-
Medical journals
- Career
Transthyretin – More Than a Thyroxine Transporter
6. 2. 2023
Transthyretin (TTR) is an extracellular protein whose main role is to transport thyroxine and retinol in the body and brain. TTR tends to form amyloid deposits, which can lead to systemic disease known as TTR amyloidosis. Recently, it has been shown that this protein has a much wider spectrum of activities in the organism than previously thought.
TTR as a Transport Barrel
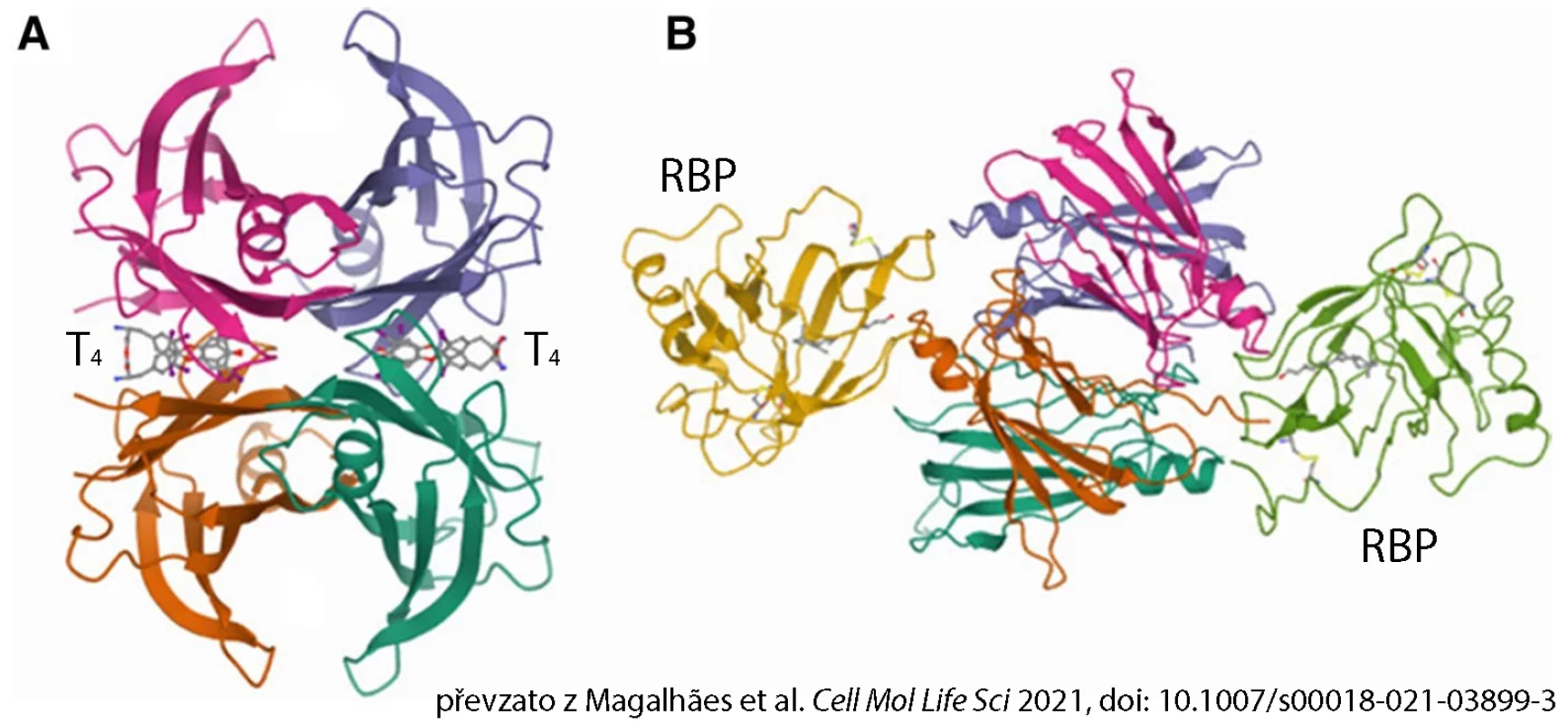
TTR is a protein composed of 4 identical subunits that together form a cylindrical hydrophobic channel containing 2 symmetrical binding sites for 2 molecules of thyroid hormone thyroxine (T4). TTR is also the main transport protein for retinol (vitamin A), which it binds by forming a complex with retinol-binding protein (RBP). One TTR tetramer can bind 2 molecules of RBP (see fig.).
The main physiological function of transthyretin, as can be deduced from its name, is considered to be transport of thyroid hormone. TTR transports approximately 15% of T4 in plasma, while in cerebrospinal fluid it binds up to 80% of this hormone.
Fig. Structure of human transthyretin tetramer in complex with:
A) 2 molecules of thyroxine (T4)
B) 2 molecules of retinol-binding protein (RBP)TTR as Scissors
However, TTR also binds other ligands, such as apolipoprotein A-I (ApoA-I). It has been shown that TTR exhibits proteolytic activity for this ligand. Cleavage of the end of ApoA-I reduces its ability to remove cholesterol and increases its potential to form plaques.
TTR is considered to be a zinc-dependent metalloprotease, and its activity has been described for a number of substrates in the nervous system, such as neuropeptide Y and amyloid beta peptide. The physiological relevance of these processes, however, is not yet sufficiently understood.
TTR as a Neuron Protector
One of the symptoms of systemic amyloidosis can be peripheral nervous system dysfunction due to the accumulation of TTR, leading to axonal degeneration. Under physiological conditions, however, TTR is present in the peripheral nervous system and appears to exhibit neuroprotective effects. It plays a role in the regeneration of damaged neurons and in axonal transport, though the mechanisms of these biological processes have not yet been elucidated.
TTR also shows neuritogenic activity in the central nervous system, particularly in hippocampal neurons. The absence of TTR in mouse models leads to an accelerated development of memory disorders associated with aging. TTR also interacts with a number of molecules important for neuroplasticity, synaptic activity, neurite growth, and neuronal activity.
TTR as a Cell Regulator
Recently, the impact of TTR on cell differentiation and proliferation, as well as immune regulation, has also been discussed. In vitro studies have further observed the ability of TTR to influence a number of signaling pathways that regulate cellular metabolism. Its effect on angiogenesis and the tumor microenvironment has also been described.
Conclusion
It is becoming evident that TTR plays a role in a range of physiological processes, from the transport of thyroxine and retinol to the maintenance of nervous system health and the regulation of cell proliferation and metabolism. To fully understand all the physiological functions of this protein, it is necessary to thoroughly explore the mechanisms of its action. Research on TTR is thus becoming an important topic in biomedical research, as changes in its levels in the body are increasingly being linked to various diseases in scientific literature.
A serious disease most commonly occurring in acquired form in the Czech Republic is cardiomyopathy caused by transthyretin amyloidosis (ATTR-CM). Early diagnosis is key to improving the prognosis of patients, both those with neurological and predominantly cardiac symptoms.
(este)
Source: Magalhães J., Eira J., Liz M. A. The role of transthyretin in cell biology: impact on human pathophysiology. Cell Mol Life Sci 2021; 78 (17–18): 6105–6117, doi: 10.1007/s00018-021-03899-3.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Neurology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career