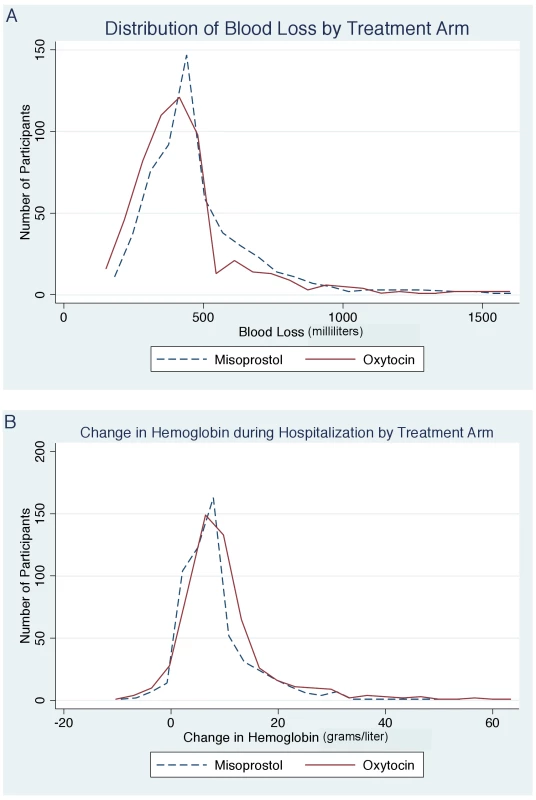
(A) Distribution of blood loss by treatment arm. (B) Change in hemoglobin during hospitalization by treatment arm. While we performed a randomized control trial and any differences in baseline characteristics occurred by chance, we did detect baseline differences in parity, requirement for episiotomy, and requirement for repair of perineal tears between study groups (Table 1). We assessed for bias from differences in baseline characteristics between groups by fitting multivariable logistic regression models. In these models, we found no meaningful difference in the odds ratio of PPH for misoprostol versus oxytocin after adjustment for parity, perineal tears, and requirement for episiotomy (adjusted odds ratio 0.54, 95% CI 0.41 to 0.72, p<0.001).
Side effects were more common in the misoprostol group than in the oxytocin group. A greater proportion of women in the misoprostol group than in the oxytocin group experienced moderate to severe shivering (56.4% versus 26.5%, RR 1.91, 95% CI 1.65 to 2.21, p<0.001), nausea and vomiting (24.2% versus 15.1%, RR 1.60, 95% CI 1.26 to 2.05, p<0.001), and temperature >37.5°C (9.3% versus 2.1%, RR 4.42, 95% CI 2.39 to 8.18, p<0.001). Afterpains were more common in the oxytocin group than in the misoprostol group (p = 0.036). No differences were found between the misoprostol and oxytocin groups in the rates of diarrhea (p = 0.155) or headache (p = 0.829).
Discussion
We demonstrated that sublingual misoprostol is inferior to oxytocin for prevention of primary PPH in women undergoing uncomplicated vaginal deliveries at a publically funded regional referral hospital in southwestern Uganda. We found a 64% increased risk of primary PPH (measured blood loss ≥500 ml at 24 h) and an absolute risk increase of 11.2% with misoprostol versus oxytocin. We also found a 33% higher rate of severe PPH (measured blood loss ≥1,000 ml) in the misoprostol group, although this difference was not statistically significant. There were no maternal deaths in either group. The rates of secondary outcomes, including mean postpartum hemoglobin, requirement for additional uterotonics, hemoglobin changes, blood transfusion, duration of third stage of labor, and retained placenta were similar in both groups. While not statistically significant, we did observe a lower absolute rate of blood transfusion and proportion of women with postpartum hemoglobin <80 g/l in the misoprostol group.
Our data contribute to a complex array of data on optimal prevention of PPH in the third stage of labor in resource-limited settings. Like many prior studies [11], we found a modest benefit for oxytocin over misoprostol. In summary, we estimate that only nine women (95% CI 6 to 16) would need to be treated with oxytocin instead of misoprostol to prevent one case of primary PPH. On the other hand, our study was restricted to relatively healthy women without significant co-morbidities, and we detected no deaths at the time of discharge in either group. Moreover, we detected a difference in mean measured blood loss of only 29 ml, 37 ml, and 52 ml at 1, 2, and 24 h postpartum, respectively, and no differences in other secondary outcomes, including severe PPH, death, hemoglobin changes, or receipt of a blood transfusion. Our data therefore signal that, among relatively healthy women undergoing uncomplicated labor, oxytocin provides modest benefit over sublingual misoprostol for prevention of PPH generally, and should be the preferred agent where feasible and available. However, although we did not include a placebo arm in our trial, the lack of significant difference in other clinically significant outcomes between the treatment groups also offers promising preliminary data that sublingual misoprostol at a dose of 600 µg is likely to be of important benefit where oxytocin is unavailable. If and whether select populations gain preferential benefit from oxytocin over misoprostol remains an important question for further investigation.
Our results are largely consistent with prior studies comparing misoprostol with oxytocin for prevention of PPH. Only one prior trial (n = 100) specifically compared sublingual misoprostol at the routinely used dose of 600 µg to oxytocin at a dose of 10 IU, and found a lower mean blood loss with oxytocin [27]. A similar study comparing lower dose sublingual misoprostol with oxytocin 10 IU found a non-significant decrease in blood loss with oxytocin at 1 h postpartum [28]. In contrast, another study also comparing low-dose sublingual misoprostol 400 µg with oxytocin 10 IU found misoprostol more effective in prevention of PPH at 2 h postpartum [21]. Importantly, that study used a powdered sublingual formulation of misoprostol and was unintentionally unblinded because of lack of proper placebos [29]. Finally, a small study (n = 60) compared sublingual misoprostol 600 µg to syntometrine in place of oxytocin, and found no difference in PPH between the two groups [30]. Unlike these prior studies, our study was powered to demonstrate non-inferiority of a clinical outcome (or lack thereof) for sublingual misoprostol at a recommended dose of 600 µg versus conventional intramuscular oxytocin 10 IU in prevention of primary PPH, as defined by WHO (measured blood loss ≥500 ml at 24 h postpartum).
While we acknowledge that a recent large systematic review comparing misoprostol with injectable uterotonics in the management of the third stage of labor has been conducted [11], the prior evidence related to use of sublingual misoprostol 600 µg has been largely limited by variability of comparator uterotonics, dosing of study drugs, and heterogeneity of outcome definitions. For example, in 72 trials discussed in this review (n = 52,678), only 663 women received sublingual misoprostol, and only 60 women did so at the recommended dose of 600 µg [30]. In summary, our data are the first, to our knowledge, powered to evaluate whether the routinely used and recommended dosing of sublingual misoprostol (600 µg) is non-inferior to oxytocin 10 IU for the outcome of PPH.
Importantly, and in contrast to our study, prior studies have demonstrated a significantly higher risk of both PPH (≥500 ml) and severe PPH (≥1,000 ml) when oral misoprostol is used versus oxytocin [11]. For example, a large WHO-lead trial [16] documented a one percentage point difference in blood loss of ≥1,000 ml at 1 h postpartum for misoprostol versus oxytocin (4% versus 3%). This is in contrast to the minimal difference (0.1%) we detected in measured blood loss at 1 h postpartum (1.9% versus 1.8%). Although we did not directly compare use of oral with sublingual misoprostol, our results—specifically the relatively small differences detected in mean measured blood loss, hemoglobin change, and rates of PPH and severe PPH—support preferential consideration of sublingual misoprostol over the oral route of administration. A potential alternative to sublingual administration of misoprostol in prevention of PPH may be a powdered formulation of sublingual misoprostol, which has shown superior efficacy compared to oxytocin [21]. A theoretical advantage of sublingual misoprostol could be improved bioavailability gained by evading first-pass metabolism [9].
Another potential explanation for differences between our study and prior data, which have shown larger effect sizes for differences between prostaglandins and oxytocin, is our exclusion of women with cesarean deliveries and multiple pregnancies. Our selection criteria could underestimate true differences in bleeding risk in the general population, and specifically in higher risk women.
We found increased rates of side effects with misoprostol versus oxytocin, as has been previously reported [11]. Although misoprostol-related shivering is typically considered a nonserious side effect, prior studies have reported fever [31],[32], secondary psychological effects including anxiety, and perceptions of lack of body control [33]. Other concerns have included resultant delays in blood transfusion, and mimicry of postpartum infection resulting in unnecessary antibiotic administration, although this may be uncommon [15].
The benefit of oxytocin over sublingual misoprostol for prevention of PPH in this trial was seen across most sub-groups. Effect sizes appeared smaller in certain sub-groups, for example, women with admission hemoglobin less than 120 g/l, those older than 35 y, those giving birth to infants with a birth weight less than 2,500 g, and those with parity greater than four, which corroborates prior work demonstrating increased risk of PPH with advanced maternal age, with anemia [34],[35], in multiparous women, and in women with infants of low birth weight [36]. Although there were observed differences in point estimates of PPH incidence in these sub-groups, we found no significant differences in the effect of the treatment across these categories.
Our study had a number of strengths. All study investigators and clinical staff were blinded to treatment allocation. We used placebos for both oxytocin injection and misoprostol pills. Although blinding might have been unmasked, particularly by known side effects (e.g., shivering), we found similar benefit for oxytocin in a sub-analysis of women without documented shivering (RR 1.62, 95% CI 1.18 to 2.23, p = 0.003). We performed the study in a prototypical, publically funded and operated hospital in a rural setting with an active maternity unit, subject to the standard limitations of public sector health care facilities in the region. As such, the study has great potential for generalizability to similar settings.
Our study had some important limitations. We observed a decrease in maternal PPH from 33% to 17% and in maternal mortality from 0.12% to 0% from the pre-study period to the study period, suggesting either presence of strict exclusion criteria, inaccurate estimation of blood loss in the pre-study period, or possibility of a Hawthorne effect, which might have resulted from use of trained MRAs in the study. We also noted clustering of blood loss measurement between 400 and 500 ml in both groups. We suspect this was an observer bias stemming from the prespecified dichotomous outcome of ≥500 ml over 24 h. While this might have diminished the overall outcome incidence, our blinding procedures make it unlikely that measurement error would bias our estimates.
Another limitation of our study was the observed rate of eligible participants declining participation (54.9%). A review of stated reasons for declining participation revealed that most (97%) participants who declined were disinterested in participating in a research study, which was perhaps not unexpected given that most women were presenting in active labor. A recent survey study on the ward (Dr. Lenard Abesiga, personal communication, 1 November 2012) demonstrated that most mothers on the maternity ward in this hospital (92%) have little or no knowledge of medicines administered during labor. Nonetheless, the high declination rate might introduce a selection bias towards relatively healthy women.
Conclusion and Recommendations
We found that sublingual misoprostol 600 µg is inferior to oxytocin 10 IU for prevention of primary PPH during active management of the third stage of labor among women undergoing uncomplicated delivery in a rural referral hospital in southwestern Uganda. Severe PPH was rare in our study population, and we detected no significant difference between those receiving sublingual misoprostol versus oxytocin (RR 1.33, 95% CI 0.69 to 2.58, p = 0.391). There were also similar rates of changes in postpartum hemoglobin, duration of the third stage of labor, requirement for additional uterotonics, and requirement for a blood transfusion. These data demonstrate that, in settings where it is available, oxytocin should remain a preferred agent for prevention of PPH. However, sublingual misoprostol appears to maintain an important role for prevention of severe PPH and other complications of PPH where oxytocin is not available, and reinforces the array of available interventions for reducing maternal morbidity and mortality.
Further work should help clarify whether and in which sub-populations preferential use of oxytocin might have the highest impact. This is particularly important in resource-limited settings where storage and availability of oxytocin remains a major challenge. Additionally, further evaluation of the actual and perceived barriers to oxytocin use for prevention of PPH in resource-limited settings will help improve its availability and use in such settings.
Supporting Information
Zdroje
1. World Health Organization, United Nations Children's Fund, United Nations Population Fund, World Bank (2012) Trends in maternal mortality: 1990–2010. WHO, UNICEF, UNFPA and The World Bank estimates. Geneva: World Health Organization.
2. United Nations Department of Economic and Social Affairs Population Division (2013) World population prospects: the 2012 revision. New York: United Nations Department of Economic and Social Affairs Population Division.
3. Uganda Ministry of Health (2010) Clinical guidelines for prevention and treatment of post partum haemorrhage using misoprostol. Kampala: Uganda Ministry of Health.
4. World Health Organization (2012) WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: World Health Organization.
5. PrataN, BellS, WeidertK (2013) Prevention of postpartum hemorrhage in low-resource settings: current perspectives. Int J Womens Health 5 : 737–752 doi:10.2147/IJWH.S51661
6. AtukundaEC, BrhlikovaP, AgabaAG, PollockAM (2013) Registration, procurement, distribution, and use of misoprostol in Uganda: an interview-based observational study. Lancet 382 : 10.
7. StantonCK, NewtonS, MullanyLC, CofieP, Tawiah AgyemangC, et al. (2013) Effect on postpartum hemorrhage of prophylactic oxytocin (10 IU) by injection by community health officers in Ghana: a community-based, cluster-randomized trial. PLoS Med 10: e1001524.
8. TangOS, Gemzell-DanielssonK, HoPC (2007) misoprostol: pharmacokinetic profiles, effects on the uterus and side-effects. Int J Gynaecol Obstet 99 (Suppl 2) S160–S167.
9. Katzung GB (2010) Basic principles of pharmacology. In: Katzung GB, Masters SB, Trevor AJ, editors. Basic and clinical pharmacology, 12th edition. New York: McGraw-Hill Medical.
10. International Federation of Gynecology and Obstetrics (2012) Prevention of postpartum hemorrhage with misoprostol. Int J Gynaecol Obstet 119 : 213–214 doi:10.1016/j.ijgo.2012.09.002
11. TuncalpO, HofmeyrGJ, GulmezogluAM (2012) Prostaglandins for preventing postpartum haemorrhage. Cochrane Database Syst Rev 8: CD000494 doi:10.1002/14651858.CD000494.pub4
12. ChuCS, BrhlikovaP, PollockAM (2012) Rethinking WHO guidance: review of evidence for misoprostol use in the prevention of postpartum haemorrhage. J R Soc Med 105 : 336–347 doi:10.1258/jrsm.2012.120044
13. DermanRJ, KodkanyBS, GoudarSS, GellerSE, NaikVA, et al. (2006) Oral misoprostol in preventing postpartum haemorrhage in resource-poor communities: a randomised controlled trial. Lancet 368 : 1248–1253 doi:10.1016/S0140-6736(06)69522-6
14. MobeenN, DurocherJ, ZuberiN, JahanN, BlumJ, et al. (2011) Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo-controlled trial. BJOG 118 : 353–361.
15. GulmezogluAM, VillarJ, NgocNT, PiaggioG, CarroliG, et al. (2001) WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet 358 : 689–695.
16. BaskettTF, PersadVL, CloughHJ, YoungDC (2007) Misoprostol versus oxytocin for the reduction of postpartum blood loss. Int J Gynaecol Obstet 97 : 2–5 doi:10.1016/j.ijgo.2006.12.016
17. VaidA, DadhwalV, MittalS, DekaD, MisraR, et al. (2009) A randomized controlled trial of prophylactic sublingual misoprostol versus intramuscular methyl-ergometrine versus intramuscular 15-methyl PGF2alpha in active management of third stage of labor. Arch Gynecol Obstet 280 : 893–897.
18. VimalaN, MittalS, KumarS (2006) Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. Int J Gynaecol Obstet 92 : 106–110.
19. VimalaN, MittalS, KumarS, DadhwalV, MehtaS (2004) Sublingual misoprostol versus methlergometrine for active management of third stage of labor. Int J Gynaecol Obstet 87 : 1–5.
20. Uganda Ministry of Health (2010) Uganda clinical guidelines: national guidelines on management of common conditions. Kampala: Uganda Ministry of Health.
21. BelladMB, TaraD, GanachariMS, MallapurMD, GoudarSS, et al. (2012) Prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin: a double-blind randomised controlled trial. BJOG 119 : 975–982 doi:10.1111/j.1471-0528.2012.03341.x
22. HojL, CardosoP, NielsenBB, HvidmanL, NielsenJ, et al. (2005) Effect of sublingual misoprostol on severe postpartum haemorrhage in a primary health centre in Guinea-Bissau: randomised double blind clinical trial. BMJ 331 : 723 doi:10.1136/bmj.331.7519.723
23. PiaggioG, ElbourneD, PockockSJ, EvansSJ, AltmanDG, et al. (2012) Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 308 : 2594–2604.
24. WesthoffG, CotterAM, TolosaJE (2013) Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst Rev 10: CD001808 doi:10.1002/14651858.CD001808.pub2
25. NordstromL, FogelstamK, FridmanG, LarssonA, RydhstroemH (1997) Routine oxytocin in the third stage of labour: a placebo controlled randomised trial. Br J Obstet Gynaecol 104 : 781–786.
26. AltmanDG, SchulzKF, MoherD, EggerM, DavidoffF, et al. (2001) The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 134 : 663–694.
27. TewatiaR, RaniS, SrivastavU, MakhijaB (2014) Sublingual misoprostol versus intravenous oxytocin in prevention of post-partum hemorrhage. Arch Gynecol Obstet 289 : 739–742.
28. ChaudhuriP, BiswasJ, MandalA (2012) Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in low-risk women. Int J Gynaecol Obstet 116 : 138–142 doi:10.1016/j.ijgo.2011.09.016
29. WeeksA (2012) Commentary on “prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin: a double-blind, randomised controlled trial”. BJOG 119 : 982–983.
30. LamH, TangOS, LeeCP, HoPC (2004) A pilot-randomized comparison of sublingual misoprostol with syntometrine on the blood loss in third stage of labor. Acta Obstet Gynecol Scand 83 : 650.
31. ElatiA, WeeksA (2012) Risk of fever after misoprostol for the prevention of postpartum hemorrhage: a meta-analysis. Obstet Gynecol 120 : 1140–1148.
32. DurocherJ, BynumJ, LeonW, BarreraG, WinikoffB (2010) High fever following postpartum administration of sublingual misoprostol. BJOG 117 : 845–852 doi:10.1111/j.1471-0528.2010.02564.x
33. AmantF (2001) The misoprostal third stage study: a randomised controlled comparison between orally administered misoprostol and standard management. A double-blind placebo controlled randomised trial of misoprostol and oxytocin in the management of the third stage labour. BJOG 108 : 338.
34. BabinszkiA, KerenyiT, TorokO, GraziV, LapinskiRH, et al. (1999) Perinatal outcome in grand and great-grand multiparity: effects of parity on obstetric risk factors. Am J Obstet Gynecol 181 : 669–674.
35. TsuVD (1993) Postpartum haemorrhage in Zimbabwe: a risk factor analysis. Br J Obstet Gynaecol 100 : 327–333.
36. StonesRW, PatersonCM, SaundersNJ (1993) Risk factors for major obstetric haemorrhage. Eur J Obstet Gynecol Reprod Biol 48 : 15–18.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 11- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Act II of the Sunshine Act
- What Could Computerized Brain Training Learn from Evidence-Based Medicine?
- Effect of Treatment of Obstructive Sleep Apnea on Depressive Symptoms: Systematic Review and Meta-Analysis
- (How) Can We Reduce Violence Against Women by 50% over the Next 30 Years?
- Association of Adenotonsillectomy with Asthma Outcomes in Children: A Longitudinal Database Analysis
- Association of FKBP51 with Priming of Autophagy Pathways and Mediation of Antidepressant Treatment Response: Evidence in Cells, Mice, and Humans
- The kSORT Assay to Detect Renal Transplant Patients at High Risk for Acute Rejection: Results of the Multicenter AART Study
- Computerized Cognitive Training in Cognitively Healthy Older Adults: A Systematic Review and Meta-Analysis of Effect Modifiers
- A Transdiagnostic Community-Based Mental Health Treatment for Comorbid Disorders: Development and Outcomes of a Randomized Controlled Trial among Burmese Refugees in Thailand
- Impact of Xpert MTB/RIF for TB Diagnosis in a Primary Care Clinic with High TB and HIV Prevalence in South Africa: A Pragmatic Randomised Trial
- Sublingual Misoprostol versus Intramuscular Oxytocin for Prevention of Postpartum Hemorrhage in Uganda: A Double-Blind Randomized Non-Inferiority Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Act II of the Sunshine Act
- Computerized Cognitive Training in Cognitively Healthy Older Adults: A Systematic Review and Meta-Analysis of Effect Modifiers
- What Could Computerized Brain Training Learn from Evidence-Based Medicine?
- Sublingual Misoprostol versus Intramuscular Oxytocin for Prevention of Postpartum Hemorrhage in Uganda: A Double-Blind Randomized Non-Inferiority Trial
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



