-
Medical journals
- Career
Bimekizumab in the Treatment of Psoriatic Arthritis
20. 6. 2024
Arthritis can appear in up to a third of patients with psoriasis, mostly after the onset of skin symptoms. Early recognition of this transition by dermatologists and general practitioners is crucial, as early intervention can prevent irreversible joint damage. In the context of effective biological therapy, there is even talk of the potential prevention of arthritis development in individuals with psoriasis.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease characterized by various manifestations on the joints—mainly arthritis, but also enthesitis or dactylitis—and axial involvement, which often occur in conjunction with psoriasis on the skin and nails (1, 2).
Risk factors for the development of arthritis include severe skin involvement, nail psoriasis, obesity, and a positive family history (3). The disease, however, is often diagnosed with a delay or not recognized at all, with approximately 15% of patients treated for psoriasis having undiagnosed arthritis (4).
For the early diagnosis of musculoskeletal involvement, it is important to identify symptoms such as joint pain and swelling, inflammation of tendons and their insertions, stiffness, and inflammatory back pain. Imaging methods (x-ray, musculoskeletal ultrasound, or MRI), along with the assessment of acute phase reactants, are helpful examinations for an accurate diagnosis (5).
Treatment Options for PsA
The treatment of PsA is guided by the type and severity of clinical manifestations (6). For mild forms of peripheral or axial involvement, nonsteroidal anti-inflammatory drugs (NSAIDs) may be sufficient. In cases where arthritis is more severe and affects multiple joints, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), such as methotrexate, are usually administered. If this treatment fails, biological anticytokine drugs targeting tumor necrosis factor (TNF), interleukin 17 (IL-17), or IL-23 are available. In cases of predominant skin involvement, IL-17 or IL-23 inhibitors are appropriate. If biological drugs fail or their administration is unsuitable, oral targeted synthetic DMARDs (tsDMARDs) that act on the level of phosphodiesterase 4 (PDE4) or Janus kinases (JAK) can be administered (6).
Bimekizumab is an innovative medicinal product approved for the treatment of psoriasis and PsA. It is a monoclonal antibody that selectively inhibits IL-17A and IL-17F—key cytokines involved in the pathogenesis of PsA (see Fig. 1) (7). This inhibition suppresses the inflammatory response and leads to an improvement in clinical symptoms of psoriatic disease.
Fig. 1 The mechanism of action of bimekizumab involves the selective binding to cytokines IL-17A, IL-17F, and the heterodimer IL-17A/F—thus the drug inhibits the activation of the IL-17RA/RC receptor complex by these cytokines, reducing the subsequent inflammatory cascade (7).

Bimekizumab in Clinical Trials
Bimekizumab was evaluated in two key randomized double-blind placebo-controlled phase III trials in patients with PsA: BE OPTIMAL and BE COMPLETE (8, 9).
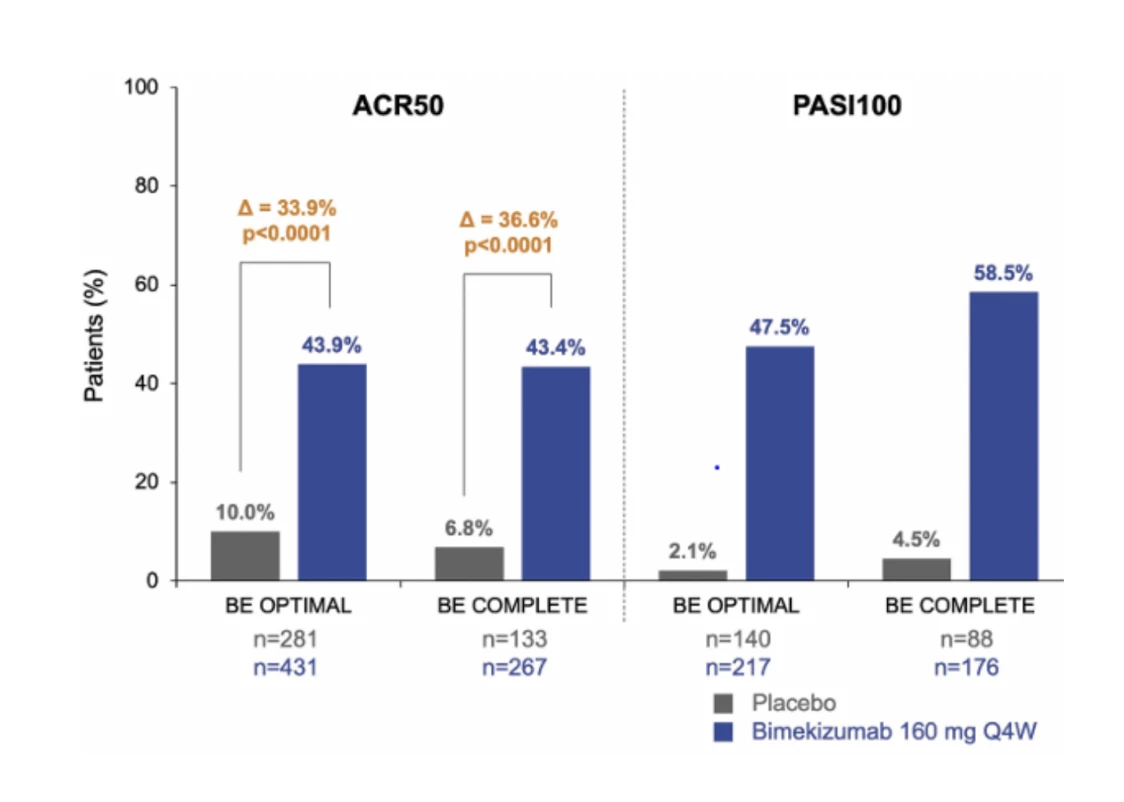
The BE OPTIMAL study (8) focused on patients who had not previously been treated with biological therapy, while the BE COMPLETE study (9) included patients with an inadequate response to TNF inhibitors. Both studies demonstrated that bimekizumab significantly improves disease symptoms, including arthritis, enthesitis, dactylitis, and skin lesions. Approximately 44% of patients achieved a 50% improvement according to the American College of Rheumatology criteria (ACR 50) after 16 weeks of treatment in both clinical trials. In the placebo group, only 7–10% of patients achieved these results, while in the active comparator group (TNF inhibitor adalimumab) in the BE OPTIMAL trial, the primary endpoint was achieved in 46% of cases.
An even more significant effect was observed in the BE OPTIMAL clinical trial when evaluating the skin score PASI 90 (90% improvement in the Psoriasis Area and Severity Index), which was achieved by 61–69% of patients on bimekizumab compared to 9–11% in the placebo group and 41% in the adalimumab group. Approximately half of the patients treated with bimekizumab achieved complete skin resolution (PASI 100), less than 5% on placebo, and 21% on adalimumab (8, 9) (see Fig. 2).
Fig. 2 Proportion of responders achieving ACR 50 and PASI 100 at week 16 and 52 in the BE OPTIMAL and BE COMPLETE clinical trials (8, 9). The results were analyzed using the NRI (non-responder imputation) method, where missing data were assumed to be treatment failures.
Bimekizumab demonstrated significantly better effects on skin symptoms of psoriasis, even when compared to IL-17A inhibition (10). This effect was observed in a clinical trial where approximately 62% of patients treated with bimekizumab achieved complete psoriasis resolution after 16 weeks compared to 49% treated with secukinumab. This difference highlights the potential of bimekizumab to provide exceptional control of skin manifestations of the disease. However, it is important to note the increased incidence of oral candidiasis in patients treated with bimekizumab compared to secukinumab.
The long-term efficacy of bimekizumab was confirmed in extended open-label phases of clinical trials. After one year of treatment, stable clinical effects were maintained, with 50% of patients achieving ACR50 and 66% achieving complete psoriasis resolution (11).
Bimekizumab was generally well-tolerated, with the most common adverse effects being upper respiratory tract infections, nasopharyngitis, headaches, and diarrhea. An increased incidence of oral candidiasis, a side effect associated with IL-17 inhibition, was observed. Candidiasis was typically mild to moderate and well-managed with standard antifungal treatment.
Conclusion
The results of clinical trials have demonstrated a very good effect of IL-17A and IL-17F inhibition using bimekizumab in patients with PsA. This includes improvements in both musculoskeletal and skin symptoms, including complete resolution of psoriasis in most patients. Overall, bimekizumab was well-tolerated with a safety profile similar to other biologics, except for a higher incidence of oral candidiasis.
prof. MUDr. Ladislav Šenolt, Ph.D.
Institute of Rheumatology and Department of Rheumatology, 1st Faculty of Medicine, Charles University in PragueReferences:
1. Fitzgerald O, Ogdie A, Chandran V et al. Psoriatic arthritis. Nat Rev Dis Primers 2021; 7 (1): 59.
2. Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol 2019; 15 (3): 153–166.
3. Zabotti A, De Marco G, Gossec L et al. EULAR points to consider for the definition of clinical and imaging features suspicious for progression from psoriasis to psoriatic arthritis. Ann Rheum Dis 2023; 82 (9): 1162–1170.
4. Villani AP, Rouzaud M, Sevrain M et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol 2015; 73 (2): 242–248.
5. Polachek A, Furer V, Zureik M et al. Role of ultrasound for assessment of psoriatic arthritis patients with fibromyalgia. Ann Rheum Dis 2021; 80 (12): 1553–1558.
6. Gossec L, Kerschbaumer A, Ferreira RJO et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann Rheum Dis 2024 May 15; 83 (6): 706–719.
7. Tanaka Y, Shaw S. Bimekizumab for the treatment of psoriatic arthritis. Expert Rev Clin Immunol 2024 Feb; 20 (2): 155–168.
8. McInnes IB, Asahina A, Coates LC et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet 2023; 401 (10370): 25–37.
9. Merola JF, Landewé R, McInnes IB et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet 2023; 401 (10370): 38–48.
10. Reich K, Warren RB, Lebwohl M et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med 2021; 385 (2): 142–152.
11. Coates LC, Landewé R, McInnes IB et al. Bimekizumab treatment in patients with active psoriatic arthritis and prior inadequate response to tumour necrosis factor inhibitors: 52-week safety and efficacy from the phase III BE COMPLETE study and its open-label extension BE VITAL. RMD Open 2024 Feb 22; 10 (1): e003855.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Rheumatology Dermatology & STDs General practitioner for adults
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


