-
Medical journals
- Career
Critical evaluation of the optimal medical therapy in the cardiac resynchronization therapy candidates – single centre experience
Authors: D. Vondráková 1; F. Málek 1; J. Dvořák 2; L. Šedivá 1; J. Kupec 1; J. Petrů 1; M. Táborský 1; P. Neužil 1
Authors‘ workplace: Department of Cardiology, Cardiovascular Centre, Na Homolce Hospital, Prague, Head Assoc. Prof. Petr Neužil, M. D., CSc., FESC 1; Czech National Institute of Health, Prague, Head Jitka Sosnovcová, Ing. 2
Published in: Vnitř Lék 2011; 57(10): 799-802
Category: Original Contributions
Overview
The aim of the present study was to evaluate the optimal medical therapy in the chronic heart failure (CHF) patients referred from the comunity centres and the outpatients cardiology clinics for the cardiac resynchronization therapy with defibrilator (CRTD) to the Department of Cardiology, Na Homolce Hospital with the device implantation between 1st January 2008 and 30st September 2009.
Methods:
The optimal medical therapy was analysed retrospectively from the medical records of 179 consecutive CHF patients NYHA class III–IV. Beta-blockers (BB) were used only in 81% subjects referred for CRTD, ACE inhibitors (ACEI) were used only in 68% patients Angiotensin receptor blockers (ARB) were used in 18 % subjects. ACEI or ARB were used in 81%, spironolacton was use in 59%. Recommended target DD for BB (carvedilol 25 mg bid) was used only in 13% subjects, recommended target DD for ACEI (enalapril 10 mg bid) was used only in 9.4% patients.Results:
In the Department of Cardiology, the optimal medical therapy was changed after CRTD, BB were used in 95% subjects at discharge (p < 0.01) and the number of patients reaching at least of 50% of recommended daily dose (DD) of BB increased (p < 0.05). ACEI were recommended after CRTD in 80% subjects after implantation (p < 0.05), the number of patients reaching at least of 50% of recommended DD for ACEIs increased too (p < 0.05). There was no significant difference in ARB use recomended in the hospital (19% after CRTD – NS). ACEI or ARB were used in 98% patients after the device implanted (p < 0.05) and spironolacton in 77% after CRTD (p < 0.05).Conclusions:
Despite optimal composition of the optimal medical heart failure therapy only small number of CRTD candidates are reaching recommended drug dose. The optimization of the medical therapy in the specialized center lead to significantly higher proportion of CHF using the optimal therapy with the increased dose of BB and ACEI.Key words:
chronic heart failure – cardiac resynchronization therapy – optimal medical therapyIntroduction
The cardiac resynchronization therapy (CRT) with automatic defibrilator (CRTD) improves outcomes in CHF patients NYHA class III and IV, with low LV EF, wide QRS complex and/or echocardiographic evidence of cardiac dyssynchrony [1]. CRTD also reduces morbidity and mortality in less symptomatic CHF patients NYHA class I and II with low LV EF ≤ 30% and QRS duration ≥ 130 ms [2]. The effectivity of device therapy is additional to optimal medical therapy, based on sympatoadrenal and renin-angiotensin-aldosteron (RAAS) blockade [2–9]. The reaching target drug dose is very often difficult because of the adverse symptomatic hypotension and bradycardia during up-titration of ACEI and BB. According to the results of CARE-HF study, ACEIs were used in 95% of patients and BB were used in about 70% of the patients treated with active CRT. The aldosteron blockade and digoxin was used in 54% and 40% of subjects in CARE-HF study [1].
Aim of the study
To evaluate the pharmacotherapy composition and the doses in the CRTD candidates referred to the Cardiocenter and to compare the medical therapy of the CRTD candidates from the referral units with a therapy after the device implantation at the specialized center.
Patients population
The pharmacotherapy was analysed retrospectively from medical records of 179 consecutive CHF patients (pts) NYHA class III and IV, referred for the first CRTD implantation to the Cardiovascular Centre Na Homolce Hospital, Prague, Czech Republic between 1-Jan-2008 to 31-Aug-2009. The mean age of the subjects was 68.47 years, 21% of pts were women, 63% pts had coronary artery disease (CAD), the mean LV EF was 29.12%, the mean QRS duration was 155 msec and the cardiac dyssynchrony was identified in 32% of the pts. The patients characteristics are shown in the tab. 1.
1. Patients characteristics, n = 179. 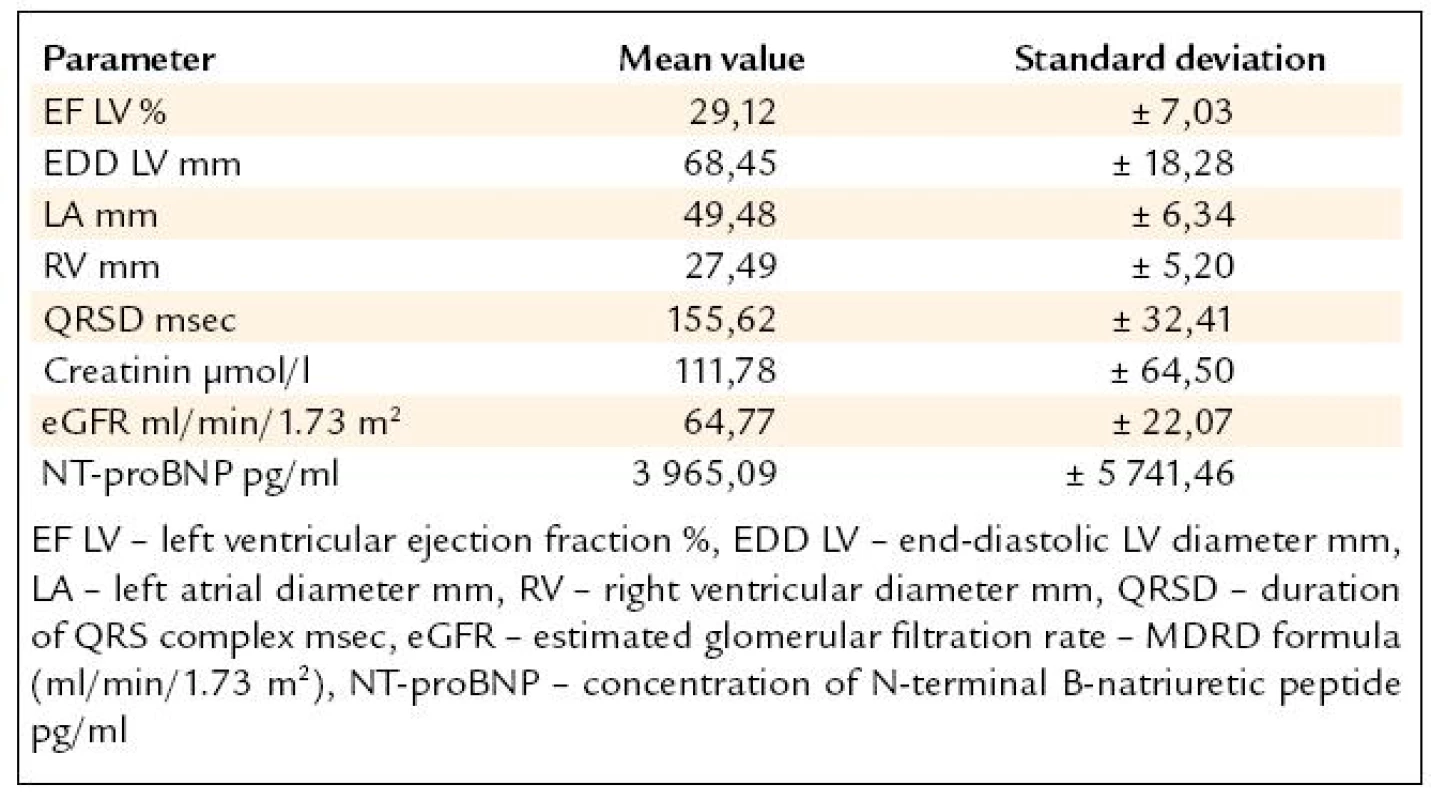
Methods
The optimal medical therapy before and after the device implantation was obtained from the patients medical records retrospectively. The therapy composition and the daily drug dose were asessed in each subject. The mean daily dose (DD) of recommended drug therapy was calculated for the standard drug (calculated daily dose CDD). The daily dose of BB was calculated for carvedilol with target dose 50 mg daily, the daily dose of ACEI was calculated for enalapril with target daily dose 20 mg daily and the ARB daily dose was calculated for losartan with target dose 50 mg daily. For the statistical analyses, unpaired t-test and McNamarra test were used with the level of significance p < 0.05.
Results
Beta-blockers (BB) were used in 81% subjects referred for the CRTD and in 95% at the discharge from the center (p < 0.01). The calculated daily dose (CDD) of carvedilol did not differ, but there was a significant increase in the number of the pts reaching at least of 50% of recommended DD (p < 0.02). ACE inhibitors (ACEI) were used in 68% patients before and in 80% subjects after the device implantation (p < 0.05). CDD for enalapril did not change, but there was a significant increase in the number of the pts reaching at least 50% of the recommended DD (p < 0.05). Angiotensin receptor blockers (ARB) were used in 18% subjects before and in 19% after the CRTD (N.S.). ACEI or ARB were used in 81% before and in 98% subjects after device implanted (p < 0.05). Spironolacton was use in 59% before and in 77% after the CRTD. The recommended target DD for BB (carvedilol 25 mg bid) was used only in 13% subjects before the device implanted, the target DD for ACE (enalapril 10 mg bid) was used only in 9.4% pts. The results are shown in tab. 2. Other therapy included: digoxin, furosemide or other diuretic, aspirin, warfarin, amiodarone, calcium channel blocker, allopurinol, statin and thyroid replacement therapy. The change of “other” medical therapy before and after CRTD is show in the tab. 3. The dose change of “other” medical therapy was not significant (N.S.). The daily dose of furosemide before CRTD was 67 mg and 59 mg after device implanted (N.S.). The daily dose for digoxin (mean 0,125 mg/day), aspirin (100 mg/day) and amiodarone (200 mg/day) was unchanged before and after CRTD.
2. Results – medical therapy before (1) and after (2) CRTD (*p < 0.05). 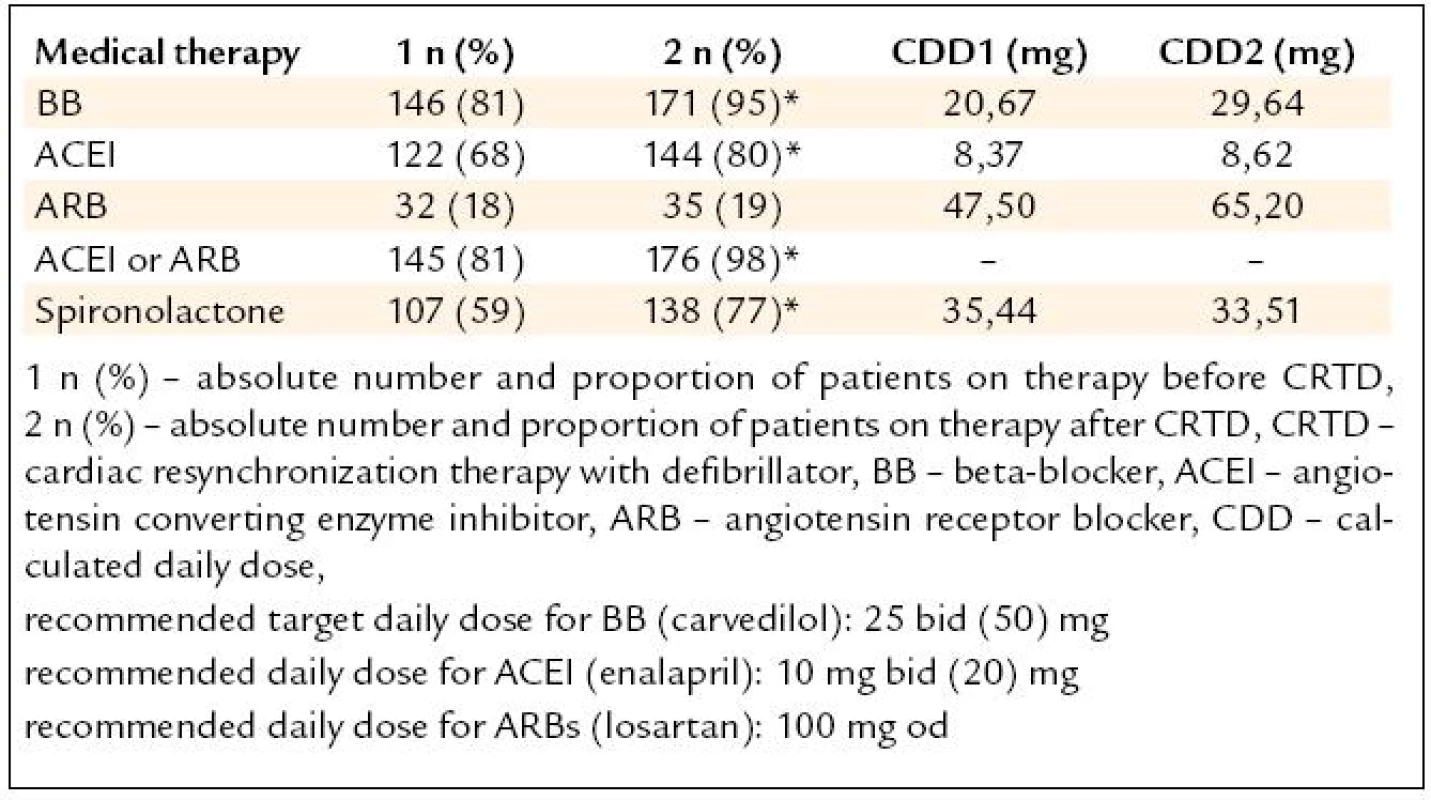
3. Results – other medical therapy before (1) and after (2) CRTD. 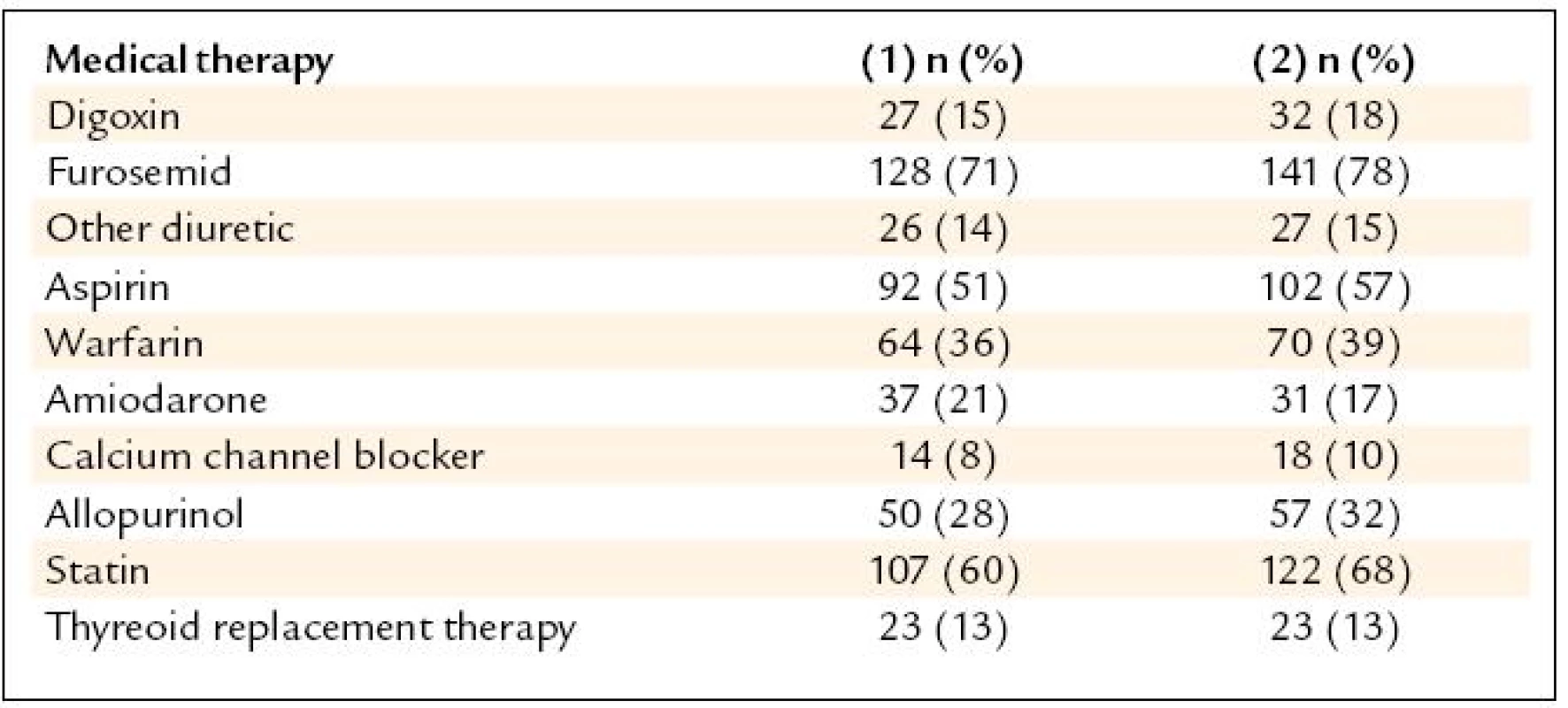
Discussion
The resynchronisation therapy is indicated for the severely symptomatic CHF patients NYHA class III and IV, with the ischemic and non-ischemic etiology, with the LV dilatation and severe systolic LV dysfunction (LV EDD > 55 mm, LV EF ≤ 35%), and QRS duration ≥ 120 msec, when the standard medical therapy is not effective. The optimal CHF pharmacotherapy is based on the drug combination with the evidence of the improvement of morbidity and mortality. The optimal drug combination should be associated with the reaching of the recommended drug dose. The dose effect of BB and ACEI was documented in clinical trials [10–13]. The up-titration of BB and ACEI to the target doses is usualy limited by the adverse symptomatic hypotension and bradycardia.
Our present study showed, that only small proportion of CHF patients referred for the CRTD reached the recommended dose of BB and ACEI despite the optimal drug composition. The reason for this under-treatment might be the concern on the risk of symptomatic bradycardia or heart failure deterioration at BB up-titration. Cardiac pacing in CRTD candidates allows BB up-titration also in very symptomatic subjects NYHA class IV, the interval between dose doubling is usualy wider in the clinical practice. NYHA class III/IV patients with advanced CHF and low cardiac output also do not tolerate up-titration of ACEI/ARB therapy because of post dose symptomatic hypotension. This could be avoided with the low dose given in the afternoons or evenings. Another reason for not reaching recommended target drug dose might be also the fact, that not all CHF patients are followed by cardiologists trained in heart failure isssues.
After the CRTD, the optimal pharmacotherapy was introduced in the significantly higher proportion of CHF patients. After the CRTD, up to 95% patients were treated with BB, but with the low daily dose calculated for carvedilol 26 mg daily. All CHF patients should be treated with RAAS blockers too. In our study, the treatment with ACEI after the CRTD was initiated in the higher proportion of CHF subjects too, with the succesfull dose up-titration. But the daily dose calculated for enalapril was also low: 8.37 mg daily. The therapy with ACEI or ARB alternatively in ACEI intolerant subjects was reached in 98% of CHF patients after the CRTD activation. The dose of ARB should be definitely higher according to results of HEAAL study [13]. Thanks to these results we are changing our practice and we are recommending to have clinical visit of the CRTD candidates at the tertiary care heart failure clinic with check-up of the optimal medical therapy.
Conclusion
Despite the optimal composition of heart failure pharmacotherapy, the only small number of NYHA class III and IV patients refered for CRTD reached recommended drug dose before the device implantation. The optimal medical therapy introduced in the specialized center was given in the significantly higher proportion of the CHF pts with the increased BB and ACEI daily dose after resynchronization pacing started. CRTD candidates should be refered for the clinical evaluation to the specialized tertiary care heart failure clinics organized at cardiocentres in advance the device implantation.
Filip Málek, M.D., Ph.D., MBA
www.homolka.cz
e-mail: filip.malek@homolka.cz
Doručeno do redakce: 24. 12. 2010
Přijato po recenzi: 10. 3. 2011
Sources
1. Cleland JGF, Daubert JC, Erdmann E et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352 : 1539–1549.
2. Špinar J, Hradec J, Meluzín J et al. Doporučení pro diagnostiku a lečbu chronického srdečního selhání ČKS 2006. Vnitř Lék 2007; 53 : 877–908.
3. Moss AJ, Hall WJ, Cannom DS et al. MADIT-CRT Trial Investigators. Cardiac--Resynchronization Therapy for the Prevention of Heart-Failure Events. N Engl J Med 2009; 361 : 1329–1338.
4. The CONSENSUS trial study group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316 : 1429–1435.
5. Granger BB, Swedberg K, Ekman I et al. CHARM investigators. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet 2005; 366 : 2005–2011.
6. Pitt B, Remme W, Zannad F et al. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348 : 1309–1321.
7. Packer M, Bristow MR, Cohn JN et al. U. S. Carvedilol Heart Failure Study Group. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996; 334 : 1349–1355.
8. CIBIS Investigators and Commities. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353 : 9–13.
9. MERIT-HF Study Group. Effect of Metoprolol CR/XL in Chronic Heart Failure. Metoprolol CR/XL. Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353 : 2001–2007.
10. Flather MD, Shibata MC, Coats AJ et al. SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005; 26 : 215–225.
11. Bristow MR, Gilbert EM, Abraham WT et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 1996; 94 : 2807–2816.
12. Rydén L, Armstrong PW, Cleland JGF et al. Efficacy and safety of high-dose lisinopril in chronic heart failure patients at high cardiovascular risk, including those with diabetes mellitus. Results from the ATLAS trial. Eur Heart J 2000; 21 : 1967–1978.
13. Konstam MA, Poole-Wilson PA, Dickstein K et al. Design of the Heart failure Endpoint evaluation of AII-Antagonist Losartan (HEAAL) study in patients intolerant to ACE-inhibitor. Eur J Heart Fail 2008; 10 : 899–906.
Labels
Diabetology Endocrinology Internal medicine
Article was published inInternal Medicine

2011 Issue 10-
All articles in this issue
- Diagnostic algorithm of syncope: integrative approach
- Treatment of acute exacerbation of the obstructive pulmonary disease with hospitalization at an Intensive Care Unit.
- The role of central nervous system in etiopathogenesis of peripheral organ diseases
- Prognostic markers in chronic lymphocytic leukemia
- Benign solitary cecal ulcer
- MR-documented remission of pituitary stalk infiltration in patients with Langerhans cell histiocytosis following treatment with 2-chlorodeoxyadenosine
- Cost of acute heart failure related readmissions
- Vaccination against hepatitis B in patients with chronic renal failure – twenty years follow-up
- Normal pulmonary circulation pressure values in healthy subjects at rest and during exercise
- Resynchronization therapy for heart failure – still many question marks
- Critical evaluation of the optimal medical therapy in the cardiac resynchronization therapy candidates – single centre experience
- Internal Medicine
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Normal pulmonary circulation pressure values in healthy subjects at rest and during exercise
- Treatment of acute exacerbation of the obstructive pulmonary disease with hospitalization at an Intensive Care Unit.
- Resynchronization therapy for heart failure – still many question marks
- Prognostic markers in chronic lymphocytic leukemia
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

