-
Medical journals
- Career
Longer survival without liver transplant in patients with PBC treated with OCA?
20. 3. 2023
Obeticholic acid (OCA) is a second-line treatment for patients with primary biliary cholangitis (PBC) who have an inadequate response to ursodeoxycholic acid (UDCA) or intolerance to it. The study presented below, the results of which were recently published, examined the effectiveness of OCA in reducing cholestatic enzymes as well as the length of survival without liver transplant in patients with PBC.
Introduction
Up to 40% of patients with PBC have an inadequate response to UDCA as first-line therapy. The goal of therapy in these patients is to prevent the progression of events related to disease decompensation. The authors of the study evaluated whether treatment with OCA was associated with a reduction in the number of deaths, liver transplants, and liver decompensations. This research complements evidence from real-world clinical practice showing that OCA administration has a positive effect on liver enzymes, fibrosis, and risk scores. Its choice is also in line with European guidelines, which indicate OCA as second-line therapy for patients with intolerance or an inadequate therapeutic response 6–12 months after UDCA administration.
Methodology and course of the study, observed population
A total of 209 patients with PBC and inadequate response to UDCA (i.e. ALP level > 1.67× the upper limit of normal) were included in the POISE phase III randomized double-blind placebo-controlled study. They subsequently took OCA at a dose of 5 mg/day (or 5 mg/day titrated to 10 mg/day) or placebo for 12 months. In the study extension (POISE-OLE), ie another 5 years, all patients (including those in the original placebo group) took OCA. The control group was represented by patients with PBC from the Global-PBC (n = 1381) and UK-PBC (n = 2135) registers. In all mentioned cohorts, there were mostly women diagnosed with PBC between the ages of 50 and 60, and most of them took UDCA at a dose of 900 or 1000 mg/day for 3.5–4 years.
The primary endpoint in the presented analysis was the time to liver transplant or patient death. The primary composite endpoint of the POISE clinical evaluation was a decrease in ALP levels to < 1.67× the upper limit of normal (and at the same time ≥ 15% reduction from baseline) and a decrease in bilirubin levels to ≤ 1× the upper limit of normal. Secondary endpoints in the POISE study and the Global-PBC cohort were disease decompensation characterized as bleeding from esophageal varices, spontaneous bacterial peritonitis, diuretic-resistant ascites, hepatic encephalopathy, and further efficacy of OCA in subgroups of patients with and without liver cirrhosis.
Results
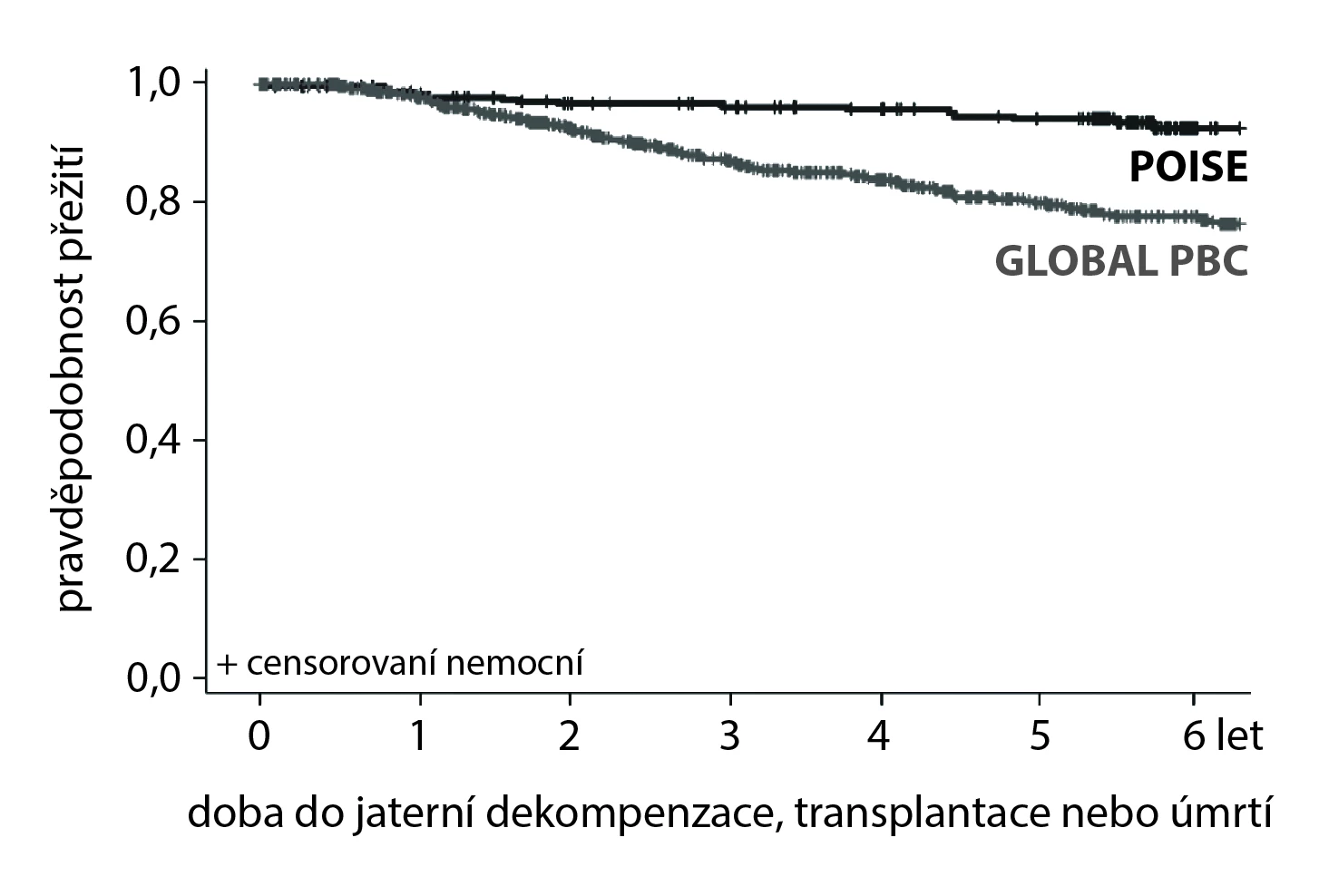
During the 6-year follow-up, 5 adverse events (2.4%; 2 liver transplants and 3 deaths) were reported in the POISE study or POISE-OLE. In the Global-PBC, or UK-PBC cohort, there were 135 (10 %; 51 liver transplants and 84 deaths) and 281 adverse events (13.2%; 119 liver transplants and 162 deaths). The hazard ratio (HR) in the POISE cohort was 0.29 (95% confidence interval [CI] 0.10–0.83; p = 0.02) and 0.30 (95% CI 0.12–0.75; p <0.01) compared to Global-PBC, or UK-PBC respectively.
HR for liver transplant or death in patients with or without liver cirrhosis in the POISE cohort vs the Global-PBC cohort was 0.20 (95% CI 0.03–1.22) and 0.31 (95% CI 0.09–1.04). HR including the aforementioned disease decompensation was set at 0.42 (95% CI 0.21–0.85; p =0.02).
Figure. Kaplan-Meier curves showing time to the first occurrence of liver decompensation, liver transplantation, or death compare POISE (clinical trial) and Global PBC (external controls (HR 0.42; 95% CI 0.21–0.85; p = 0.02))
Conclusion
Patients with primary biliary cholangitis treated with obeticholic acid as a second-line therapy exhibit not only a laboratory reduction in cholestatic enzymes but also longer survival without events (decompensation, liver transplant, or death) compared to the control group. The results of this analysis support the further long-term use of OCA to optimize the prognosis of patients with PBC.
(mafi)
Source: Murillo Perez C. F., Fisher H., Hiu S. et al. Greater transplant-free survival in patients receiving obeticholic acid for primary biliary cholangitis in a clinical trial setting compared to real-world external controls. Gastroenterology 2022; 163 (6): 1630–1642.e3, doi: 10.1053/j.gastro.2022.08.054.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Gastroenterology and hepatology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


