-
Medical journals
- Career
The viability of ovarian carcinoma cells A2780 affected by titanium dioxide nanoparticles and low ultrasound intensity
Authors: Vladan Bernard; Vojtěch Mornstein
Authors‘ workplace: Masaryk University, Faculty of Medicine, Department of Biophysics, Brno, Czech Republic
Published in: Lékař a technika - Clinician and Technology No. 1, 2016, 46, 21-24
Category: Original research
Overview
The effect of titanium dioxide nanoparticles and ultrasound was studied on human ovarian carcinoma cells A2780 in vitro. The viability of cells has been studied by a standard 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide viability assay in different modes of treatment: application of nanoparticles alone, exposure to ultrasound field alone, application of nanoparticles followed by an exposure to ultrasound, and an exposure to ultrasound followed by addition of nanoparticles. The viability was measured 48 and 72 hours after the exposure. The titanium dioxide nanoparticles used were smaller than 100 nm in diameter, ultrasound was applied at a therapeutical intensity of 1 W∙cm-2 and frequency of 1 MHz; the cells were treated in a 37 °C thermostated water bath in a configuration with far field ultrasound exposure. The final concentration of titanium dioxide nanoparticles was 50 μg/mL. The results showed that a combined effect of titanium dioxide nanoparticles and ultrasound influenced the viability of human carcinoma cells more than the application of titanium dioxide nanoparticles or ultrasound alone.The outcomes showed a significant difference between experimental groups with different sequences of application or exposure of nanoparticles or ultrasound. Maximal decrease of viability was achieved by application of experimental protocol with exposure to ultrasound first, followed by application of nanoparticles. It seems to indicate the possibility to intensify the effect of nanoparticles on cell viability by previous ultrasound exposure.
Keywords:
nanomaterials, titanium dioxide, ultrasound, viabilityIntroduction
The cancer is one of the major threats to mankind today, although considerable amount of money is spent for the development of new therapies. A general incidence of this disease is not linked with age or gender. Ovarian cancer is the second most frequent malignancy of female genital tract and the first one among all the other gynaecologic malignancies in the mortality rate [1]. Except standard chemotherapy and radiation, treatment of cancer is performed also by using of nanotechnology [2, 3]. In this case nanoparticles play an important role [4]. An interest in them is often related to their oligodynamic effect, which can be manifested as an antibacterial effect. It is also known that the metallic nanoparticles are able to affect the viability of tissue cells and whole organism [5]. Titanium dioxide (TiO2) belongs among the nanoparticles with a potential in cytotoxic therapy. In recent years TiO2 has been applied in photodynamic therapy of cancer cells [6]. It is known that TiO2 particles under UV light exposure can produce free electrons necessary for the formation of reactive oxygen species such as hydrogen peroxide. This effect can be used to kill tumor cells due to cell membrane peroxidation [7]. The use of TiO2 in photodynamic therapy looses its efficiency, however, in the case of an insuffiencient penetration of ultraviolet radiation into the tissues - as would be in the case of ovarian cancer cells. TiO2 is namely used as a photocatalysts agents and is able to convert solar energy to chemical energy to oxidize or reduce materials, and is widely used for many applications including production of materials and environmental purification [8]. It is reason, why is widespread in the environment with aditional possible of undesirable risks.
A promising trend in anti-cancer therapy appears to be the combined effect of chemotherapy and lowpower ultrasound. The outcome of the experiments showed the possibility of intensifying cytostatic therapy by applications of ultrasonic field in vitro [9], [10]. This effect of ultrasound field is comprehensively described in the theory of sonodynamic therapy [11]. The combined action of nanomaterials and ultrasound was studied in limited extent yet. The basic question asked in our in vitro study is whether the cell viability of human ovarian cancer cell lines can be affected by a combined application of low intensity ultrasound (below the cavitation threshold) and the nanoparticles TiO2 or not. It is also intended to show whether the succession of application of ultrasound and TiO2 nanoparticles plays a role in the viability effect.
Material and methods
Cell culture
Human ovarian carcinoma cell line A2780, obtained from the European Cell Culture Collection was used. RPMI-1640 medium with L-glutamine (Bio Tech, Ltd., Prague, Czech Republic) supplemented with 10% fetal calf serum (Bio Tech) and 100 μg/mL strep-tomycin/penicillin (Bio Tech) was used. The cell line was grown in cell culture flasks in 5% CO2 at temperature of 37 °C. The cells were detached from glass by trypsinization (trypsin obtained from Bio Tech) with passivation by complete RPMI medium.
Nanoparticles
Titanium dioxide TiO2 nanoparticles of size < 100 nm were obtained in a dry crystalline form (Sigma Aldrich, Prague, Czech Republic). The 1 mg/mL stock suspension in PBS was prepared and stored in the darkness.
Ultrasound exposure
BTL-07 therapeutic ultrasound generator (Beautyline Ltd., Prague, Czech Republic) working at a frequency of 1 MHz with a 4 cm2 probe was used as the source of ultrasound. The cells were exposed for 10 min to the far field of a horizontal beam of continuous-wave ultrasound at intensity of 1 W∙cm-2 in a 37 °C thermostated water bath. The exposure was carried out in a polyethylene tube fastened to a rotating holder (3 rpm). This experimental set-up provided uniform exposure of the entire volume of the cell suspension. Ultrasound intensity and acoustic pressure was measured by means of a calibrated PVDF hydrophone, type MH28-6 (Force Institute Copenhagen, Denmark) by using the calibration protocol.
Experimental design
The cells of A2780 line were incubated for up to 72 h after the following modes of treatment: sample ti - addition of titanium dioxide only; sample us -10 minute ultrasound exposure only; sample ti+us - addition of titanium dioxide and subsequent 10 minute ultrasound exposure; sample us+ti -10 minute ultrasound exposure followed by addition of titanium dioxide; sample contr - without addition of titanium dioxide and ultrasound exposure.
Cell viability assay
The following procedure was used to compare the viability of samples us, ti, ti+us, us+ti and control cells contr: A cell suspension was obtained by trypsinization of cells adhering to the flask bottom. To each well of a 96-well plate containing 5∙104 cells in RPMI medium, a volume of titanium dioxide stock suspension was added to achieve a final concentration of 50 μg/mL. An equal volume of PBS free of nanoparticles was added to the control cells. The cells were affected by ultrasound before seeding on 96-well sample plate for us and us+ti. The cells in ti+us sample were affected by ultrasound with presence of titanium dioxide in cultivation medium and then seeded. After incubation for 48 and 72 hours, the cells were washed in PBS and evaluated by a standard MTT test of viability [12]. Using an EL800 microplate reader (Bio-Tek, USA), the absorbance of a blue product in each well was recorded at 570 nm.
Statistical analysis
The absorbance value for each experimental group was converted into cell viability as follows: the median absorbance value of the control group contr was taken as 100%; the absorbance of each experimental group was expressed as a percentage of control value, i.e., its viability relative to that of the control group. Because of a non-normal distribution of the values for individual groups, the non-parametric Mann-Whitney U-test at a significance level of p = 0.05 was used. The statistical software STATISTICA 10 was used to calculate the median and the upper and lower quartiles.
The data of viability of each experimental group shown in graphs were obtained from repeated independent experiments, each experiment involving analysis of samples on 96-well plate.
Results
The data obtained in the experiments permit a comparison of the viability of A2780 cells line in the experimental groups ti, ti+us, us+ti and us with respect to the control group contr following incubation times 48 and 72 hours (Fig. 1). The graphs show a dependence of both median and interquartile range values of relative cell viability on the incubation time and the experimental design.
Fig. 1: Cell viability of the cell lines A2780 for incubation time 48 and 72 hours. Ultrasound intensity of 1 W·cm<sup>-2</sup>. Experimental groups of samples are following: contr – control group; ti – cells incubated with titanium dioxide only; ti+us – cells exposed to ultrasound in the presence of titanium dioxide; us+ti – cells exposed to ultrasound followed by incubation with titanium dioxide; us – cells exposed to ultrasound only. 
At first sight, it is evident that the value of the viability decreases over time in almost all of the samples. It can be seen from the results on Fig. 1 that the effect on the cell viability caused by TiO2 nanoparticles is a rapid process. Maximum decrease was already achieved at 48 hours without significant change over time interval 48–72 h. The decrease of viability for ultrasound affected group is confirmed and occurs also at longer times.
The results of the 48 hours experiment show a statistically significant difference at a level p < 0.05 (Tab. 1) for all experimental groups with the exception of us compare with control group contr. The maximum decrease in viability was observed for the sample ti, the measured value of viability was 63% compared to the control group. The viability value of sample us+ti was at 69%, value of sample ti+us was at level 75%, both of them with significance difference (p < 0.05).
1. Significance of differences in viability values, as assessed by the Mann-Whitney test. 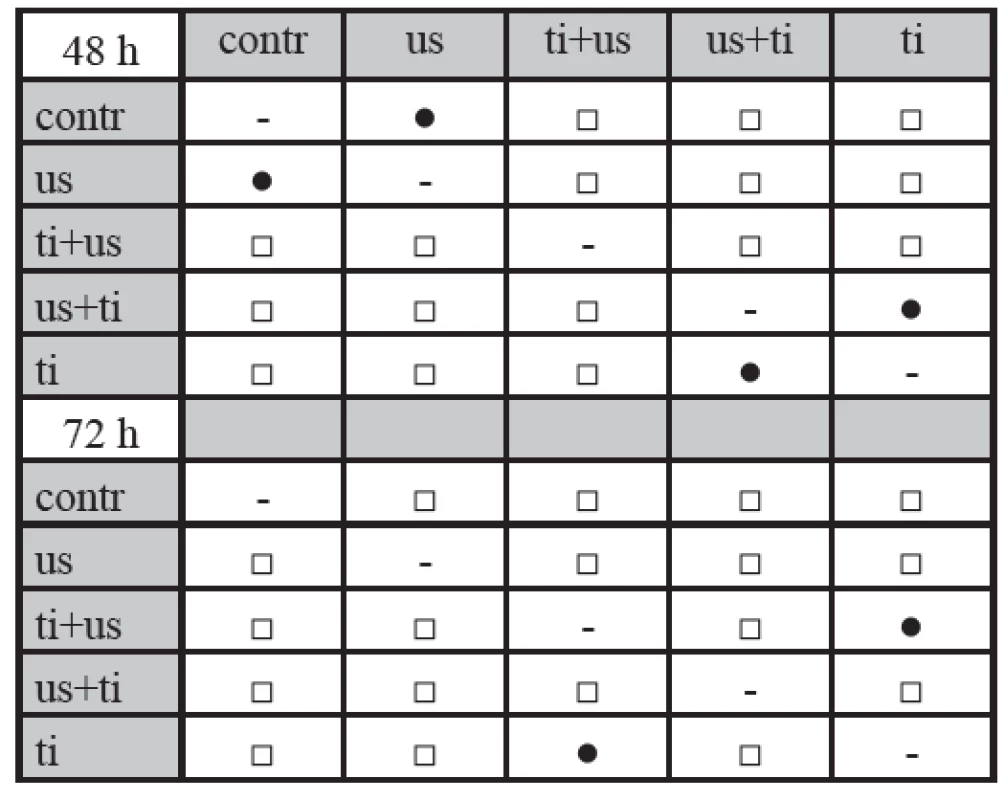
Results of Mann-Whitney test for the experimental group: contr - control group; ti - incubated cells with titanium dioxide only; ti + us - exposed cells to ultrasound in the presence of titanium dioxide; us + ti - cells exposed to ultrasound followed by incubation with titanium dioxide; us - cells exposed to ultrasound only. Symbol □ indicates statistically significant difference at p < 0.05 for a respective pair of the experimental group, symbol ● indicates no significance; incubation time 48 and 72 hours. The experimental results of the 72 hours experiment show statistically significant difference (p < 0.05) between all of the experimental groups with exception of the ti and us+ti pair. Decrese of viability value was seen for all ultrasound treated samples compared with graph for 48 hours. Maximal decrease of viability value was observed for experimental group us+ti at the value of 42%. Ti+us sample showed the viability 51% and ti sample 61% compared with control group contr.
The acoustic pressure in the position of the polyethylene tube was 0.91 + 0.04 MPa (at this value, intensity loss of 30% due to absorption in the material of the tube has been counted).
Discussion
The main objective of the present study was to verify the expected effect of the combined action of TiO2 nanoparticles and low intensity ultrasound on carcinoma cells A2780 in vitro. The experiments were carried out by monitoring the value of viability of different experimental groups, such as ultrasound alone, TiO2 nanoparticles alone or a combination of these factors. Effect of TiO2 nanoparticles on cell viability is known, as well as effect of ultrasound action alone [6, 11]. There is actually an article describing the impact of the combined effect of ultrasound and TiO2 too [13], but the authors of this article use the near field of ultrasound with higher values of acoustic pressure in the cell suspension compared with our previous experiments using far field of ultrasound with low intensity [1]. The observed far field effect seems to reduce the importance of cavitation evoked sonoluminescence, which was assumed action mechanism in [13] experiments. Thus, activation of TiO2 as a photosenzitier can, not be an explanation of our results. We believe that the cumulative effect of nanoparticles and ultrasound is caused by the mechanical action of ultrasound on the cellular structures, cytoplasic membrane perhaps, in this case. The results showed that a combined effect of TiO2 nanoparticles and ultrasound influenced the viability of human carcinoma cells more than the application of TiO2 nanoparticle and ultrasound alone. In the experiments, there was no discernable change in viability for the experimental group ti at the time of 48h and 72 h. This result shows the rapid affecting of viability without an additional effect in long time exposures to nanoparticles. On the contrary, there was observed certain trend in value of viability over time 48 h and 72 h for the sample us+ti and ti+us, where the viability value decreased in both groups. The outcomes show a significant difference between experimental groups with different sequences of application or exposure to nanoparticles and ultrasound – us+ti and ti+us. Maximal decrease of viability was shown by using experimental protocol with exposure to ultrasound followed by application of nanoparticles (the sample us+ti). These results show the significant effect of the ultrasound on the process of action of nanoparticles on cells. This fact seems to indicate the possibility of intensifying cellular viability effect of nanoparticles by using ultrasound which can be advantageous in the targeted treatment of cancer. At the same time, it may be inappropriate and even dangerous simultaneous application of ultrasound in case of the presence of nanoparticles in the body of a healthy individual because it is known that many commercial products contain nanoparticles such as the cosmetic products [14] or even foods [15]. At low levels of acoustic pressure in the cell suspension medium authors predict possible mechanical manifesttations of ultrasound on cell structure and viability. In view of the frequent occurrence of nanoparticles in the human environment and the findings, that the combined effect of TiO2 nanoparticles and ultrasound on cells depend on the experimental design we can find still another questions to answer. It is the reason for the need for further research in this field, namely in terms of the in vivo animal model experiments.
Conclusion
Presented results show the significant effect of the ultrasound on the process of action of nanoparticles on cells. The combined effect of TiO2 nanoparticles and ultrasound on cells was founded and described. This fact seems to indicate the possibility of intensifying cellular viability effect of nanoparticles by using ultrasound which can be advantageous in the targeted treatment of cancer. At the same time, it may be inappropriate and even dangerous simultaneous application of ultrasound in case of the presence of nanoparticles in the body of a healthy individual.
Acknowledgments
This project was financially supported by a grant of GACR Nr. 13-04408P.
Mgr. Vladan Bernard, Ph.D.
Biofyzikální ústav
Lékařská fakulta
Masarykova univerzita
Kamenice 126/3,
625 00 Brno
E-mail: vbernard@med.muni.cz
Phone: +420 549 496 577
Sources
[1] Ozols, R.F., Bookman, M.A., Connolly, D.C., Daly, M.B., Godwin, A.K., Schilder, R.J., Xu, X., Hamilton, T.C. Focus on epithelial ovarian cancer. Cancer Cell, 2004, 5, 19-24.
[2] Parida, S., Das. T.K. Nanotechnology and cancer. Apollo Medicine, 2008, 5, 251-2.
[3] Thomas, D.G., Pappu, R.V., Baker, N.A. NanoParticle Ontology for cancer nanotechnology research. Journal of Biomedical Informatics, 2011, 44, 59-74.
[4] Fukumori, Y., Ichikawa. H. Nanoparticles for cancer therapy and diagnosis. Advanced Powder Technol., 2006, 17, 1–28.
[5] Lanone, S., Rogerieux, F., Geys, J., Dupont, A., Maillot-Marechal, E., Boczkowski, J., Lacroix G., Hoet, P. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Particle and Fibre Toxicology, 2009, 6, 14.
[6] Kubota, Y., Shuin, T., Kawasaki, C., Hosaka, M., Kitamura, H., Cai, R., Sakai, H., Hashimoto, K., Fujishima, A. Photokilling of T-24 human bladder cancer cells with titanium dioxide. Br J Cancer, 1994, 70 1107-1111.
[7] Liu, L., Miao, P., Xu, Y., Tian, Z., Zou, Z., Li, G. Study of Pt/TiO2 nanocomposite for cancer-cell treatment. Journal Photochem Photobio B., 2010, 98, 207-210.
[8] Nakata, K., Ochiai, T., Murakami, T., Fujishima, A. Photoenergy conversion with TiO2 photocatalysis: New materials and recent applications. Electrochim Acta, 2012, 84, 103–111.
[9] Bernard, V., Škorpíková, J., Mornstein, V. The combined effect of ultrasound exposure and cisplatin on human ovarian carcinoma cells A2780. Folia Biol. - Prague, 2008, 54, 97-101.
[10] Bernard, V., Mornstein, V., Bourek, A. Effect of near field ultrasound on carboplatin treatment of ovarian carcinoma cells. Indian J Biochem Bio, 2013, 50, 284-288.
[11] Rosenthal, I., Sostaric, J.Z., Riesz, P. Sonodynamic therapy-a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem, 2004, 11, 349-363.
[12] Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods, 1983, 16, 55-63.
[13] Harada, Y., Ogawa, K., Irie, Y., Endo, H., Feril Jr., L.B., Uemura, T., Tachibana, K. Ultrasound activation of TiO2 in melanoma tumors. J Control Release, 2011, 149, 190-195.
[14] ]Mihranyan, A., Ferraz, N.,Strømme M. Current status and future prospects of nanotechnology in cosmetics. Prog Mater Science, 2012, 57, 875–910.
[15] Blasco, C., Picó, Y. Determining nanomaterials in food. Trends in Analytical Chemistry, 2011, 30, 84-99.
Labels
Biomedicine
Article was published inThe Clinician and Technology Journal

2016 Issue 1-
All articles in this issue
- Optical nerve segmentation using The Active shape method
- The viability of ovarian carcinoma cells A2780 affected by titanium dioxide nanoparticles and low ultrasound intensity
- Raman label-free visualisation of Titanium dioxide nanoparticles uptake in BJ cell LINES
- NEW METHOD FOR ESTIMATION OF FLUENCE COMPLEXITY IN IMRT FIELDS
- Measuring regularity of fine upper limb movements with a haptic platform for motor learning and rehabilitation
- The Clinician and Technology Journal
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- The viability of ovarian carcinoma cells A2780 affected by titanium dioxide nanoparticles and low ultrasound intensity
- Raman label-free visualisation of Titanium dioxide nanoparticles uptake in BJ cell LINES
- Optical nerve segmentation using The Active shape method
- NEW METHOD FOR ESTIMATION OF FLUENCE COMPLEXITY IN IMRT FIELDS
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

