-
Medical journals
- Career
Brain natriuretic peptide is a marker of poor prognosis in decompensated liver cirrhosis
Authors: M. Radvan 1; P. Svoboda 1
Authors‘ workplace: Interní oddělení, Nemocnice Třebíč 1; Interní oddělení, Nemocnice Kyjov 2
Published in: Gastroent Hepatol 2013; 67(2): 104-110
Category: Hepatology: Original Article
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Doručeno: 30. 1. 2013
Přijato: 27. 3. 2013Overview
Background:
Patients with liver cirrhosis suffer from various cardiac abnormalities, which may influence their outcome. This condition, known as cirrhotic cardiomyopathy, is usually asymptomatic, subclinical. The aim of our study was to determine parameters associated with mortality in decompensated liver cirrhosis with particular focus on echocardiographic parameters and brain natriuretic peptide level (BNP).Methods:
For the purposes of our prospective non-intervention one-centre study we have included 61 patients with decompensated liver cirrhosis. Severity of disease was assessed according to Child-Turcotte-Pugh score and Model for End Stage Liver Disease (MELD). Cardiac functions were evaluated using echocardiography (inclusive non-invasive hemodynamic assessment and tissue Doppler measurement). BNP level at admission was recorded. In-hospital mortality and 1-year all-cause mortality were evaluated.Results:
We identified several negative prognostic markers of in-hospital and 1-year mortality: MELD score (p < 0.0001; p = 0.0007) and all it’s components, Child-Pugh score (p = 0.004; p = 0.002), age (p = 0.03; p = 0.006) and lnBNP (p = 0.001; p = 0.004). The echocardiographic parameters associated with in-hospital mortality were the left atrium diameter (p = 0.03) and higher cardiac index (p = 0.04). Both these parameters were, however, neutral in evaluation of 1-year mortality. Left ventricle diastolic dysfunction grade was found as another possible negative marker (p = 0.06 for in-hospital and p = 0.04 for 1-year mortality assessment). Prolongation of QTc was also associated with poor prognosis (p = 0.003; p = 0.002). BNP level was accompanied with hyperdynamic circulation (p = 0.05), systolic pulmonary artery pressure (p = 0.03), diastolic function of left ventricle (p = 0.01) and left atrial diameter (p = 0.02).Conclusions:
High level of BNP is associated with in-hospital and 1-year mortality of patients with decompensated liver cirrhosis. Addition of BNP to MELD or to other scoring systems could improve the prognostic accuracy. Definitive conclusion concerning BNP role in liver diseases requires further research.Key words:
cirrhosis – cardiomyopathy – brain natriuretic peptide – BNP – QTc – MELD – prognosis
According to 2006 Vital Statistics Report cirrhosis was the 12th leading cause of death in the United States [1]. Patients with liver cirrhosis suffer from various cardiac abnormalities: decrease of systemic vascular resistance, decrease of arterial pressure, increased basal cardiac output, but insufficient contractile response to stimuli. This is called cirrhotic cardiomyopathy [2]. The condition is usually asymptomatic, subclinical. It may be revealed by treatment procedures including volume overload, transjugular intrahepatic portosystemic shunt placement, liver transplantation and others [3]. Clinical impact of these hemodynamic abnormalities on prognosis of cirrhotics is unknown, but heart failure following liver transplantation is the third most frequent death cause after the procedure [4].
Liver cirrhosis is frequently associated with fluid retention, especially ascites, which is the most common complication of cirrhosis that leads to hospital admission [5] and which can strongly influence the prognosis [6,7], in particular when it is refractory to the treatment [8]. Brain natriuretic peptide (BNP) is a good marker of volume overload in patients with heart failure [9] and it is associated with the prognosis [10]. BNP level is also increased in other noncardiac clinical situations: lung cancer, renal failure [11,12], sepsis [13] and septic shock [14], and also in liver cirrhosis [15].
The aim of our study was to determine parameters associated with in-hospital and 1-year mortality in decompensated liver cirrhosis in patients with preserved left ventricle systolic function. Our study was especially targeted at echocardiographic parameters and brain natriuretic peptide level (BNP).
Patients and methods
We prospectively evaluated patients older then 18 years of age admitted to our department with decompensated liver cirrhosis (ascites or/and bleeding or/and icterus). Patients were excluded form the study if there was a history or clinical signs of cardiovascular disease, hemodynamic instability, enlargement of cardiac shadow on chest X-ray, systolic dysfunction of left (ejection fraction < 0,5) or right ventricle (tricuspid annular plane systolic excursion < 20 mm), advanced oncologic disease. In eligible patients standard clinical evaluation including body weight and height, blood pressure, pulse, 12-lead electrocardiogram (ECG), gross neurologic examination including assessment of encephalopathy was performed. Informed consent in writing was obtained from each patient, the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, which was approved by the local institutional review committee.
Conventional laboratory parameters were measured, BNP was determined by MEIA – (Microparticle Enzyme Immunoassay, AxSYM, Abbott, Illinois, USA) glomerular filtration was estimated using MDRD (Modification of Diet in Renal Disease Study) formula. For measuring the severity of disease, the Child-Pough score and Model of End Stage Liver Disease (MELD) scale was used. The presence of diabetes mellitus, hypertension and esophageal varices was monitored. In-hospital mortality and 1-year all-cause mortality were evaluated.
Echocardiography
Transthoracal echocardiography with Envisor C (Philips, MA, USA) was performed soon after the patient was admitted to the hospital, but not later then the 2nd day after admission. All studies were performed according the guidelines of American Society of Echocardiography [16]. Standard two dimensional images were obtained for the measurement of left atrium, left and right ventricular dimension, and interventricular septum thickness. Left ventricular systolic and diastolic volumes were measured and left ventricular systolic fraction was calculated by experienced echocardiographist. Diastolic function of left ventricle was evaluated using Doppler analysis of transmitral flow and pulsed wave tissue Doppler of medial and lateral part of mitral annulus in standard way [17]. Pulmonary systolic pressure was measured using the tricuspid regurgitation velocity evaluated by continuous wave Doppler.
Non-invasive hemodynamic assessment was used to determine stroke volume: measurement of left ventricular outflow tract (LVOT) from parasternal short axis assuming circular shape of LVOT; measurement of LVOT velocity and time velocity integral using pulsed wave Doppler from apical long-axis view. Cardiac index was obtained by multiplying stroke volume by heart rate and cardiac index by dividing cardiac output by body surface area. All values were established as arithmetic mean of at least three measured values.
Statistical analysis
Statistical analysis was performed using SPSS software for Windows version 13.0 (SPSS Inc., Chicago, USA). The two-tailed unpaired (between groups) and paired (inside each group) Student’s t-test and the Mann-Whitney test were used to assess differences in continuous variables between both groups. Pearson’s correlation coeficients were calculated. The BNP levels were logaritmized to achieve a normal distribution in a study sample. Normally distributed data are expressed as arithmetic mean ± standard deviation, and other quantitative data are expressed as median (range); p < 0.05 were considered to be statistically significant.
Results
A total of 81 patients were screened for the enrolment. 20 patients were excluded for one or more reasons in exclusion criteria mentioned above (6 patients with heart failure in medical history, 6 patients with heart failure newly diagnosed, 1 with acute myocardial infarction, 5 with advanced malignancy, and 2 patients for hemodynamic instability). A total of 61 patients with decompensated liver cirrhosis were enrolled. Study group consists of 20 female (33%) and 41 male (67%); the mean age 54 years (24–84). The cause of cirrhosis was in case of (a) 48 (78%) patients an alcohol abuse, (b) 5 patients (8%) chronic hepatitis, (c) 4 patients an autoimmune origin, (d) 2 (3%) patients a non alcoholic fatty liver disease, while in two cases (3%) the etiology remained unexplained. Baseline characteristics are summarized in tab. 1.
1. Baseline data. Tab. 1. Charakteristika souboru. 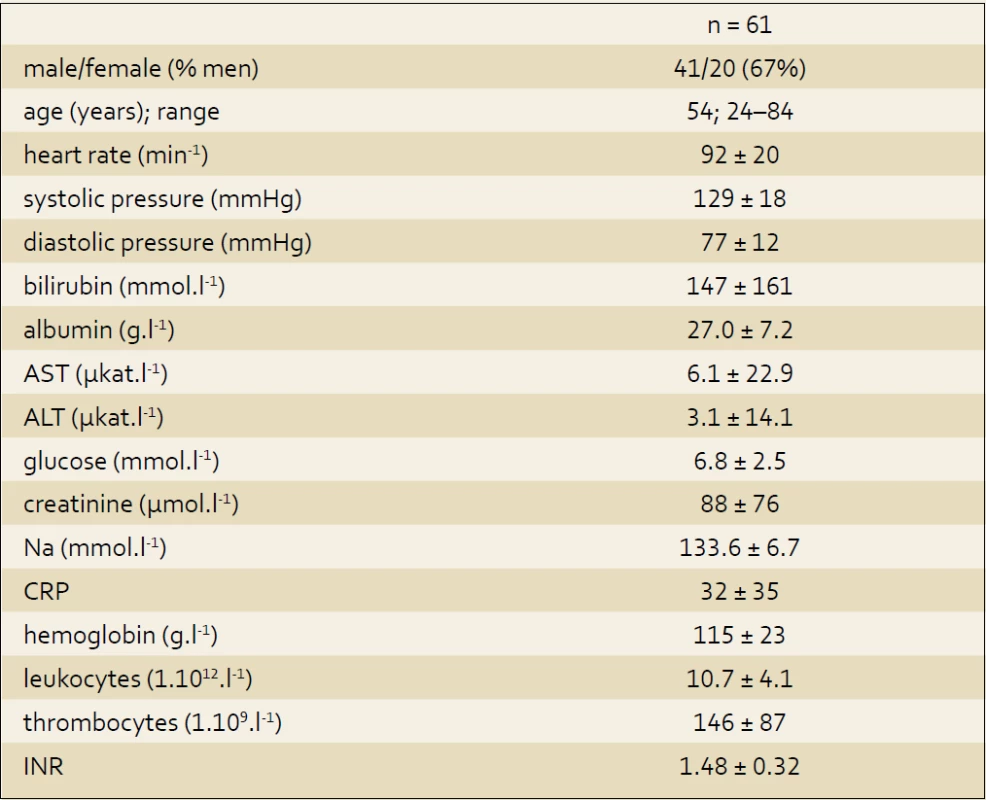
The total of 9 patients died during the hospital stay (end stage liver disease or its complications as a cause of death in all cases, by autopsy verified in 8 cases), next 10 died during one year of follow up (6 liver failures, 2 fatal bleedings, 1 sepsis, 1 bronchopneumonia). Another 3 patients have been lost to follow up.
We identified several negative prognostic markers of in-hospital and 1-year mortality: MELD score (p < 0.00001; p = 0.0007) and its components – renal functions represented by creatinine level (p = 0.0001; 0.04), coagulation by INR (p = 0.01; 0.05), and liver function by bilirubin level (p < 0.00001; 0.008). Age (p = 0.03; p = 0.006) and Child’s score (p = 0.004; p = 0.002) were also markers of poor prognosis. LnBNP was found to be a strong marker of death in decompensated cirrhosis in both evaluations (p = 0.001; p = 0.004). Lower natrium level was found to be marker of death in one year (p = 0.02), but there was no association with in hospital mortality.
From echocardiographic parameters was the in-hospital mortality associated with the left atrium diameter (p = 0.03) and higher cardiac index (p = 0.04). Both of these parameters were neutral in evaluation of 1-year mortality. Another important echocardiographic parameter – left ventricle diastolic function – was also found to be negative prognostic marker (p = 0.06 for in-hospital and p = 0.04 for 1-year mortality assessment).
Estimated systolic pulmonary pressure (PASP) in our study group was 28 ± 13 mmHg, (range 12–65 mmHg) and the PASP value was not associated with the outcome. There were 10 patients with mild, 2 patients with moderate and one patient with severe pulmonary hypertension. Prolongation of corrected QT interval on ECG was associated with poor prognosis (p = 0.003; p = 0.002) in both evaluation. Complete results are summarized in tab. 2a and 2b.
Tab. 2a. In-hospital mortality. Tab. 2a. Hospitalizační mortalita. 
Tab. 2b. 1-year mortality (all-cause). Tab. 2b. Roční mortalita. 
Higher level of BNP correlated with hyperdynamic circulation (p = 0.05), systolic pulmonary artery pressure (p = 0.03), diastolic function of left ventricle (p = 0.01) and left atrial diameter (p = 0.02). BNP level rose with age (p = 0.04) and severity of ascites. There was no correlation to albumin level (p = 0.67). Pearson’s correlations coefficients of studied parameters and BNP are summarized in the tab. 3.
2. Pearson´s correlations. Tab. 3. Korelace dle Pearsona. 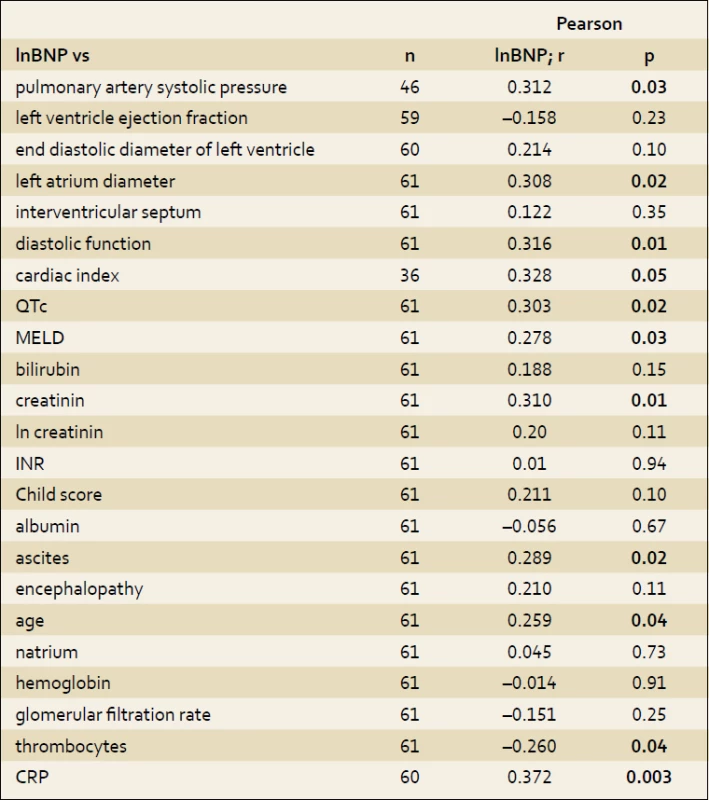
Discussion
Orthotopic liver transplantation (OLT) is a treatment of choice for end stage liver disease. The major constraint to meet the demand for transplants is the availability of donated organs [18]. The expansion of the donor pool is a major challenge for the future, but in present situation of lack of organs the selection of patients in greatest clinical need is also important. Nowadays, there are several scoring systems used for patient with liver failure considered for liver transplantation; the most widely used is Model of End Stage Liver Disease, originally developed for patients undergoing TIPS [19]. New prognostic factors for patients with decompensated cirrhosis awaiting OLT are looking for. For example, persistent ascites and low serum sodium are considered to be one of those markers of early death in cirrhosis [20]. Estimation of prognosis of liver failure is important not only for OLT, but also for the therapy of specific complications of cirrhosis, risk stratification of extrahepatic surgery, indications for TIPS and others.
In our study we found several negative prognostic factors of decompenesated liver cirrhosis. Not surprisingly, MELD score and its components were strong predictors of death in the evaluations of in-hospital and 1-year mortality. Also Child’s score and its constituents, with the exception of albumin and ascites in short term prognosis, confirmed clinical value. Low natrium level was found to be associated with earlier death only in evaluation of 1-year mortality, but was neutral for short term prediction. Another prognostic marker is age: also not surprisingly, older patients died earlier despite the same severity of liver disease.
In our study we were especially interested in echocardiographic parameters: the patients who died during index hospitalization had larger left atrium diameter (p = 0.03), which indirectly represents left ventricle filling, while diastolic function alone in short term evaluation only closely did not achieved statistical significance (p = 0.06). In 1-year assessment was the grade of diastolic dysfunction significantly associated with the mortality.
Cardiac index, as a marker of hyperdynamic circulation, was significantly higher in non-survivals (p = 0.04). In this context, we would like to point out, that there were only patients with preserved left ventricle systolic function enrolled in our study. Natural history of cirrhotic cardiomyopathy is described as early phase of hyperdynamic circulation, which is typically of a temporary nature, followed by the end stage decrease of cardiac output and subsequently death. The simple evaluation of hemodynamic as a prognostic marker is impossible.
Pulmonary artery systolic pressure or left ventricle systolic ejection fraction (LVEF) were not found to be a prognostic markers in our group preselected non-cardiac cirrhotics. Nevertheless the LVEF is not as accurate marker of systolic function as usual in other clinical conditions, because of cirrhosis induced peripheral vasodilatation, which is in fact „autotreatment“ of left ventricle dysfunction. From cardiologic point of view the situation is comparable with severe mitral regurgitation, which afford left ventricle to maintain good ejection fraction despite systolic dysfunction. Methods (and maybe also selection of studied group) we used in our study, made in fact impossible to find strong echocardiographic predictors of death. On the other hand, some morphologic and function parameters were associated with prognosis quite well. The absolute difference between groups is too small to be important in every day practice. We assume that the echocardiographic parameters in patients with preserved left ventricle ejection fraction can be so far hardly used in clinical judgement.
BNP was strongly associated with worse outcome of the patient with decompensated liver cirrhosis in both evaluations. There are several possible mechanisms standing behind. One of them is a latent heart failure, while the dysfunction of two organs simply must have worse prognosis comparing with isolated liver disease. Also the kidney dysfunction may be important, but in the present study we did not find any correlation between BNP and GFR. The other reason for higher BNP in liver disease may be the hyperkinetic circulation (which is common in decompensated cirrhosis) whereas our assumption is also supported by the results reached: BNP level rise with cardiac index. Possible mechamism may also be the secondary response to resistance to natriurteic peptide described in liver cirrhosis [21]. Altered biodegradation of natriuretic peptides due to liver failure is another potential cause, even when the major roles in metabolism of BNP play specific circulating receptors for natriuretic peptides type C and dipeptydyl-pepitidase IV [22].
Saner et al [23] have found higher mortality rate in liver transplant patients with increased serum BNP. They point out the risk of heart failure after operation, especially in previously asymptomatic patients. They establish the cut of value for cirrhotic cardiomyopathy at the level 391 ng/L, which represented the presence of diastolic dysfunction [24]. Nevertheless in our view the isolated BNP level has in fact low practical significance for estimation of severity of liver disease or the prognosis. In our study the BNP of survivors was 245 ± 396 ng/L, range 17–2,778 ng/L, comparing with non-survivors: 876 ± 787 ng/L, range 80–2,161 ng/L. Even if the difference was found to be highly statistically significant (p = 0.001 for lnBNP), looking at the wide range, it is clear, that isolated value of BNP has only low predictive value, as it is impossible to establish critical cut off value. However, although the isolated BNP has low predictive value, its implementation in complex scoring systems may improve prognostic accuracy. Another possible candidate for complex scores is QTc – routinely measured parameter easily to be derived from ECG, while long QTc is also associated with the worse outcome. Simultaneously this may be indirect warning against using drugs that may prolong the QT interval of the patients with severe liver disease.
There are not many other studies concerning natriuretic peptides in cirrhosis. Henriksen et al [25] described elevated circulating levels of proBNP and BNP of patients with cirrhosis, these studies assume cardiac dysfunction rather then hyperdynamic circulation as the major cause. Wong et al [26] also found higher BNP level in cirrhosis, just as correlation between BNP and interventricular septal thickness and left ventricle diameter. Woo et al [27] found that plasma NT-proBNP levels are higher in cirrhotic patients and they are likely to be related to the severity of disease. Fejfar et al [28] described increase of BNP level after successful TIPS creation; they conclude that hyperkinetic circulation is the major cause.
Yilmaz recently [29] found higher BNP level in case of cirrhotic patients comparing with healthy controls. Pimenta et al [30] described BNP as an independent predictor of medium-term prognosis in hospitalized patients; predictors of higher BNP were age, cardiac output and haemoglobin level. They suggest BNP in risk stratification of advance cirrhosis. Padillo et al [31] in a study using dobutamin stress ventriculography found, that high BNP level is associated with decrease of ejection fraction.
The changes of pulmonary circulation in liver cirrhosis are noteworthy as well. There are two major clinical conditions: portopulmonal hypertension (high pulmonary pressure following portal hypertension) and hepatopulmonary syndrome (pulmonary vasodilatation leading to dyspnoea) with different pathophysiology and clinical signs. The incidence of pulmonary hypertension in our study is almost the same as mentioned in the literature [32]. We found association of PASP and lnBNP (p = 0.03), which may mean that hemodynamic changes in pulmonary circulation influence BNP level or more possible, the pulmonary hypertension is rather of postcapillary etiology and influenced by the left ventricle diastolic dysfunction: PASP and diastolic function (p = 0.002). Another reason for pulmonary hypertension linked with the liver disease is hyperdynamic circulation, because we also found association between PASP and cardiac index (p = 0.02). Currently we conclude that the PH in liver cirrhosis is of multifactor origin.
Patients with liver cirrhosis suffer from various cardiac abnormalities, in papers usually called cirrhotic cardiomyopathy, and we know that these alterations are usually reversible after successful liver transplantation. Provided that the BNP is marker of these changes, and the BNP is also the marker of poor prognosis, we must take in to account, that the patients with high level of BNP should be regarded as preferential for transplant procedure.
In future the BNP may be also possible therapeutic target in decompensated liver cirrhosis: neprylisin (normally responsible for degradation of natriuretiuc peptides) may be inhibited to increase the BNP level leading to higher natriuresis and better ascites control. First experiences from patients with heart failure and hypertesnion are promising [33].
Conclusions
BNP level is a possible independent marker of death of patients with liver cirrhosis, but the isolated BNP is of little value. Also diastolic function of left ventricle and QTc can help us in estimation of individual outcome. Addition of BNP and QTc to MELD or to other scoring systems could improve the prognostic accuracy. Our data can be used as one of the sources for the appropriate calculation of regression correlations. Definitive conclusion concerning BNP role in liver diseases requires further research.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Submitted: 30. 1. 2013
Accepted: 27. 3. 2013
MUDr. Martin Radvan, Ph.D.
Interní oddělení Nemocnice Třebíč
Purkyňovo nám. 133/2
674 01 Třebíč
martinrad@post.cz
Sources
1. Minino AM, Heron MP, Smith BK. Deaths: preliminary data for 2004. Natl Vital Stat Rep 2006; 54 : 1–7.
2. Henriksen JH, Miller S. Systemic Hemodynamic Complications in Liver Cirrhosis. Scand Cardiovasc J 2009; 43(4): 218–225.
3. Baik SK, Fouad TR, Lee SS. Cirrhotic cardiomyopathy. Orphanet J Rare Dis 2007; 2: 15.
4. Rayes N, Bechstein WO, Keck H et al. Cause of death after liver transplantation: an analysis of 41 cases in 382 patients. Zentralbl Chir 1995; 120 : 435.
5. Gines P, Quintero E, Arroyo V et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology 1987; 7: 12–18.
6. Planas R, Montoliu S, Balleste B et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol 2006; 4 : 1385–1394.
7. Salerno F, Borroni G, Moser P et al. Survival and prognostic factors of cirrhotic patients with ascites: a study of 134 outpatients. Am J Gastroenterol 1993; 88: 514–519.
8. Rimola A, Garcia-Tsao G, Navasa M et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol 2000; 32 : 142–153.
9. Knudsen CW, Riis JS, Finsen AV et al. Diagnostic value of a rapid test for B-type natriuretic peptide in patients presenting with acute dyspnoe: efect of age and gender. Eur J Heart Fail 2004; 6(1): 55–62.
10. Koglin J, Pehlivanli S, Schwaiblmair M et al. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol 2001; 38(7): 1934–1941.
11. Jensen KT, Carstens J, Ivarsen P et al. A new, fast and reliable radioimunoassay of brain natriuretic peptide in human plasma, reference values in healthy subjects and in patients with different disease. Scand J Clin Lab Invest 1997; 57(6): 529–540.
12. Horl WH. Natriuretic peptides in acute and chronic kidney disease and during renal replacement therapy. J Investig Med 2005; 53(7): 366–370.
13. Shor R, Rozenman Y, Bolshinsky A et al. BNP in septic patients without systolic myocardial dysfunction. Eur J Intern Med 2006; 17(8): 536–540.
14. Ueda S, Nishio K, Akai Y et al. Prognostic value of increased plasma levels of brain natriuretic peptide in patients with septic shock. Shock 2006; 26(2): 134–139.
15. Yildiz R, Yildrim B, Karincagolu M et al. Brain natriuretic peptide and severity of disease in non-alcoholic cirrhotic patients. J Gastroenterol Hepatol 2005; 20(7): 1115–1120.
16. Lang RM, Bierig M, Devereux RB et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18(12): 1440–1463.
17. Oh JK, Seward JB, Tajik AJ. The Echo Manual, 3rd ed., Rochester 2007 : 122–1126.
18. Saab S, Han SH, Martin P. Liver transplantation. Selection, listing criteria, and preoperative management. Clin Liver Dis 2000; 4(3): 513–532.
19. Kamath PS, Wiesner RH, Malinchoc M et al. A Model to Predict Survival in Patients with End-stage Liver Disease. Hepatology 2001; 33 : 464–470.
20. Heuman DM, Abou Assi SG, Habib A. et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology 2004; 40(4): 802–810.
21. La Villa G, Riccardi D, Lazzeri C et al. Blunted natriuretic response to low-dose brain natriuretic peptide infusion in nonazotemic cirrhotic patients with ascites to avoid sodium retention. Hepatology 1995; 22(6): 1745–1750.
22. Mair J. Biochemistry of B-type natriuretic peptide-where are we now? Clin Chem Lab Med 2008; 46(11): 1507–1514.
23. Saner FH, Neumann T, Canbay A et al. High brain-natriuretic peptide level predicts cirrhotic cardiomyopathy in liver transplant patients. Transpl Int 2011
24. Maisel AS, Koon J, Krishnaswamy P et al. Utility of B-natriuretic peptide as a rapid, point-of-care test for screening patients undergoing echocardiography to determine left ventricular dysfunction. Am Heart J 2001; 141 : 367.
25. Henriksen JH, Gotze JP, Fuglsang S et al. Increased circulating pro-brain natriuretic peptide (pro BNP) and brain natriuretic peptide (BNP) in patients with cirrhosis: relation to cardiovascular dysfunction and severity of disease. Gut 2003; 52(10): 1511–1517.
26. Wong F, Siu S, Liu P et al. Brain natriuretic peptide: is it a predictor of cardiomyopathy in cirhosis? Clin Sci (London) 2001; 101(6): 621–628.
27. Woo JJ, Koh YY, Kim HJ et al. N-terminal Pro B-type Natriuretic Peptide and the Evaluation of Cardiac Dysfunction and Severity of Disease in Cirrhotic Patients. Yonsei Med J 2008; 49(4): 625–631.
28. Fejfar T, Safka V, Solar M et al. Brain natriuretic peptide: marker of portal hypertensive circulatory dysfunction? Prague hepatology meeting 2006; abstrakt: Ces a Slov Gastroent a Hepatol 2006; 60(9): 123.
29. Yilmaz VT, Eken C, Avci AB et al. Relationship of increased serum brain natriuretic peptide levels with hepatic failure, portal hypertension and treatment in patients with cirrhosis. Turk J Gastroenterol 2010; 21(4): 381–386.
30. Pimenta J, Paulo C, Gomes A et al. B-type natriuretic peptide is related to cardiac function and prognosis in hospitalized patients with decompensated cirrhosis. Liver Int 2010; 30(7): 1059–1066.
31. Padillo J, Rioja P, Muñoz-Villanueva MC et al. BNP as marker of heart dysfunction in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 2010; 22(11): 1331–1336.
32. Krowka MJ, Mandell SM, Ramsay MAE et al. A report from the hepatopulmonary syndrome/portopulmonary hypertension multicenter liver transplant data database: arterial oxygenation, pulmonary hemodynamics and outcomes of patiens “denied” versus those who underwent transplantation. Liver Transpl 2004; 9(10): 174–182.
33. Solomon SD, Zile M, Pieske B et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012
Labels
Paediatric gastroenterology Gastroenterology and hepatology Surgery
Article was published inGastroenterology and Hepatology
2013 Issue 2-
All articles in this issue
- Standard diagnostic and therapeutic process of HCV chronic infection – update
- Danis oesophageal stent in treatment of variceal bleeding
- What´s new in hepatology?
- Guidelines for anti-aggregative therapy by acetylsalicylic acid. Czech Society of Gastroenterology Statement
- Cholangiopancreatoscopy using SpyGlassTM direct visualization system: description of the method and initial experience
- Multifocal hepatocellular carcinoma imitating hepatal cirrhosis
- Surgical treatment of adrenal metastases from hepatocellular carcinoma
- Cap-assisted water immersion colonoscopy – a prospective, randomized trial
- Metastatic affection of small intestine as the first symptom of generalization of malignant melanoma
- Endsocopic treatment of Zenker´s diverticulum
- Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline
- An invasive therapy of chronic pancreatitis is indicated if pain, suspicion on malignancy, biliary tract obstruction, leak and cystoid appeared
- Health status and pancreatic cancer
- 3rd Prague Endoscopy Workshop (and 14th Endoscopy Workshop in IKEM)
- Anniversary of Professor MUDr. Marie Brodanová, DrSc.
- The Mutaflor – Escherichia coli strain Nissle 1917, serotype 06:K5:H1
- Brain natriuretic peptide is a marker of poor prognosis in decompensated liver cirrhosis
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Danis oesophageal stent in treatment of variceal bleeding
- The Mutaflor – Escherichia coli strain Nissle 1917, serotype 06:K5:H1
- Cholangiopancreatoscopy using SpyGlassTM direct visualization system: description of the method and initial experience
- Multifocal hepatocellular carcinoma imitating hepatal cirrhosis
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

