-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Pregnancy outcomes in women with inflammatory bowel disease treated with biosimilar infliximab
Výsledky těhotenství u pacientek s idiopatickými střevními záněty léčených biosimilárním infliximabem
Úvod:
Biologická terapie těhotných pacientek s idiopatickými střevními záněty (IBD – inflammatory bowel disease) nemá negativní vliv na výsledek těhotenství ani na vývoj plodu. Stále však chybí data o efektivitě a bezpečnosti biosimilárního infliximabu (IFX) v této situaci.Metody:
Retrospektivní observační studie zahrnovala 20 žen s IBD léčených v průběhu těhotenství biosimilárním IFX (CT-P13), u kterých byly k dispozici informace o výsledku těhotenství. Sledovány byly údaje o aktivitě choroby, léčbě, výsledcích těhotenství a data o novorozenci. Hladiny IFX z pupečníkové krve byly měřeny pomocí metody ELISA.Výsledky:
Do studie bylo zařazeno 20 těhotných žen (16 s Crohnovou nemocí, 4 s ulcerózní kolitidou) průměrného věku 28,7 ± 4,1 let, z nich 55 % (11) bylo prvorodiček. Střední doba trvání onemocnění k počátku těhotenství byla 6,0 ± 5,3 let, 70 % (14) žen mělo anamnézu perianálního postižení. V období početí trpělo 30 % (6) žen aktivní chorobou, 65 % (13) bylo v remisi a u jedné pacientky byla nově diagnostikována těžká kolitida těsně po početí. Kromě této pacientky, které byla podána záchranná terapie IFX, byly všechny ženy léčeny dlouhodobě, v průměru 2,3 ± 2,7 let před otěhotněním. Z 20 těhotenství vzniklo celkem 19 živě narozených dětí (průměrná váha 3 305 ± 493 g), z toho 18 v termínu a 1 předčasně (s nízkou porodní váhou). Jedno těhotenství skončilo spontánním potratem. Aktivní choroba matky v období početí byla spojena s nižší porodní váhou (3 549 ± 392 g u pacientek v remisi vs. 2 921 ± 390 g s aktivní chorobou; p = 0,0043). K císařskému řezu bylo přistoupeno v 70 % (14) případů, z toho 79 % (11) u žen s anamnézou perianálního postižení. Nebyly hlášeny žádné perinatální komplikace a z vrozených vad se vyskytl 1 případ rozštěpové vady patra.Závěr:
V této prioritní práci zaměřené na hodnocení efektu a bezpečnosti biosimilárního IFX u těhotných pacientek s IBD nebyla v rámci mantinelů daných omezenou velikostí vzorku zjištěna žádná nová bezpečnostní rizika takového léčebného postupu.Klíčová slova:
těhotenství – biosimilars – infliximab – idiopatické střevní zánětyDoručeno:
24. 1. 2018Přijato:
30. 1. 2018
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bio medicínských časopis .
Authors: M. Kolár 1; M. Bortlik 1–3; D. Ďuricová 1
; M. Lukas Jr. 1; V. Hrubá 1
; N. Machková 1; K. Malickova 1,4; M. Lukas 1,4
Authors place of work: IBD Clinical and Research Centre, ISCARE I. V. F. a. s., Prague, Czech Republic 1; Institute of Pharmacology, 1st/sup> Medical Faculty, Charles University, Prague, Czech Republic 2; Department of Internal Medicine, Military Hospital, Charles University, Prague, Czech Republic 3; Institute of Medical Biochemistry and Laboratory Diagnostics, 1st Medical Faculty and General Teaching Hospital, Charles University, Prague, Czech Republic 4
Published in the journal: Gastroent Hepatol 2018; 72(1): 20-26
Category: IBD: původní práce
doi: https://doi.org/10.14735/amgh201820Summary
Background:
In pregnant women with inflammatory bowel disease (IBD), exposure to biologic therapy, especially anti-TNF antibodies, has not been associated with adverse events. However, data on the efficacy and safety of biosimilar infliximab (IFX) in this particular population of IBD patients are lacking.Methods:
This retrospective study included 20 women treated with biosimilar IFX (CT-P13) during pregnancy for whom information about pregnancy outcome was available. Data on disease activity, treatment, and pregnancy and newborn outcome were recorded. Cord blood levels of anti-TNF were measured by ELISA.Results:
Twenty pregnant women (16 with Crohn’s disease and 4 with ulcerative colitis) with a mean age of 28.7 ± 4.1 years were included, 55% of whom were primigravidae. The mean disease duration at the time of pregnancy was 6.0 ± 5.3 years, and 70% of the women had a history of perianal disease. At the time of conception, 30% had active disease and 65% were in remission; one of the women was newly diagnosed with acute severe colitis after conception. Besides this patient, to whom IFX rescue therapy was administered during the first trimester, all women had already been treated with IFX before becoming pregnant, with a mean treatment duration of 2.3 ± 2.7 years. There were 19 live births (mean weight, 3,305 ± 493 g), 18 at term and 1 pre-term (with a low birth weight). One pregnancy ended in spontaneous abortion. Disease activity at conception was associated with lower birth weight (3,549 ± 392 g for those in remission vs. 2,921 ± 390 g for those with active disease; p = 0.0043). Cesarean section was performed in 70% of the women, 79% of whom had a history of perianal disease. No perinatal complications and birth defects were reported, except for a single case of cleft palate.Conclusion:
To our knowledge, this is the first report of pregnancy outcomes in women exposed to biosimilar IFX. Under the constraints inherent in the limited sample size, no new safety concerns have so far arisen.Key words:
pregnancy – biosimilar – infliximab – inflammatory bowel diseaseIntroduction
Inflammatory bowel disease (IBD) most often affects young people between 20 and 40 years of age and women are thus affected with IBD during the peak of their reproductive years [1]. In the past years, it has been shown that active disease during conception and pregnancy is a significant risk factor for adverse pregnancy outcomes such as low birth weight (< 2,500 g), pre-term birth, small for gestational age status, and spontaneous abortion [2–4]. Control of the disease activity is thus crucial, especially in females that want to become pregnant. However, fear from negative impact of medication on foetus development can frequently lead to poor adherence and consequently inadequate control of the disease. This makes proven safety of the therapy administered during pregnancy a key factor for both pregnant IBD patients and physicians.
Treatment with anti-tumor necrosis factor (anti-TNF) has been found to be a very effective modality for both induction and maintenance therapy of IBD. Since infliximab (IFX) and adalimumab belong to IgG1 monoclonal antibodies, they are actively transported across the placenta with an exponential growth of concentration in foetal blood during 3rd trimester [5]. So far, in pregnant women with IBD, exposure to anti-TNF antibodies, has not been associated with adverse events. However, data on efficacy and safety of biosimilar IFX (CT-P13) in this particular population of IBD patients is lacking, since it has been accepted by EMA for treatment of IBD in September 2013 and by the Food and Drug Administration (FDA) only in April 2016. Our study is thus focused on the evaluation of pregnancy outcomes of women with IBD treated with biosimilar IFX during pregnancy.
Patients and methods
All consecutive female patients with Crohn’s disease (CD) and ulcerative colitis (UC) who became pregnant while treated with biosimilar IFX or in whom therapy with biosimilar IFX was initiated during pregnancy for acute severe attack of IBD at ISCARE IBD Clinical and Research Centre between January 1, 2015 and December 31, 2016 and in whom pregnancy outcome was available were included. General treatment scheme was guided by available ECCO statements, however, individual decisions regarding the IFX cessation were made by a treating physician according to the respective patient’s requirements or wishes.
Data on demographics, disease characteristics and concomitant medication were collected. Disease state was retrospectively evaluated from available medical records using Physician’s Global Assessment (PGA) as either remission or active disease and data on C reactive protein (CRP) and faecal calprotectin (FC) were retrieved from the pre-conception visits, visits in individual trimesters and post-partum period up to 22 weeks. The 1st trimester was defined as the time from conception up to 13 weeks of pregnancy. The 2nd trimester included the time from the 14th to 27th week and 3rd trimester lasted from the 28th week until delivery.
Data on pregnancy outcome included gestational age, presence of congenital defects and weight and height of the newborn. Low birth weight was defined as weight less than 2,500 g and preterm delivery as less than 37 weeks of gestation.
Measurement of IFX levels
At the time of delivery, maternal and cord blood samples were collected to measure serum levels of biosimilar IFX and anti-IFX antibodies (ATIs). The measurement was conducted by quantitative Enzyme-linked immunosorbent assay (ELISA) kit SHIKARI Q-Inflixi and Q-ATI, resp., manufactured by Matriks Biotek, Turkey. The lowest detectable limit of Q-Inflixi kit was 0.03 µg/mL. The presence of ATIs was determined qualitatively by comparing the share of optical density of a sample at 450 nm and the mean optical density at 450 nm of negative controls with an index of 3. If the share was ≥ 3 the sample was positive for ATIs.
Statistical analysis
Standard descriptive statistical analyses were performed, including frequency distributions for categorical data and calculation of mean and standard deviation and/or range for continuous variables. Difference between birth weight was analysed using an unpaired t-test assuming normal distribution of the variable. Non-parametric Spearman correlation was used to evaluate the relationship between maternal and foetal IFX levels as well as cord IFX levels and time from last IFX administration to delivery. Laboratory values of subjects during the follow-up were compared using Mann-Whitney test. The statistical tests were performed using GraphPad Prism (version 7.04). A p-value (two-tailed) < 0.05 was considered statistically significant.
Results
Twenty women were included in the analysis, 16 with CD (80.0%) and 4 with UC (20.0%). The mean age at conception was 28.7 ± 4.1 years and 11 (55.0%) patients were primigravidae. The mean disease duration at inclusion was 6.0 ± 5.3 years, 14 out of 16 CD patients (87.5%) had history of perianal disease and one single woman (5.0%) underwent abdominal surgery in the past. The demographic and clinical characteristics of the patients are outlined in Tab. 1.
Tab. 1. Demographic and clinical characteristics of patients at conception. Tab. 1. Demografické a klinické charakteristiky pacientek při početí. 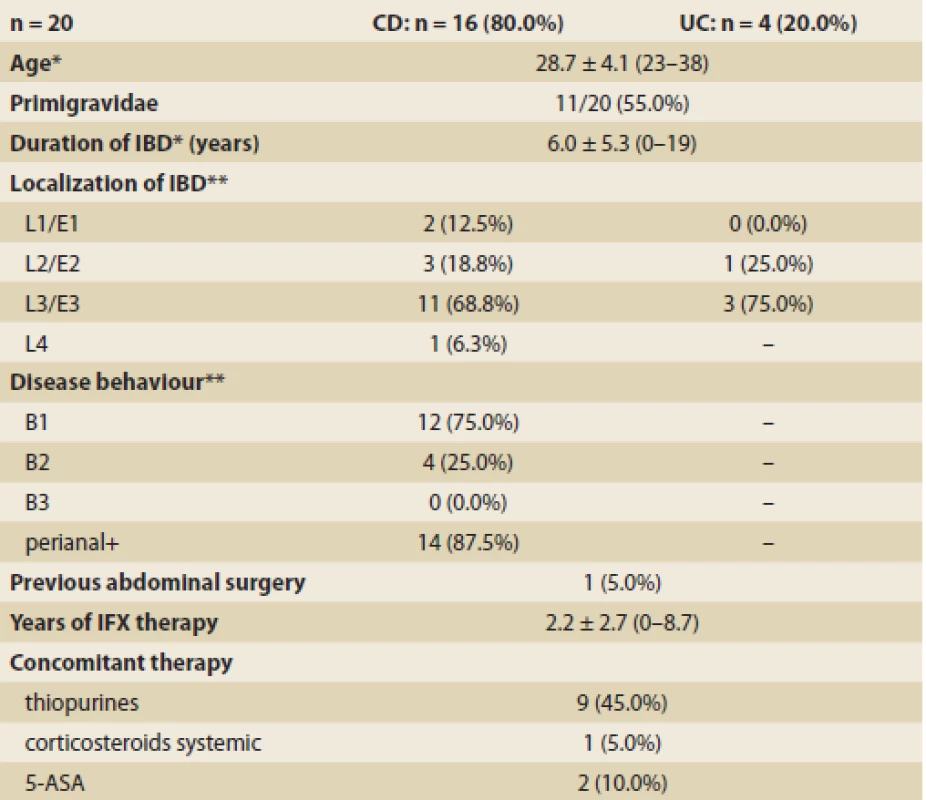
IBD – inflammatory bowel disease, CD – Crohn’s disease, UC – ulcerative colitis, IFX – infl iximab *mean ± SD (range) **Montreal classification Pregnancy outcomes
Twenty recorded pregnancies resulted in 19 live births. One case (5.0%) ended in spontaneous abortion. Only 5 deliveries (26.3%) were vaginal, while 14 (73.7%) were using section method. All section deliveries occurred in women with CD, 11 of them had a history of perianal disease. All but one live births were at-term at mean 39.0 ± 1.3 weeks of gestation. A single case of pre-term birth was registered (36 weeks) and this birth also resulted in a newborn with low birth weight (2,210 g). The mean birth weight and height of all newborns were 3,305 ± 493 (2,210–4,200) g and 49.6 ± 2.3 (45–53) cm, resp. Congenital defect was detected in one case (5.3%) and included cleft palate. No perinatal complications were observed. Pregnancy and newborn outcomes are outlined in Tab. 2.
Tab. 2. Pregnancy and newborn outcomes. Tab. 2. Výsledky těhotenství a parametry novorozenců. 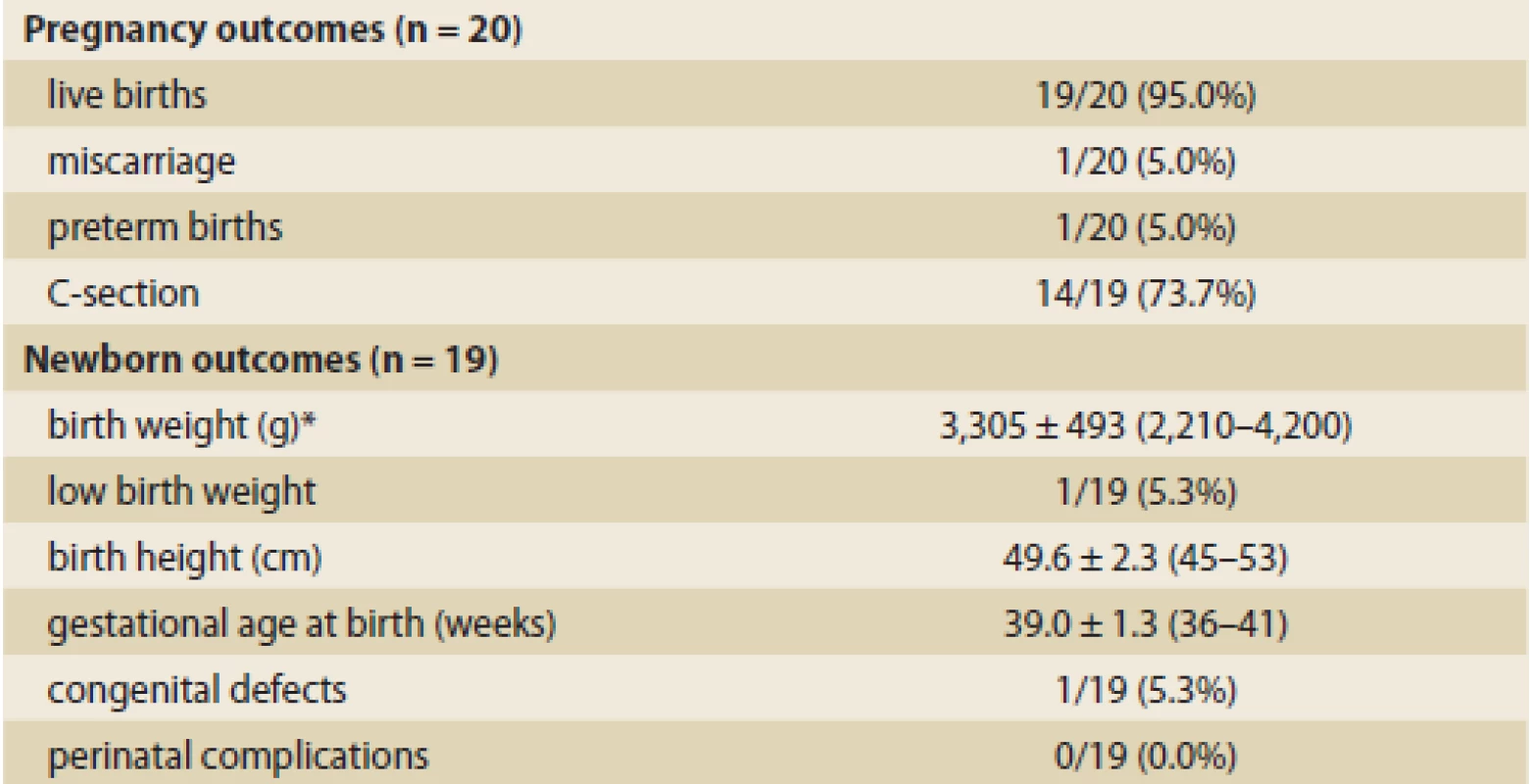
*mean ± SD (range) Disease activity
At conception, the disease was active in 6 out of 20 patients (30.0%) according to PGA, 13 patients (65.0%) were in remission and 1 case (5.0%) was yet undiag-nosed. During pregnancy, the majority of women remained in stable remission (12/20; 60.0%), 3 patients (15.0%) still had active disease and in 3 cases the condition improved (15.0%). One patient (5.0%) experienced worsening of the disease and 1 woman (5.0%) was newly diagnosed with acute severe UC. Within 6 weeks after delivery, further disease course was stable in 11 patients (57.9%) including 1 who didn’t continue IFX after delivery due to adverse skin effects. However, 8 patients (42.1%) experienced relapsing disease symptoms.
Graph 1 and 2 show and compare levels of CRP and FC in patients who had in respective time periods active disease and in patients who were in displayed stages in remission (defined by PGA). CRP slightly increased in all patients during pregnancy, however, the mean value in patients in remission didn’t exceed 5 mg/L and was only significantly higher in patients with active disease comparing to the patients in remission from 2nd trimester to early post-partum period.
Graph 1. Levels of C reactive protein (CRP) in patients in remission and patients with active disease (as defined by PGA) in the respective time periods. PP + x, weeks postpartum. Graf 1. Hladiny C reaktivního proteinu (CRP) u pacientů v remisi a s aktivní chorobou (na základě PGA) v příslušných časových obdobích. PP + x, týdny po porodu. 
Graph 2. Levels of faecal calprotectin (FC) in patients in remission and patients with active disease (as defined by PGA) in the respective time periods. PP + x, weeks postpartum. Graf 2. Hladiny fekálního kalprotektinu (FC) u pacientů v remisi a s aktivní chorobou (na základě PGA) v příslušných časových obdobích. PP + x, týdny po porodu. 
The mean FC value in patients in remission didn’t vary significantly throughout the whole course of pregnancy and didn‘t exceed 250 µg/g in all but two visits. It was significantly lower comparing to the FC of patients with active disease in the majority of the visits despite low numbers of subjects.
Comparing birth weights of newborns born to mothers in remission and mothers with active disease at conception (approximated by the disease activity during the nearest visit), newborns of mothers with active disease at conception had significantly lower birth weight (2,921 ± 390 g vs. 3,549 ± 392 g; p = 0.0043) (Graph 3).
Graph 3. Difference in mean birth weights of newborns born to mothers in remission and to those with active disease at conception (as defined by PGA). Graf 3. Rozdíl v průměrných porodních hmotnostech novorozenců pacientek v remisi a s aktivní chorobou (na základě PGA) v období početí. 
IFX therapy
Before conception, the mean duration of therapy with IFX was 2.2 ± 2.7 years. In 12 patients (60.0%) the biosimilar IFX was started de novo and 8 (40.0%) were switched from the original preparation (before conception). Concomitant corticosteroids were given in 1 case (5.0%), 2 patients (10.0%) had 5-ASA, 9 (45.0%) were on concomitant immunosuppression with azathioprine and 9 patients (45.0%) had no other treatment. In 3 patients IFX had to be intensified during pregnancy. Sixteen (80.0%) patients interrupted the regular IFX dosing interval before delivery, while in 3 patients (15.0%) the disease activity required continuation of IFX during the whole pregnancy. One patient stopped IFX in the 1st trimester and didn’t continue after pregnancy due to skin adverse effects of the therapy. The patient was later indicated for ileocecal resection. In one patient the IFX was newly started due to acute severe UC newly diagnosed soon after conception. This patient achieved remission and success-fully completed pregnancy. The mean time between the last infusion and delivery was 12.1 ± 6.6 (range 2.1–33.6) weeks and was negatively correlated with cord blood IFX levels (rS = –0.6759; p = 0.0069) (Graph 4). The treatment was reinitiated 7.5 ± 4.3 (1.0–18.4) weeks after delivery. Retrieved newborn IFX levels at delivery were 11.2 ± 15.0 µg/mL. Corresponding maternal levels were 7.8 ± 15.0 µg/mL. Foetal and maternal IFX levels were strongly positively correlated (rS = 0.9607; p < 0.0001) (Graph 5).
Graph 4. Spearman correlation of cord blood IFX levels and time in weeks between the last IFX administration and delivery. Graf 4. Spearmanova korelace hladin IFX z pupečníkové krve a času v týdnech mezi poslední dávkou IFX a porodem. 
Graph 5. Spearman correlation of cord blood and maternal IFX levels at the time of delivery. Graf 5. Spearmanova korelace hladin IFX z pupečníkové krve a hladin v mateřské krvi odebrané během porodu. 
Discussion
Both IFX and adalimumab have been labelled as pregnancy category B by the U.S. FDA indicating there were no well-controlled trials and no harmful signs from animal studies, however this classification is being gradually abandoned and replaced by new Pregnancy and lactation labelling rule with a more thorough description and regular updating. In the beginning of the anti-TNFs era, some studies reported higher frequency of adverse pregnancy outcomes related to the administration of anti-TNF therapy during pregnancy [7]. However, methodology of some of those studies had several limitations. Since then, the majority of published studies have confirmed no association between anti-TNF therapy during pregnancy and adverse pregnancy outcomes including one of our own studies and a 2016 meta-analysis [4,6,8–10].
Shortly after CT-P13 has been authorized for use in clinical practice, many concerns arose due to the applied principle of extrapolation of limited clinical results and approval of the biological in all indications. However, currently available data have proven the same efficacy, safety and immunogenicity of CT-P13 and original IFX [11]. In our current study of pregnancy and newborn outcomes in 20 women with IBD treated with CT-P13 we observed one (5.0%) spontaneous abortion, one (5.0%) pre-term birth with low birth weight and one (5.0%) congenital anomaly. In a previous study on outcomes of pregnancy in women treated with original IFX from the same centre, Bortlik et al. reported 12% rate of spontaneous abortions, 3% of low birth weights and 3% of congenital malformations [10]. Earlier, Schnitzler et al. reported abortion rate of 21% in women with direct exposure to original IFX, however, didn’t register any congenital anomalies [2]. A meta-analysis from 2016 reported mean rate of congenital anomalies of 3%, 17% of pre-term births and 14% of low birth weights. No new safety concerns regarding the use of CT-P13 in pregnancy have been detected in our current study and the results are thus in concordance with other available data on the use of original IFX in pregnant patients with IBD.
Due to the active transport of IFX into the foetus, especially during the second half of pregnancy, foetal IFX concentrations at delivery usually exceed maternal levels, being detectable up to 6 months after birth [12,13]. In our study, we detected newborn IFX levels at delivery of 11.2 ± 15.0 µg/mL with the last dose being administered 12.1 ± 6.6 weeks before delivery. Corresponding maternal levels were 7.8 ± 15.0 µg/mL, and the time to delivery negatively correlated with cord blood IFX levels (rS = –0.6759; p = 0.0069). This fact is reflected in both the ECCO guidelines [14] and the guidelines of Czech Society of Gastroenterology [15], which both recommend the discontinuation of anti-TNF at the end of the 2nd trimester if there is no need to continue due to disease activity or high relapse risk.
It has been argued that high and long-lasting newborn IFX levels might negatively impact newborn health, including infections, allergies or other immune-mediated conditions. In a recent work, Duricova et al. compared 69 anti-TNF exposed and 42 unexposed children of mothers with IBD during the 1st year of their life and found no increased risk in infectious complications in children exposed to anti-TNF in utero [16]. Also, no increased risk of combination therapy with thiopurines was reported. On the other hand, Julsgaard et al. found an almost 3-fold risk for infant infections during the first 12 months after being exposed to combination therapy compared to anti-TNF monotherapy [17]. Another recent paper also suggests that serologic response to vaccines in newborns does not appear to be affected by in utero exposure to biologic therapy [18]. However, results on these issues are still only scarce and further investigation is warranted, particularly in the case of biosimilar biologics.
Our study also confirms some already well-demonstrated facts, however it is worth emphasizing that results are also markedly present even in such a small study of 20 subjects. Previous studies have shown that active disease is the main driver of adverse pregnancy outcomes [4,19–22]. We have found that the newborns of mothers with active disease at the time of conception had significantly lower birth weight compared to those born to mothers in remission. This finding underscores the need of maintaining disease remission at conception and during pregnancy and provides an eventual rationale for continuing IFX throughout the whole course of pregnancy.
It has been previously described that many biomarkers of inflammation including CRP, albumin, haemoglobin, and leukocytes change secondary to the physiological adaptation in pregnancy in both healthy women and patients with IBD [23]. However, FC levels don’t seem to be affected by pregnancy and thus appear to be a more reliable marker of inflammation in pregnant women [24]. In our study, we observed a numerical increase in CRP in both patients with active disease and in remission, however, patients with active IBD could have been identified by significantly higher CRP, particularly during pregnancy. On the other hand, FC remained stable and low in patients in remission in our cohort throughout whole course of pregnancy. FC was higher during all visits in patients with active disease.
In conclusion, our study found no new safety concerns regarding use of biosimilar IFX (CT-P13) during pregnancy in terms of birth outcomes, however, further evaluation of a larger cohort of patients is warranted. We suggest additional follow-up of the infants to determine the potential negative impact on their health outcomes.
The authors declare they have no potential con icts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for bio medical papers.
Submitted: 24. 1. 2018
Accepted: 30. 1. 2018
MUC. Martin Kolář
IBD Clinical and Research Centre
ISCARE I.V.F. a. s.
Jankovcova 1569/2c
170 04 Prague 7
Czech Republic
E-mail: martin.kolar@gmail.com
Zdroje
1. Molodecky NA, Soon IS, Rabi DM et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142 (1): 46–54. doi: 10.1053/j.gastro.2011.10.001.
2. Schnitzler F, Fidder H, Ferrante M et al. Outcome of pregnancy in women with inflammatory bowel disease treated with antitumor necrosis factor therapy. Inflamm Bowel Dis 2011; 17 (9): 1846–1854. doi: 10.1002/ibd.21583.
3. Bröms G, Granath F, Linder M et al. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm Bowel Dis 2014; 20 (6): 1091–1098. doi: 10.1097/MIB.0000000000000060.
4. de Lima-Karagiannis A, Zelinkova-Detkova Z, van der Woude CJ. The effects of active IBD during pregnancy in the era of novel IBD therapies. Am J Gastroenterol 2016; 111 (9): 1305–1312. doi: 10.1038/ajg.2016.254.
5. Malek A, Sager R, Kuhn P et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol 1996; 36 (5): 248–255.
6. Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor α therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis 2016; 10 (8): 979–988. doi: 10.1093/ecco-jcc/jjv234.
7. Carter JD, Ladhani A, Ricca LR et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol 2009; 36 (3): 635–641. doi: 10.3899/jrheum.080545.
8. Komoto S, Motoya S, Nishiwaki Y et al. Pregnancy outcome in women with inflammatory bowel disease treated with anti-tumor necrosis factor and/or thiopurine therapy: a multicenter study from Japan. Intest Res 2016; 14 (2): 139–145. doi: 10.5217/ir.2016.14.2.139.
9. Seirafi M, de Vroey B, Amiot A et al. Factors associated with pregnancy outcome in anti-TNF treated women with inflammatory bowel disease. Aliment Pharmacol Ther 2014; 40 (4): 363–373. doi: 10.1111/apt.12833.
10. Bortlik M, Machkova N, Duricova D et al. Pregnancy and newborn outcome of mothers with inflammatory bowel diseases exposed to anti-TNF-α therapy during pregnancy: three-center study. Scand J Gastroenterol 2013; 48 (8): 951–998. doi: 10.3109/00365521.2013.812141.
11. Kolar M, Duricova D, Bortlik M et al. Biosimilar infliximab in anti-TNF-naïve IBD patients – 1-year clinical follow-up. Gastroenterol Hepatol 2016; 70 (6): 514–522. doi: 10.14735/amgh2016514.
12. Mahadevan U, Wolf DC, Dubinsky M et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013; 11 (3): 286–292. doi: 10.1016/j.cgh.2012.11.011.
13. Zelinkova Z, de Haar C, de Ridder L et al. High intra-uterine exposure to infliximab following maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther 2011; 33 (9): 1053–1058. doi: 10.1111/j.1365-2036.2011.04617.x.
14. van der Woude CJ, Ardizzone S, Bengtson MB et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis 2015; 9 (2): 107–124. doi: 10.1093/ecco-jcc/jju006.
15. Bortlík M, Ďuricová D, Kohout P et al. Guidelines for the administration of bio logical therapy in patients with infl ammatory bowel diseases: third, updated edition. Gastroenterol Hepatol 2016; 70 (1): 11–26. doi: 10.14735/amgh201611.
16. Duricova D, Dvorakova E, Kozeluhova J et al. P734 Anti-TNFa exposure during pregnancy is not associated with increased infection risk in exposed children during the first year of life. Presented at: ECCO Congress Vienna. 14–17 February, 2018.
17. Julsgaard M, Christensen LA, Gibson PR et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology 2016; 151 (1): 110–119. doi: 10.1053/j.gastro.2016.04.002.
18. Beaulieu DB, Ananthakrishnan AN, Martin C et al. Use of biologic therapy by pregnant women with inflammatory bowel disease does not affect infant response to vaccines. Clin Gastroenterol Hepatol 2018; 16 (1): 99–105. doi: 10.1016/j.cgh.2017.08.041.
19. Kane SV. Inflammatory bowel disease, women, and pregnancy. Gastroenterol Hepatol (N Y) 2013; 9 (11): 741–743.
20. Moser MA, Okun NB, Mayes DC et al. Crohn‘s disease, pregnancy, and birth weight. Am J Gastroenterol 2000; 95 (4): 1021–1026.
21. Vermeire S, Carbonnel F, Coulie PG et al. Management of inflammatory bowel disease in pregnancy. J Crohns Colitis 2012; 6 (8): 811–823. doi: 10.1016/j.crohns.2012.04.009.
22. Arai K, Takeuchi Y, Oishi C et al. The impact of disease activity of Crohn‘s disease during pregnancy on fetal growth. Clin J Gastroenterol 2010; 3 (4): 179–181. doi: 10.1007/s12328-010-0158-9.
23. Klajnbard A, Szecsi PB, Colov NP et al. Laboratory reference intervals during pregnancy, delivery and the early postpartum period. Clin Chem Lab Med 2010; 48 (2): 237–248. doi: 10.1515/CCLM.2010.033.
24. Julsgaard M, Hvas CL, Gearry RB et al. Fecal calprotectin is not affected by pregnancy: clinical implications for the management of pregnant patients with inflammatory bowel disease. Inflamm Bowel Dis 2017; 23 (7): 1240–1246. doi: 10.1097/MIB.0000000000001 136.
Štítky
Dětská gastroenterologie Gastroenterologie a hepatologie Chirurgie všeobecná
Článek News in 2018Článek Správná odpověď na kvíz
Článek vyšel v časopiseGastroenterologie a hepatologie
Nejčtenější tento týden
2018 Číslo 1- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
-
Všechny články tohoto čísla
- Quality of life in patients with inflammatory bowel diseases in the Czech Republic – a multicentre study
- Pregnancy outcomes in women with inflammatory bowel disease treated with biosimilar infliximab
- Guidelines of the IBD working group of the Slovak Society of Gastroenterology on the management of Crohn’s disease
- Therapeutic drug monitoring in the treatment of inflammatory bowel disease with infliximab
- Efficacy of ustekinumab in a patient refractory to other biological treatment
- News in 2018
- Gastroenterology and renal diseases
- A comparison of cost-effectiveness analysis of two strategies – immediate and delayed initiation of treatment of hepatitis C in the Czech Republic
- Can we increase the effectiveness of anti-TNF therapy?
- The role of CT colonography in large bowel investigation
- Endoscopic treatment of Bouveret syndrome
- In memoriam: Assoc. Prof. Zdenek Slezak, CSc.
- The selection from international journals
- Mladá žena s expanzivním procesem v pravém hypogastriu
- Správná odpověď na kvíz
- Kreditovaný autodidaktický test: IBD
- VSL#3 – the first highly concentrated therapeutic probiotic with multiple bacterial strains and a proven therapeutic efficacy
- Gastroenterologie a hepatologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- VSL#3 – the first highly concentrated therapeutic probiotic with multiple bacterial strains and a proven therapeutic efficacy
- Gastroenterology and renal diseases
- The role of CT colonography in large bowel investigation
- Guidelines of the IBD working group of the Slovak Society of Gastroenterology on the management of Crohn’s disease
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



