-
Medical journals
- Career
Engraftment of acute and chronic myeloid leukemia in NOD scid gamma mice
Authors: M. Čulen 1; D. Dvořáková 2; L. Semerád 1,2; Z. Šustková 1,2; J. Bouchnerová 1,2; M. Palacková 1,2; J. Mayer 1,2,3; Z. Ráčil 1,2,3
Authors‘ workplace: Department of Internal Medicine, Hematology and Oncology, Faculty of Medicine, Masaryk University, Brno 1; Department of Internal Medicine, Hematology and Oncology, Faculty Hospital Brno, Brno 2; Masaryk University, Central European Institute of Technology, Brno 3
Published in: Transfuze Hematol. dnes,21, 2015, No. 1, p. 6-12.
Category: Comprehensive Reports, Original Papers, Case Reports
Overview
The immunodeficient mouse represents an established animal model for leukemic transplantation studies. It is used both as a self-contained research tool for the study of leukemia genesis and progression, as well as for confirmation of cell leukemogenic capability. The model as such has seen many modifications over the years, and while engraftment of primary acute and chronic myeloid leukemia samples is commonly feasible still not all samples can provide leukemic repopulation. This review specifically focuses on acute and chronic myeloid leukemia xe-nograft experiments using the current standard NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mouse model and aims to provide a basic insight into the topic. It also discusses the various specifics relevant for conducting such experiments.
Key words:
xenograft, NSG mouse, AML, CMLINTRODUCTION
Myeloid leukemias are a group of hematological malignancies affecting the myeloid lineage of white blood cells. They can be simply divided into acute and chronic myeloid leukemia (AML, CML), where the former is characteristic by its rapid progression and high hete-rogeneity, with respect to both genetics and patient prognosis, and the latter represents a homogeneous disease, in 95% cases defined by the presence of the chromosomal translocation known as “Philadelphia chromosome” (Ph). A comprehensive description or understanding of AML is so far lacking, given its extensive heterogeneity. This provides very broad research options. On the other hand, in CML, tyrosine-kinase inhibitors represent a successful approach for disease suppression and research now mainly focuses on eliminating residual leukemic stem cells, which should eventually provide a definite cure for this disease.
The immunodeficient mice have played an important role in the study of leukemias as they allow in vivo modeling of patient disease. The importance of the first experiments involving severe combined immunodeficient (SCID) mice was that they allowed transplantation of primary patient leukemic samples without graft rejection [1]. Later, the residual immunity of this strain was suppressed in the NOD.CB17-Prkdcscid (NOD/SCID, NS) mouse and a further deletion of the γ-common chain yielded the NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mouse, which currently represents a standard model for leukemic xenograft assays. The NSG mouse lacks mature T cells, B cells and functional natural killer (NK) cell. It has impaired cytokine signaling and does not spontaneously develop thymic lymphomas as the older NS model did. This fact has significantly extended the life span of the animals [2].
A direct comparison of the NSG model with NS and a related NOD.Cg-PrkdcscidB2mtm1Unc/J, (NOD/scid/b2 null, NSB) mouse strain was performed by Agliano et al., showing that the NSG mouse provided the highest number of successfully engrafted mice and also engrafted the highest number of primary acute myeloid leukemia (AML), acute lymphoid leukemia (ALL) and cell line samples [3]. In contrast, Risueno et al. showed engraftment of the same number of primary AML samples in all three NSG, NS and NSB strains, but still with the highest engraftment levels in the NSG mice, which was also confirmed in other studies [4–6].
Although the xenograft models are mainly used as an assay to confirm the presence of hematopoietic or leukemic stem cells, over the years, they have mediated several important discoveries involving leukemic stem cells (LSC). For example, it was shown that AML LSC are not only present in the CD34+38- population, which phenotypically defines healthy hematopoietic cells, but that even cells with progenitor phenotype and also patient samples without CD34 expression are capable of recapitulating leukemia in immunodeficient mice, thus underlining the complex nature of this disease [6, 7]. Similarly, in samples with mutated nucleophosmin (NPM), which represent around 60% of cytogenetically normal AML samples generally associated with low CD34 expression, it was shown that in some cases the CD34+ fraction gave rise only to multilineage engraftment, whereas only CD34- cells initiated leukemia [18–10]. In CML, leukemic engraftment was then achieved with samples from patients with complete molecular response after long-term imatinib (IM) treatment, providing further confirmation that CML LSCs are not eradicated by therapy [11–13].
1. Engraftment kinetics of particular CML stages in NS mice [18] ![Engraftment kinetics of particular CML stages in NS mice [18]](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/92092066b915de2ea2d11dfff7af5cad.png)
Values show median percentages of human cells, as detected in mouse bone marrow using flow cytometry by human CD45 staining. *Ph+ = Philadelphia chromosome positive. Xenografts of AML
For AML, many factors and aspects influencing the engraftment of primary samples in mice have been studied. Despite the fact that the studies differed at some level in various technical details, as evidenced in Table 2 and discussed further in the text, some general points can be assembled. Regarding engraftment rates, Sanchez et al. documented 66% engrafting samples from a group of 35 patients [5]. Similarly, Risueno et al. reported a 61% engraftment rate [4]. But also higher success rates of 81.5 and 90.9% have been reported [14, 15]. It should be noted, however, that the engraftment rates depend on many factors. The most important is probably the selection of patient samples, which can be supported by the study of Mitchell et al. on 307 AML samples, using the NS strain [16]. The authors have shown that poor cytogenetic prognosis and worse patient outcome, as defined by achievement of complete remission, overall survival (OS) and event free survival (EFS), were strongly correlated with better engraftment characteristics, and this link between patient prognosis and engraftment was also proven by Woiterski et al. and Pearce et al. [15, 17]. Interestingly, the study by Mitchell et al. did not prove a positive effect of higher blast count on better engraftment, while many studies have considered this factor in their samples [5, 15, 16].
2. Summary of technical approaches in AML NSG xenografts 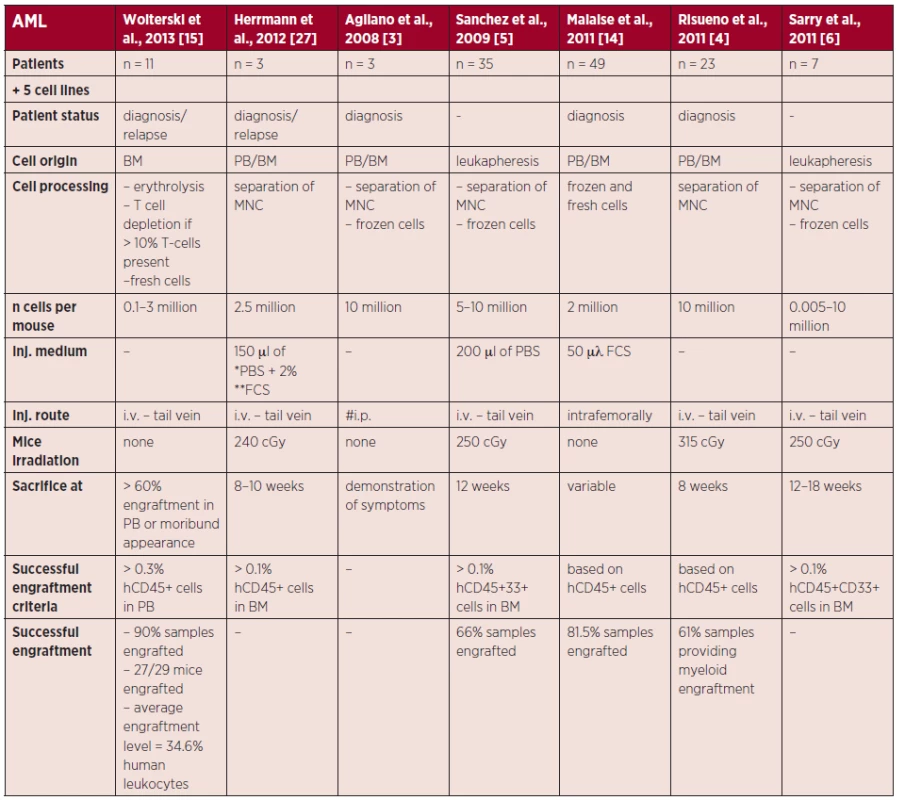
The table shows technical details of engraftment experiments for each selected study and states the origin of the injected leukemic cells, preparation of the cells prior to engraftment, information on the engraftment assays and engraftment results. Engraftment was assessed by flow cytometry, based on the frequency of either human leukocytes (stained for human CD45) or human myeloid cells (stained for human CD45 and CD33) present in the given mouse tissue (if specified). *PBS = phosphate buffered saline **FCS = fetal calf serum #i.p. = intraperitoneal Also, pediatric AML samples were shown to engraft better than adult samples (100 vs. 76.3% engrafted samples) [14].
Xenografts of CML
In general, the information on the xeno-graft assays of CML is scarce, as most of the studies used the model as an in vivo test method, while robust studies specifically investigating the engraftment of CML are lacking. One of the very few comprehensive studies of CML engraftment was performed by Dazzi et al. who also compared the engraftment kinetics of chronic phase (CP), accelerated phase (AP), blast crisis (BC) CML and the BV173 cell line in NS mice (see Table 1) [18]. The authors used rather high cell numbers of 5 million CD34+ cells per mouse obtained by leukapheresis, which however is not as commonly available as normal peripheral blood (PB) samples or bone marrow (BM) samples. Interesting data were then obtained by Chu et al. who tested engraftment of patients after imatinib treatment, showing that all six tested samples gave multilineage engraftment, with a predominant B-cell fraction and BCR/ABL1 being detected for all engrafted samples. Interestingly, they reached engraftment levels from around 2.5% up to around 50%, while using low and commonly obtainable cell doses of 86–173 thousand CD34+ cells per mouse [11]. Further comprehensive CML xenograft data were obtained by Herrmann et al. in an elegant study, where the authors injected a lineage depleted (Lin-) stem cell fraction, further divided into LSC and hematopoi-etic stem cells (HSC) based on expression of the CD26 marker [19]. The injection of 0.1–0.8 million Lin-CD26+ cells, proposed as LSC provided myeloid engraftment with confirmed BCR/ABL1 transcripts and engraftment levels ranging from 0.05 to 0.31% of hCD45+ at 16 or 28 weeks after injection. The lin-CD26 - fraction, proposed as healthy stem cells, then provided myeloid engraftment or predominantly B-cell engraftment in 2/6 and 4/6 samples, respectively, where none of the samples were positive for BCR/ABL1. Rather low engraftment level (5% hCD45+) was also achieved in a study with as much as 6 million CD34+ cells per one mouse, which provides yet another indication that low engraftment levels are generally more common for CML xeno-graft assays [20].
Effect of particular parameters on engraftment
Cell dose
The effect of the number of injected cells was investigated, for example, by Pearce et al. who have shown on NS mice that non-engrafting samples do not engraft irrespective of the cell dose [17]. Nevertheless, in samples capable of engraftment, the injected sample has to carry LSC/s to provide engraftment – and thus successful engraftment depends on the LSC frequency and the cell dose of the sample. This was nicely documented by Sarry et al. in a comprehensive study where only 3/6 patients engrafted with 0.1 million mononuclear cells (MNC) injected, but 6/7 of the same patient samples engrafted with 0.5 million cells and engraftment of all samples in all mice was achieved with 10 million cells [6].
Injection route
The effect of the injection route has so far not been directly assessed for myeloid leukemia xenograft assays, although it has been shown that intra femoral injection leads to better engraftment of HSC [21, 22]. However, the high technical demands of the intra bone injection are a limiting factor and intravenous (i. v.) injection through the tail vein remains the standard route. Intraperitoneal injection was also documented, but with a high cell load (10 million MNC/mouse), and its efficacy has not been directly compared with other routes [3].
Pre-treatment
Irradiation of mice recipients prior to cell injection to enhance the engraftment is a common practice, although it was challenged for AML in the NSG mice in some works. For example, Watanabe et al. showed a stable HSC mediated engraftment in non-irradiated mice; Agliano and colleagues then showed equal engraftment of the HL-60 cell line in irradiated and non-irradiated mice [3, 23]. Moreover, there were further studies completely omitting irradiation [14, 15]. According to the authors’ knowledge, the effect of irradiation was not tested for CML xenografts and all reported CML studies employed myeloablation through irradiation (see Table 3).
3. Summary of technical approaches in CML xenografts 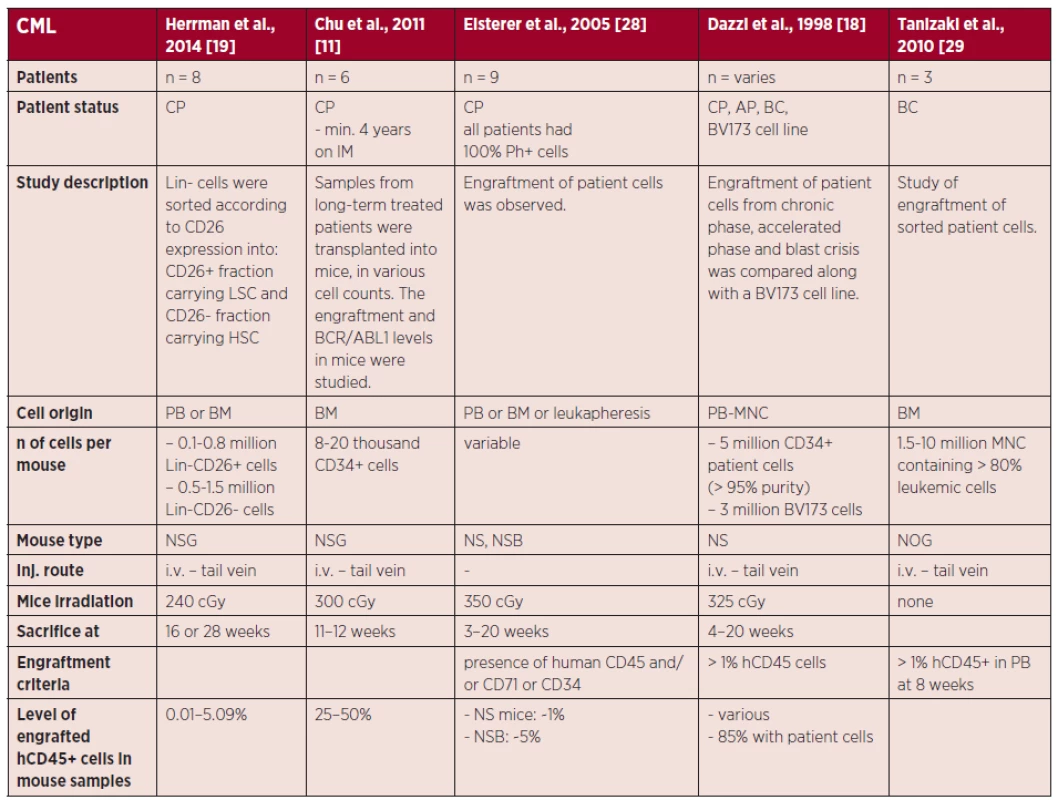
The table shows technical details of engraftment experiments for each selected study and states the origin of the injected leukemic cells, preparation of the cells prior to engraftment, information on the engraftment assays and engraftment results. Engraftment was assessed by flow cytometry, based on the frequency of either human leukocytes (stained for human CD45) or human myeloid cells (stained for human CD45 and CD33) present in the given mouse tissue (if specified). In previous models, such as the NS mouse, CD-122 antibody was commonly used for depletion of NK cells and had a significant effect on engraftment improvement. Nevertheless, due to the innate deletion of γ-common chain in the NSG mice, this pre-treatment is no longer needed in this model [21, 24].
Cell handling
One of the options for achieving higher engraftment rates is the injection of fresh cells. It was demonstrated that fresh samples showed better engrafting rates (96.3% for fresh vs. 63.6% for thawed samples) with shorter time to engraftment (8 and 19 weeks for fresh and frozen samples respectively) [14]. These results are striking, although the use of fresh cells is often not feasible.
Sex of recipient mice
It was demonstrated that NSG females engraft HSC better than males, where the difference was most pronounced with the use of limiting cell numbers [21, 25]. With cell doses above 10 million of cord blood MNC, the effect of sex was not observed [26]. In contrast, Woiterski et al. reported higher engraftment in males for both non-limiting numbers of HSC and samples of acute leukemias, where in the latter case this may have been caused by the higher frequency of high-risk patient samples injected into male than female recipients [15]. Based on the findings with the high-enough cell doses, the selection of a specific sex of the mouse recipients for AML may be questioned. But for CML, where often only limiting cell doses are available, the use of female mice would be highly recommended.
Conclusions
The xenograft models represent thus far the ultimate assays for demonstrating the repopulating ability of leukemic cells or their specific subpopulations. For AML and CML, the NSG mouse represents a standard model although it does not offer successful engraftment of all samples. This is primarily dependent on the sample itself, while certain technical details also play role. There is no doubt that new modifications and improvements of the recipient animals will bring a new standard in this field and already a modification of the NSG mouse has been presented, denoted NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ (NSGS, NSG-SGM3). This relatively new model should eliminate the “lymphoid bias” i.e. the preferential growth advantage of lymphoid cells in the NSG mouse through stable production of human interleukin-3 (IL-3), human granulocyte/macrophage-stimulating factor (GM-CSF) and human Steel factor (SF). These three soluble factors have already been shown to improve engraftment in previous SCID based mouse models either after administration or transgenic introduction [30, 31]. The new NSGS strain has so far shown to provide more pronounced engraftment of AML samples as well as engraftment of samples not repopulated in the NSG mouse. However, more works would be required to prove or specify its benefits [32, 33]. Another modification expected to significantly improve the engraftment options has been described and involves implantation of a humanized niche into immunodeficient mice [34, 35]. Although the model is technically more chal-lenging, it offers vast research potential through modification of the implanted niche and such studies, involving engraftment of primary patient samples, may radically broaden our options for simulation of patient leukemia in the future.
Acknowledgments
This work was supported by the project „Employment of Best Young Scientists for International Cooperation Empowerment“ (CZ.1.07/2.3.00/30.0037) co-financed from the European Social Fund and the state budget of the Czech Republic.
The work was further supported by grant project MUNI/A/0830/2013.
Authors’ participation in preparing this manuscript
M. Č. – manuscript preparation, other authors (D. D., L. S., Z. Š., J. B., M. P., J. M., Z. R.) – revisions and proof-checking.
Doručeno do redakce: 25. 11. 2015
Přijato po recenzi: 26. 1. 2015
Martin Čulen, PharmDr., Ph.D.
Department of Internal Medicine, Hematology and Oncology
Faculty of Medicine, Masaryk University
Kamenice 5
625 00 Brno
e-mail: mculen@gmail.com
Sources
1. Kamel-Reid S, Letarte M, Sirard C, et al. A model of human acute lymphoblastic leukemia in immune-deficient scid mice. Science 1989; 246 : 1597–1600.
2. Shultz LD, Schweitzer PA, Christianson SW, et al. Multiple defects in innate and adaptive immunologic function in nod/ltsz-scid mice. J Immunol 1995; 154 : 180–191.
3. Agliano A, Martin-Padura I, Mancuso P, et al. Human acute leukemia cells injected in nod/ltsz-scid/il-2rgamma null mice generate a faster and more efficient disease compared to other nod/scid-related strains. Int J Cancer 2008; 123 : 2222–2227.
4. Risueno RM, Campbell CJV, Dingwall S, et al. Identification of t-lymphocytic leukemia-initiating stem cells residing in a small subset of patients with acute myeloid leukemic disease. Blood 2011; 117 : 7112–7120.
5. Sanchez PV, Perry RL, Sarry JE, et al. A robust xenotransplantation model for acute myeloid leukemia. Leukemia 2009; 23 : 2109–2117.
6. Sarry JE, Murphy K, Perry R, et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in nod/scid/il2rgammac-deficient mice. J Clin Invest 2011; 121 : 384–395.
7. Terpstra W, Prins A, Ploemacher RE, et al. Long-term leukemia-initiating capacity of a cd34-subpopulation of acute myeloid leukemia. Blood 1996; 87 : 2187–2194.
8. Taussig DC, Vargaftig J, Miraki-Moud F, et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the cd34(-) fraction. Blood 2010; 115 : 1976–1984.
9. Dvorakova D, Racil Z, Borsky M, et al. Clonal heterogeneity in patients with cytogenetically normal acute myeloid leukemia with NPM1 mutations. Leukemia Lymphoma 2013; 54 : 1056–1060.
10. Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 2005; 352 : 254–266.
11. Chu S, McDonald T, Lin A, et al. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood 2011; 118 : 5565–5572.
12. Graham SM, Jorgensen HG, Allan E, et al. Primitive, quiescent, philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to sti571 in vitro. Blood 2002; 99 : 319–325.
13. Bhatia R, Holtz M, Niu N, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood 2003; 101 : 4701–4707.
14. Malaise M, Neumeier M, Botteron C, et al. Stable and reproducible engraftment of primary adult and pediatric acute myeloid leukemia in nsg mice. Leukemia 2011; 25 : 1635–1639.
15. Woiterski J, Ebinger M, Witte KE, et al. Engraftment of low numbers of pediatric acute lymphoid and myeloid leukemias into nod/scid/il2rcgammanull mice reflects individual leukemogenecity and highly correlates with clinical outcome. Int J Cancer 2013; 133 : 1547–1556.
16. Mitchell A, Chen WC, McLeod J, et al. Leukemic engraftment in nod.Scid mice is correlated with clinical parameters and predicts outcome in human aml. Blood 2013; 122 : 50.
17. Pearce DJ, Taussig D, Zibara K, et al. Aml engraftment in the nod/scid assay reflects the outcome of aml: Implications for our understanding of the heterogeneity of aml. Blood 2006; 107 : 1166–1173.
18. Dazzi F, Capelli D, Hasserjian R, et al. The kinetics and extent of engraftment of chronic myelogenous leukemia cells in non-obese diabetic/severe combined immunodeficiency mice reflect the phase of the donor’s disease: An in vivo model of chronic myelogenous leukemia biology. Blood 1998; 92 : 1390–1396.
19. Herrmann H, Sadovnik I, Cerny-Reiterer S, et al. Dipeptidylpeptidase iv (cd26) defines leukemic stem cells (lsc) in chronic myeloid leukemia. Blood 2014; 123 : 3951–3962.
20. Chen M, Gallipoli P, DeGeer D, et al. Targeting primitive chronic myeloid leukemia cells by effective inhibition of a new ahi-1-bcr-abl-jak2 complex. J Natl Cancer Inst 2013; 105 : 405–423.
21. McDermott SP, Eppert K, Lechman ER, Doedens M, Dick JE. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood 2010; 116 : 193–200.
22. Bueno C, Montes R, de la Cueva T, Gutierrez-Aranda I, Menendez P. Intra-bone marrow transplantation of human cd34(+) cells into nod/ltsz-scid il-2rgamma(null) mice permits multilineage engraftment without previous irradiation. Cytotherapy 2010; 12 : 45–49.
23. Watanabe S, Ohta S, Yajima M, et al. Humanized nod/scid/il2rγnull mice transplanted with hematopoietic stem cells under nonmyeloablative conditions show prolonged life spans and allow detailed analysis of human immunodeficiency virus type 1 pathogenesis. J Virol 2007; 81 : 13259–13264.
24. Taussig DC, Miraki-Moud F, Anjos-Afonso F, et al. Anti-cd38 antibody–mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood 2008; 112 : 568–575.
25. Notta F, Doulatov S, Dick JE. Engraftment of human hematopoietic stem cells is more efficient in female nod/scid/il-2rgc-null recipients. Blood 2010; 115 : 3704–3707.
26. Ballen KK, Valinski H, Greiner D, et al. Variables to predict engraftment of umbilical cord blood into immunodeficient mice: Usefulness of the non-obese diabetic–severe combined immunodeficient assay. Brit J Haematol 2001; 114 : 211–218.
27. Herrmann H, Kneidinger M, Cerny-Reiterer S, et al. The hsp32 inhibitors sma-znpp and peg-znpp exert major growth-inhibitory effects on d34+/cd38+ and cd34+/cd38 - aml progenitor cells. Curr Cancer Drug Targets 2012; 12 : 51–63.
28. Eisterer W, Jiang X, Christ O, et al. Different subsets of primary chronic myeloid leukemia stem cells engraft immunodeficient mice and produce a model of the human disease. Leukemia 2005; 19 : 435–441.
29. Tanizaki R, Nomura Y, Miyata Y, et al. Irrespective of cd34 expression, lineage-committed cell fraction reconstitutes and re-establishes transformed philadelphia chromosome-positive leukemia in nod / scid / il-2rγc−/−mice. Cancer Sci 2010; 101 : 631–638.
30. Feuring-Buske M, Gerhard B, Cashman J, et al. Improved engraftment of human acute myeloid leukemia progenitor cells in beta 2-microglobulin-deficient nod/scid mice and in nod/scid mice transgenic for human growth factors. Leukemia 2003; 17 : 760–763.
31. Terpstra W, Prins A, Visser T, et al. Conditions for engraftment of human acute myeloid leukemia (aml) in scid mice. Leukemia 1995; 9 : 1573–1577.
32. Wunderlich M, Chou FS, Link KA, et al. Aml xenograft efficiency is significantly improved in nod/scid-il2rg mice constitutively expressing human scf, gm-csf and il-3. Leukemia 2010; 24 : 1785–1788.
33. Klco JM, Spencer DH, Miller CA, et al. Functional heterogeneity of genetically defined subclones in acute myeloid leukemia. Cancer Cell 2014; 25 : 379–392.
34. Vaiselbuh SR, Edelman M, Lipton JM, Liu JM. Ectopic human mesenchymal stem cell-coated scaffolds in nod/scid mice: An in vivo model of the leukemia niche. Tissue Eng Part C Methods 2010; 16 : 1523–1531.
35. Groen RWJ, Jaques J, Yuan H, et al. Mouse versus human extrinsic cues dictate transformation potential in bcr-abl/bmi1-induced leukemia in humanized xenograft models. Blood 2013; 122 : 515.
Labels
Haematology Internal medicine Clinical oncology
Article was published inTransfusion and Haematology Today

2015 Issue 1-
All articles in this issue
- First-line treatment for severe aplastic anemia in children: bone marrow transplantation from a matched family donor versus immunosuppressive therapy
- Management and clinical outcomes in patients treated with apixaban vs warfarin undergoing procedures
- Recombinant long-acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trial
- Infectious complications of induction therapy in acute myeloid leukaemia patients without the use of antibiotic prophylaxis
- Invasive fungal infections following autologous hematopoietic stem cell transplantation in patients with multiple myeloma
- Interference of blood plasma components in turbidimetric D-dimer estimation
- Excellent prognosis of late relapses of ETV6/RUNX1-positive childhood acute lymphoblastic leukemia: lessons from the FRALLE 93 protocol
- Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients
- The toxicity of very prolonged courses of PEGasparaginase or Erwinia asparaginase in relation to asparaginase activity, with a special focus on dyslipidemia
- Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients
- Incorporation of humanized niche as a strategy for improving leukemic engraftment in immunodeficient mice
- Engraftment of acute and chronic myeloid leukemia in NOD scid gamma mice
- Transfusion and Haematology Today
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Infectious complications of induction therapy in acute myeloid leukaemia patients without the use of antibiotic prophylaxis
- Interference of blood plasma components in turbidimetric D-dimer estimation
- Invasive fungal infections following autologous hematopoietic stem cell transplantation in patients with multiple myeloma
- Engraftment of acute and chronic myeloid leukemia in NOD scid gamma mice
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

