-
Medical journals
- Career
Neuroprotective Efficacy and Safety of Vinpocetine in Patients with Acute Ischemic Stroke
13. 5. 2024
A meta-analysis of 4 randomized placebo-controlled studies demonstrated the benefits of vinpocetine in patients with ischemic stroke (iCMP). Administering vinpocetine within 14 days of the onset of the stroke significantly reduced the level of disability and the proportion of those who died or were dependent on others after 1 and 3 months. The results showed benefits in several other parameters and indicated very good tolerability of this drug used to increase cerebral blood flow.
Introduction
Approximately 85% of strokes are of ischemic etiology. The primary goal of their treatment is to restore perfusion to brain tissue and minimize the extent of brain infarction. Besides intravenous application of recombinant tissue plasminogen activator (r-tPA) to achieve thrombus recanalization and restore perfusion—which must be administered within a tight time window and carries the risk of bleeding complications—researchers are also seeking neuroprotective strategies to prevent the progression of ischemic brain tissue injury. One compound with neuroprotective effects is vinpocetine. However, the previous meta-analysis of studies on this drug in stroke patients was published more than 10 years ago, prompting the authors of the cited study to conduct a new literature review and meta-analysis of randomized placebo-controlled studies evaluating vinpocetine for this indication.
Methods and Objectives
The aim of the study was to estimate the combined efficacy, tolerability, and safety of vinpocetine administered during the acute phase or early recovery phase in patients with ischemic stroke. The primary outcome was the level of disability in the vinpocetine group compared to the placebo group at 1 month post-stroke. Secondary outcomes included the level of disability at 3 months, the proportion of patients who died or were dependent on others in daily activities (defined as significant disability) at 1 and 3 months, overall mortality, changes in neurological and cognitive functions, and the incidence of anxiety or depression.
A systematic literature review was conducted in the MEDLINE, Web of Science, EMBASE, and Cochrane Central Register of Controlled Trials databases up to August 15, 2021. The inclusion criteria were the randomized controlled trial design and the evaluation of the efficacy and safety of vinpocetine compared to a control group in patients with acute ischemic stroke. Vinpocetine treatment had to be initiated within 14 days of the stroke. Disability level was assessed using the modified Rankin Scale, neurological deficit severity was assessed using the NIHSS (National Institutes of Health Stroke Scale), cognitive function changes were assessed with the MMSE (Mini Mental State Examination) and MoCA (Montreal Cognitive Assessment) scales, anxiety and depression were assessed with the Beck Scale and HADS (Hospital Anxiety and Depression Scale), and cerebral blood flow was measured using transcranial Doppler ultrasonography.
Analyzed Studies and Patient Population
Four placebo-controlled studies (1 double-blind, 1 single-blind, and 2 open-label) met the inclusion criteria, including a total of 601 patients treated with vinpocetine and 236 with placebo. Bias risk was high in 1 of these studies and low in the remaining 3.
Patients with significant psychiatric comorbidities, dementia, severe uncompensated cardiac, renal, liver, or endocrine disease, and CNS bleeding confirmed by imaging were excluded from these studies.
The baseline demographic characteristics of patients were comparable in both therapeutic groups. These characteristics included age, gender, BMI, time from first symptoms to hospitalization and randomization, localization and extent of brain infarction, a history of cardiometabolic diseases, GCS (Glasgow Coma Scale), NIHSS, MMSE, and HADS scores.
In the vinpocetine group, 61% were men, and the average age was 62.4 years. The average time from the onset of stroke symptoms to hospitalization was 28.9 hours and to randomization was 1.9 days. The baseline GCS score was 13.7, NIHSS 11.2, MMSE 24.3. The median dose was 30 mg/day (range 10–40 mg), and the median duration of follow-up was 3 months (range 1–6 months).
Findings
Efficacy
The vinpocetine group showed a lower percentage of patients who died or had significant disability at 1 month (relative risk [RR] 0.80; 95% confidence interval [CI] 0.65–0.99; p = 0.04; Figure 1A) and at 3 months (RR 0.67; 95% CI 0.48–0.92; p = 0.02; Figure 1B). Additionally, the level of disability was lower in the vinpocetine group compared to the placebo group at 1 month (p = 0.001; Figure 2A) and at 3 months (p = 0.04; Figure 2B).
Vinpocetine also demonstrated benefits in reducing neurological deficits according to the NIHSS (p = 0.03) and cognitive deficits according to the MMSE (p = 0.04). Transcranial measurements of blood flow velocity in cerebral arteries, performed in only 1 study, showed significant increases with vinpocetine treatment.
Results for overall mortality and changes in anxiety and depression were comparable, though a trend towards improvement with vinpocetine was observed in the latter evaluated in a single study.
Figure 1 Proportion of patients with ischemic stroke who died or had significant disability with vinpocetine treatment compared to placebo at 1 month (A) and at 3 months (B) post-stroke
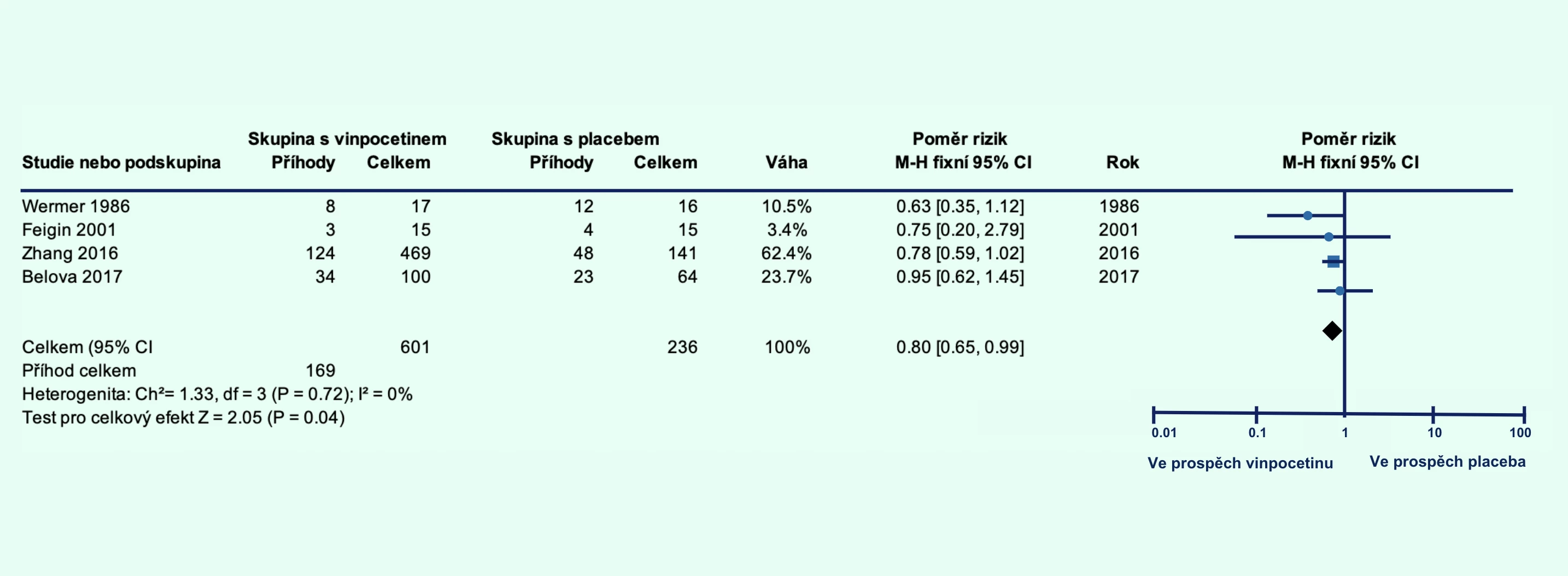
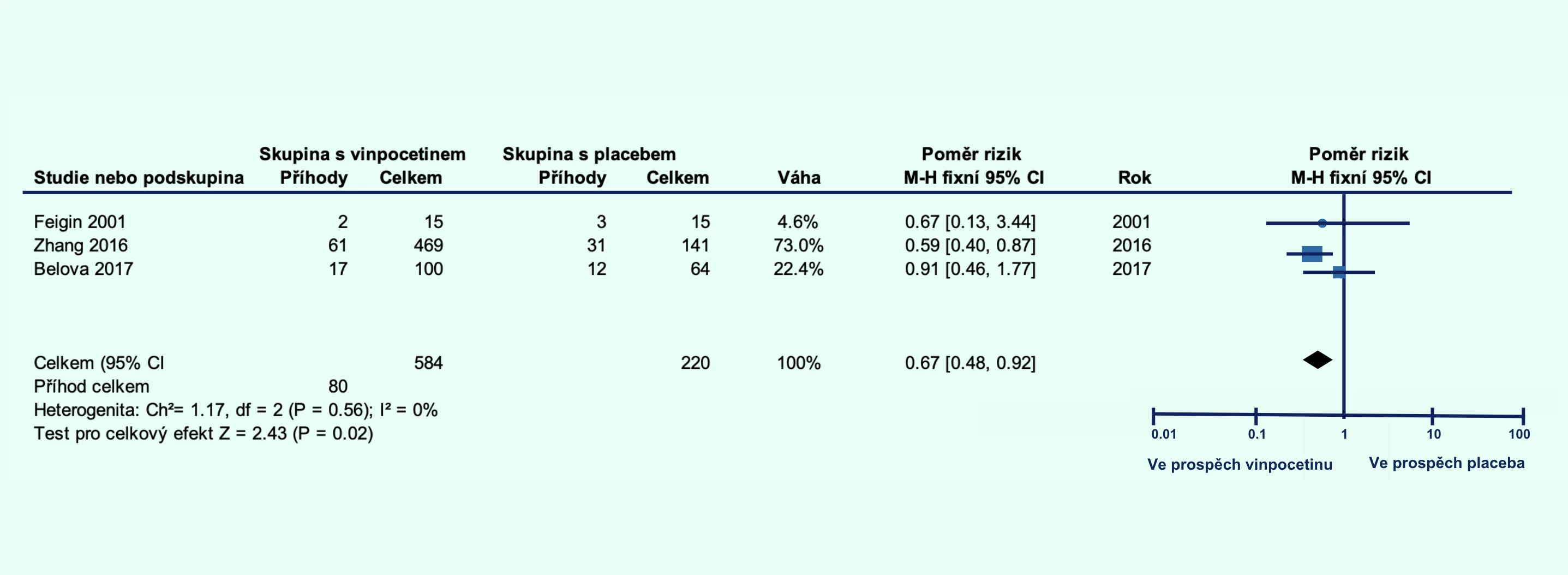
Note: CI – confidence interval; FEM – fixed-effect model; MH – Mantel–Haenszel method
Figure 2 Level of disability in patients with ischemic stroke treated with vinpocetine compared to placebo at 1 month (A) and 3 months (B) post-stroke
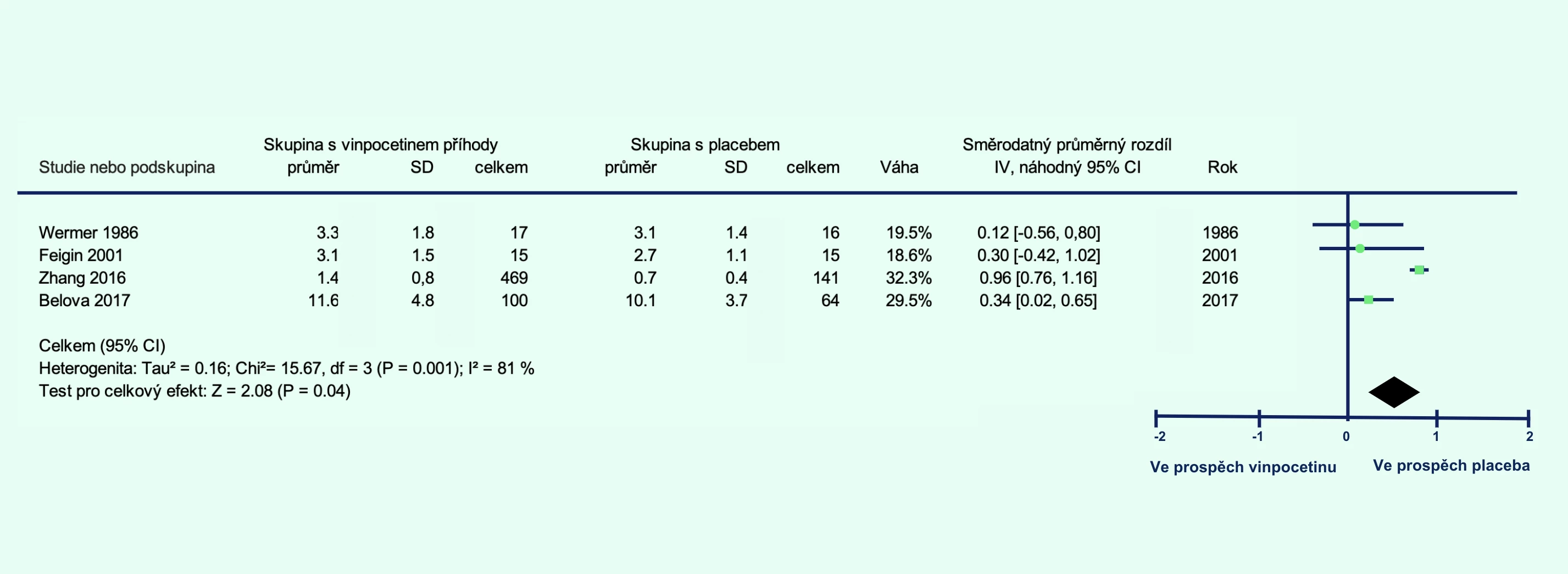
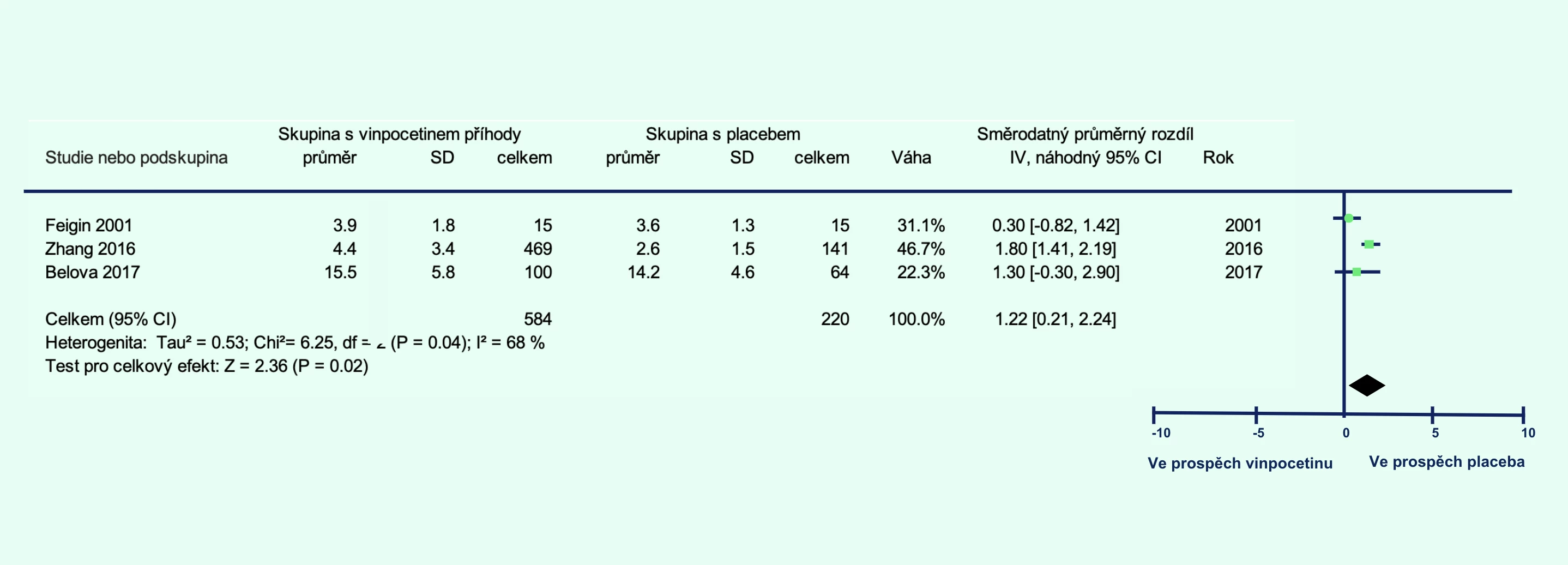
Note: CI – confidence interval; IV – inverse variance; REM – random-effects model; SD – standard deviation
Safety
Vinpocetine showed an excellent safety profile. Adverse events (AEs) occurred in only 0.5% of treated patients, with some clearly not causally related to this therapy. Compared to placebo, the frequency of AEs was comparable (RR 1.10; 95% CI 0.07–6.31; p = 0.95). None of the serious AEs were deemed causally related to vinpocetine.
Conclusion
In a meta-analysis of randomized controlled studies, vinpocetine showed promising efficacy in patients with ischemic stroke when administered within 14 days of the event. Compared to placebo, it reduced disability at 1 and 3 months post-stroke. Its administration was associated with greater reductions in neurological and cognitive deficits and improved cerebral blood flow. Tolerability was comparable to placebo. The authors suggest that additional double-blind placebo-controlled studies with larger patient cohorts should be conducted before recommending the routine administration of vinpocetine in patients with acute ischemic stroke.
(zza)
Source: Panda P. K., Ramachandran A., Panda P., Sharawat I. K. Safety and efficacy of vinpocetine as a neuroprotective agent in acute ischemic stroke: a systematic review and meta-analysis. Neurocrit Care 2022; 37 (1): 314-325, doi: 10.1007/s12028-022-01499-y.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Neurology General practitioner for adults Psychiatry Anaesthesiology, Resuscitation and Inten Surgery Orthopaedics Nurse Traumatology Trauma surgery Pain management
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


