-
Medical journals
- Career
Soudně toxikologické závěry pitvy po předávkování směsí léků včetně clomipraminu, chlorpromazinu a flunitrazepamu
Authors: Hiroshi Kinoshita 1; Minori Nishiguchi 1; Shogo Kasuda 1; Motonori Takahashi 1; Harumi Ouchi 1; Takako Minami 1; Kiyoshi Matsui 1; Takehiko Yamamura 1; Hiroyuki Motomura 2; Nao Ohtsu 1; Shie Yoshida 1; Nobuyuki Adachi 1; Takehiko Ohta 1; Motoo Komeda 1; Kiyoshi Ameno 3; Shigeru Hishida 1
Authors‘ workplace: Department of Legal Medicine, Hyogo College of Medicine, 1-1 Mukogawa-cho, Nishinomiya, Hyogo, 66 -8501, Japan 1; Forensic Science Laboratory, Hyogo Prefectural Police Headquarters, 4-1, Shimoyamate -dori 5-chome, Chuo-ku, Kobe, 650-8510, Japan 2; Department of Forensic Medicine, Faculty of Medicine, Kagawa University, 1750-1 Miki, Kagawa, 761-0793, Japan 3
Published in: Soud Lék., 53, 2008, No. 3, p. 28-30
Overview
Autoři uvádějí případ smrtelné otravy clomipraminem, chlorpromazinem a flunitrazepamem. Ve femorální krvi byla kvantitativní toxikologickou analýzou stanovena koncentrace chlomipraminu 3,24 μg/ml, chlorpromazinu 0,36 μg/ml a 7–aminoflunitrazepamu (metabolitu flunitrazepamu) 0,61 μg/ml a velké množství léků bylo také zjištěno v žaludečním obsahu. Uzavřeli jsme, že příčinou smrti bylo kombinované požití clomipraminu, chlorpromazinu a flunitrazepamu.
Klíčová slova:
chlorpromazine – clomipramin – flunitrazepam – otravaIntroduction
Accidental and suicidal poisoning cases due to multiple psychiatric drug ingestion are large problems in the fields of psychiatric drug treatments and forensic toxicology. Clomipramine is a chlorinated analogue of imipramine, and its therapeutic and toxic effects are similar to those of the imipramine [2,10]. Chlorpromazine, a phenothiazine derivative, is widely used in the treatment of psychotic disorders [1]. Flunitrazepam, an analogue of nitrazepam, is used as a hypnotic agent[3]. Here we report a case of death involved the combined toxicity of clomipramine, chlorpromazine and flunitrazepam.
Case history
A 22-year-old woman (height 165 cm, weight 40 kg) with a history of eating disorders, was found dead in her house. She had been prescribed antidepressants. A number of empty packets of prescribed drugs were found near the corpse. The postmortem interval was approximately 2 days. Autopsy findings indicated no evidence of external injury. The lungs were severely congested, and pleural effusions were observed. The stomach contained 350 ml of a green-brownish fluid. Drug screening testing using a TriageTM (Biosite Diagnostic Inc, San Diego, USA) panel was positive for benzodiazepines and tricyclic antidepressants. Postmortem samples including heart blood, femoral blood, left and right pleural effusion, stomach contents and urine were collected for toxicological examination and kept at -40 °C until analysis.
Materials and Methods
Toxicological analysis was performed using a high performance liquid chromatography drug analysis system (Class-VP system, Shimadzu, Kyoto, Japan) [8]. The system operation was in accordance with the manufacturer’s specifications. Quantitation of ethanol was performed using a head-space gas-chromatography.
Results and Discussion
Toxicological analysis identified clomipramine, chlorpromazine and 7-aminoflunitrazepam, a metabolite of flunitrazepam, but no ethanol was detected in the blood. Table 1 shows the quantitation of clomipramine, chlorpromazine, flunitrazepam and its metabolite, 7-aminoflunitrazepam, in the victim’s blood, pleural effusion and stomach contents, and also summarizes their fatal and therapeutic levels [2, 5, 14, 16]. Although 7-aminoflunitrazepam was identified, no flunitrazepam was detected in the blood or pleural effusion. This observation may be due to postmortem bioconversion of flunitrazepam to 7-aminoflunitrazepam [13], and, therefore, 7-aminoflunitrazepam is an important marker of flunitrazepam usage [4].
The victim’s femoral blood concentrations of clomipramine, chrolpromazine and 7-aminoflunitrazepam were 3.24, 0.36, and 0.61 μg/ml respectively. These clomipramine and flunitrazepam concentrations exceed fatal levels [2, 5], while the chrolpromazine is within therapeutic levels [16]. As shown in Table 1, the concentrations of clomipramine and chlorpromazine in heart blood and pleural effusion were about 1.3 and over 2 fold higher than in the femoral blood, while 7-aminoflunitrazepam concentrations in the same were about 3 fold higher than in the femoral blood. These variations are due to postmortem redistribution of each drug from lung or liver [7, 9, 11] and postmortem diffusion of each drug from the stomach [12], because a more than 650-fold higher concentration of clomipramine and chlorpromazine was detected in the stomach contents than in the femoral vein, due to large amounts of those drugs being left in the stomach. It was possibly due to the cholinergic effects of clomipramine on stomach emptying and peristalsis [6, 10].
Table 1. Drug concentrations in each sample (μg/ml) and their fatal and therapeutic levels 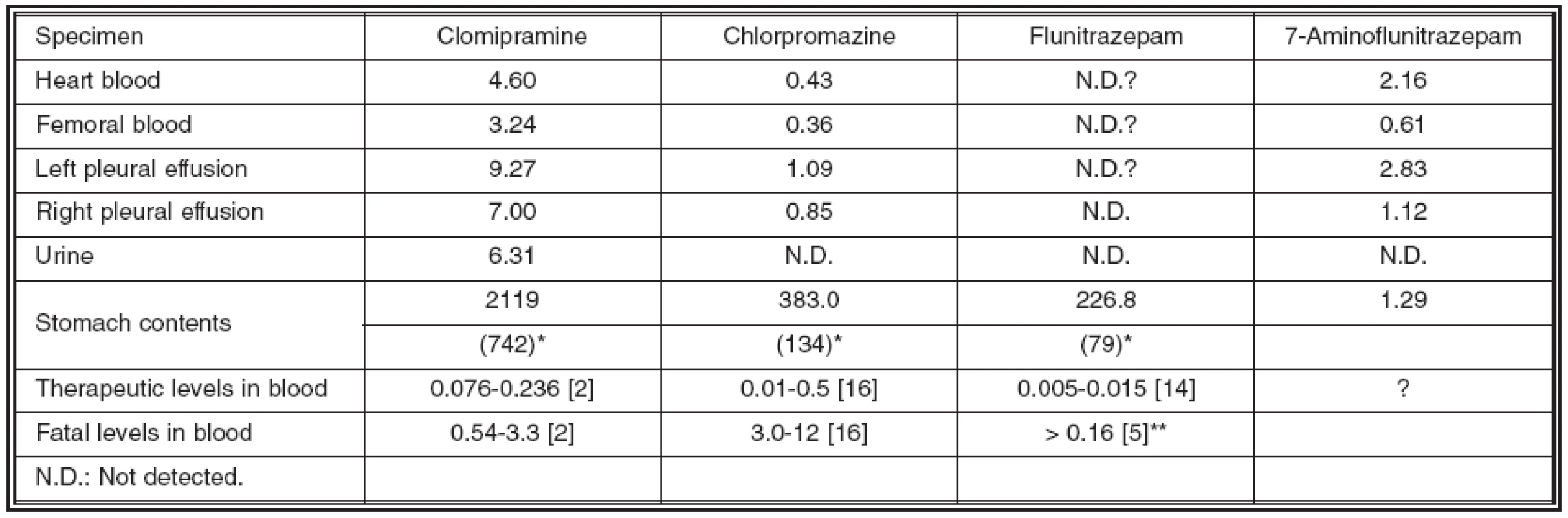
* Each figure in parentheses represents the total amount of drug in the stomach (mg). ** Combined total levels of Flunitrazepam and 7-Aminoflunitrazepam [5] In this case, it was apparent that the victim died during the absorption phase following oral ingestion, based on the detection of the quite high concentrations and large amounts of drugs in the stomach. In the absorption phase, drug concentration in the portal vein is the highest among various site of blood, and the concentration in the hepatic vein remains relatively high, even though the drug is metabolized in the liver as the first pass metabolism [15]. These concentration gradients may be one possible reason why drug concentrations in heart blood are higher than in femoral blood.
We have also estimated the victim’s total amounts of ingestion of clomipramine, chlorpromazine and flunitrazepam, using forensic toxicokinetic factors. Estimation of the antemortem blood concentration of flunitrazepam was 0.67 μg/ml, calculating from 7-aminoflunitrazepam data in the femoral blood. The calculated amounts of clomipramine, chlorpromazine and flunitrazepam, using values of the distribution volume (Vd) for clomipramine (17 L/kg), chlorpromazine (10-35 L/kg) and flunitrazepam (3.4-5.5 L/kg) [1–3], the victim’s body weight and femoral blood levels, were approximately 2176 mg, 144–504 mg and 91–147 mg, respectively. The total ingested dose of each drug is the sum of the above value and the dose left in the stomach. We therefore estimated that she had ingested at least 2910 mg of clomipramine, 278 mg of chlorpromazine and 170 mg of flunitrazepam.
From the autopsy findings and the results of the toxicological examination, we concluded that death was mainly due to the combined toxicity of clomipramine with flunitrazepam, while chlorpromazine may also have partially contributed. The present case indicates that we should pay more attention to the toxicity of combinations of multiple psychotropic drugs. We also have to consider the postmortem diffusion or redistribution of drugs for the evaluation of postmortem data.
All correspondence concerning this paper should be addressed to:
Dr. H. Kinoshita,
Department of Legal Medicine, Hyogo College of Medicine,
1-1, Mukogawa-cho, Nishinomiya, Hyogo, 663-8501, Japan
TEL: +81-798-45-6578
FAX: +81-798-49-3279
e-mail: kinochin@hyo-med.ac.jp
Sources
1. Baselt RC.: Chlorpromazine. In: Baselt RC editor. Disposition of toxic drugs and chemicals in man, (5th ed). Foster City, CA: Chemical Toxicology Institute, 2000, pp. 173-177.
2. Baselt RC.: Clomipramine. In: Baselt RC editor. Disposition of toxic drugs and chemicals in man, (5th ed). Foster City, CA: Chemical Toxicology Institute, 2000, pp. 192-194.
3. Baselt RC.: Flunitrazepam. In: Baselt RC editor. Disposition of toxic drugs and chemicals in man, (5th ed). Foster City, CA: Chemical Toxicology Institute, 2000, pp. 360-362.
4. Drummer OH, Syrjanen ML, Cordner SM.: Deaths involving the benzodiazepine flunitrazepam. Am J Forensic Med Pathol 1993; 14 : 238-243.
5. Drummer OH, Odell M.: Benzodiazepines and related drugs. In The forensic pharmacology of drug of abuse. London: Arnold, 2001, pp. 103-175.
6. Frommer DA, Kulig KW, Marx JA, Rumack B.: Tricyclic antidepressant overdose. JAMA 1987; 257 : 521-526.
7. Jones GR, Pounder DJ.: Site dependence of drug concentrations in postmortem blood – a case study. J Anal Toxicol 1987; 11 : 186-190.
8. Kinoshita H, Taniguchi T, Kubota A, Nishiguchi M, Ouchi H, Minami T, Utsumi T, Motomura H, Nagasaki Y, Ameno K, Hishida S.: An autopsy case of imipramine poisoning. Am J Forensic Med Pathol 2005; 26 : 271-274.
9. Moriya F, Hashimoto Y.: Redistribution of basic drugs into cardiac blood from surrounding tissues during early-stages postmortem. J Forensic Sci 1999; 44 : 10-16.
10. Peters MD II, Davis SK, Austin LS.: Clomipramine: an antiobsessional tricyclic antidepressant. Clin Pharm 1990; 9 : 165-178.
11. Pounder DJ, Jones GR.: Post-mortem drug redistribution – a toxicological nightmare. Forensic Sci Int 1990; 45 : 253-263.
12. Pounder DJ, Fuke C, Cox DE, Smith D, Kuroda N.: Postmortem diffusion of drugs from gastric residue an experimental study. Am J Forensic Med Pathol 1996; 17 : 1-7.
13. Robertson MD, Drummer OH.: Postmortem drug metabolism by bacteria. J Forensic Sci 1995; 40 : 382-386.
14. Schulz M, Schmoldt A.: Therapeutic and toxic blood concentrations of more than 800 drugs and other xenobiotics. Pharmazie 2003; 58 : 447-474.
15. Shinohara T, Ijiri I, Fuke C, Kiriu T, Ameno K.: Studies on ethanol absorption from the intestine - blood ethanol and acetaldehyde concentrations in the various vessels. Arukoru Kenkyuto Yakubutsu Ison. 1992; 27 : 71-80.
16. Winek CL, Wahba WW, Winek CL Jr, Balzer TW.: Drug and chemical blood-level data 2001. Forensic Sci Int 2001; 122 : 107-123.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inForensic Medicine

2008 Issue 3
Most read in this issue- Poranění brokovou zbraní – jednotné střely
- Soudně toxikologické závěry pitvy po předávkování směsí léků včetně clomipraminu, chlorpromazinu a flunitrazepamu
- Využitie biochemických markerov v diagnostike náhlej srdcovej smrti
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

