-
Medical journals
- Career
First evaluation of completeness and sensitivity of the measles surveillance system in the Czech Republic, January 1, 2018 until June 30, 2019
Authors: M. Liptáková 1,2; M. Špačková 2; V. Príkazský 2; R. Limberková 2; S. Repelová 2; H. Orlíková 2; S. Balasegaram 3; J. Částková 2
Authors‘ workplace: European Programme for Intervention Epidemiology Training, European Centre for Disease Prevention and Control, Stockholm, Sweden 1; National Institute of Public Health, Prague, Czech Republic 2; UK Health Security Agency, London, United Kingdom 3
Published in: Epidemiol. Mikrobiol. Imunol. 71, 2022, č. 2, s. 109-117
Category: Original Papers
Overview
Aim: The aim of study was to evaluate completeness and estimate sensitivity of the measles surveillance using the new electronic version of the national notification system of infectious diseases (ISIN) in order to assess its performance.
Material and Methods: The completeness of measles reporting in the ISIN for demographic characteristics (week and region of reporting, age and gender), date of onset, complications, hospitalisations, vaccination status, used laboratory methods and country of import from January 2018 to June 2019 was assessed. The register from National Reference Laboratory (NRL) and the ISIN were compared using the capture-recapture method (CRM). Cases were matched using unique personal identifier. The total number of measles cases in the population was assessed using the Chapman’s formula. Sensitivity of reporting was calculated by dividing the number of reported cases by the CRM estimated true number of cases.
Results: In the ISIN, 765 measles cases were registered within specified time period. For many variables 100% completeness was found. The data were missing mainly for vaccination status (20%), serology results (55%) and used laboratory methods (8%). The NRL confirmed 653 patient samples in respected period. Within both registries (ISIN and NRL) the total 612 cases were matched. Estimated real number of measles cases using the CRM was 816 (95% CI: 809-823) compared to 806 reported cases. The estimated surveillance system sensitivity was 98.8%. Five percent (n = 41) of cases tested positively in the NRL were not reported to the ISIN.
Conclusions: We found high level of reported measles data completeness in the ISIN for most variables. Estimated real and reported number of cases was in a good correlation and calculated sensitivity of the ISIN was on very high level. Though, the data sources used in the study were not independent on each other, therefore results may not be fully accurate. The technical changes (more mandatory fields and more logical syntax to check data) in the ISIN to improve data completeness are being recommended. Data providers should report all measles cases to the ISIN with maximum precision in entering individual variables and investigating laboratories should send samples for confirmation to the NRL in required cases.
Keywords:
surveillance – measles – applied epidemiology – capture-recapture method – analytical study
INTRODUCTION
Measles is an acute, highly contagious viral disease capable of causing large epidemics mainly in unvaccinated or under-vaccinated populations. Measles virus (Morbillivirus) is transmitted from person to person via airborne route, or by direct contact with nasal and throat secretions of infected individuals. Infectivity is in susceptible individuals close to 100% and globally, measles remains a leading cause of childhood deaths even though a safe and cost-effective vaccine is available. In 2018, there were more than 140 000 measles deaths globally, mostly among children under the age of five [1]. Immunization is the only effective preventive measure against measles and eliminating the disease remains one of the top immunization priorities for the World Health Organization (WHO) European Region [2]. In 2017, the EU/EEA member states experienced a resurgence of measles with the majority of cases (87%) occurring in gaps in cohorts of individuals that missed-out vaccination [3]. In the Czech Republic, cases increased from 0 in the year 2010 to 146 cases in 2017 (notification rate per 1 000 000 population was 13.8), 207 cases in 2018 (notification rate 19.5), 590 cases in 2019 (notification rate 55.4) and decreased to only 4 cases in 2020 (notification rate 0.4) [4, 5]. Increased number of measles cases was seen due to measles outbreaks [6, 7]. The European Regional Verification Commission concluded in 2018, that the transmission of measles virus was re-established in the Czech Republic [8]. The reasons for decreasing vaccination coverage [9–11] are most probably parental refusal of vaccination [12, 13] which is also influenced by anti-vaccination activities [14]. Further, there is impact of waning immunity [15]. To combat the disease in future, the stable immunization programs and surveillance systems are key elements. Special emphasis should be put on information of possible disease importation, travel history within the considerable incubation period, and on the vaccination history.
In the Czech Republic, former Czechoslovakia, a long - -term measles surveillance program was introduced simultaneously with implementation of mandatory measles vaccination program into the National Immunization Program in 1969 [16, 17]. Nowadays, since 1st January 2018 the two-dose vaccination regimen is scheduled for children 13–18 months and 5–6 years of age. Reporting of infectious diseases (including measles) is mandatory [18]. The current measles surveillance system in the Czech Republic consists of comprehensive, nationwide, system which is case-based and harmonised with the EU legislation [19] and according to the Decree 473/2008, Annex 4 – measles [20]. New version of electronic Czech national web-based notification system of infectious diseases “Information system of infectious diseases” (ISIN), that replaced previous electronic system called EpiDat, was launched on 1st January 2018. Healthcare providers take biological material and ensure its transport to the laboratory. The investigating (regional) laboratory examine the samples and, in the case of a laboratory-confirmed case, arrange that a serum aliquot is sent to the National Reference Laboratory (NRL). Healthcare providers, those who first diagnose the disease, are obliged to report cases to one of 14 Regional Public Health Authorities (RPHAs) that further perform epidemiological investigation, including control of vaccination, in order to determine the source of the infection and the route of the transmission. RPHAs report the case-based information into the ISIN. Confirmation of measles cases is performed by the NRL for measles, mumps, rubella and parvovirus B19. Internal data validity is generally controlled for the first time thoroughly at data entry into the system: e. g. date of onset cannot be set later than date of notification, invalid dates cannot be uploaded and duplicities shall be checked automatically based on the unique personal identification number (UPIN).
The National Institute of Public Health (NIPH) validates the data and export them to the TESSy (The European Surveillance System) on monthly basis and provides annual analysis to the WHO and to the Ministry of Health of the Czech Republic (MoH). Monitoring data validity within the surveillance system is extremely important in order to avoid false alarms and unnecessary public health interventions [21, 22]. Surveillance reflects the actual epidemiological situation and appropriate public health strategies are designed accordingly.
The aim of this study was to evaluate data quality by measuring sensitivity, completeness, and validity of measles surveillance system in the Czech Republic using the capture-recapture method (CRM) and to make recommendations for improvements if necessary.
MATERIAL AND METHODS
The measles data maintained within period 1st January 2018 to 30th June 2019 in ISIN were analysed. The population under surveillance was the whole Czech population using annual census population data from the Czech Statistical Office (CSO).
Descriptive analysis of the data was performed. A case was defined as any patient with clinical symptoms of measles reported to the ISIN under the the 10th revision of the International Classification of Diseases (ICD-10) code “B05” with reporting week between 1st January 2018 to 30th June 2019, based on 2008 EU case definitions. The case is defined as 1) possible: any person meeting the clinical criteria; 2) probable: any person meeting the clinical criteria with an epidemiological link; 3) confirmed: any person not recently vaccinated and meeting the clinical and the laboratory criteria [19]. All cases compliant with the EU case definition were retrieved and analysed during the period under the study. Demographic characteristics, i. e. age, gender, region of reporting (based on place of residence), place of isolation and complications, vaccination status, number of vaccine doses administered, date of vaccination, date of laboratory testing, laboratory results of serologic tests and imported/autochthonous cases were analysed using absolute numbers and relative frequencies. Data for laboratory methods were retrieved solely from the ISIN, therefore we mention only the most frequent results.
Completeness of ISIN data was measured as proportion of completed (having a valid value) and unfilled (missing) variables. Following variables were checked: date of onset, week of reporting, patient’s age, gender, region of reporting, place of isolation and complications, vaccination status, number of vaccine doses if vaccinated, date of vaccination, date of laboratory testing, laboratory results of serologic tests, genotyping if performed, outbreak/sporadic cases, type of case (imported, autochthonous) and travel history (country of import).
The capture-recapture method was used to assess the total number of cases. The capture probabilities of cases within each source were estimated. To identify common cases, we compared the two data sources using the UPIN as a common identification key to match the data from the ISIN and the NRL. Two‐list CRM technique was used as illustrated by the Venn diagram (Figure 1).
Figure 1. Schematic description of the distribution of the total number of cases in a two-source capture-recapture model (Venn diagram) [24] ![Figure 1. Schematic description of the distribution of the total number of cases in a two-source capture-recapture model
(Venn diagram) [24]](https://pl-master.mdcdn.cz/media/image_pdf/569c905a6264c192cd6f7d9ca54c174b.jpg?version=1659511168)
N – the total number of cases in the population
L1 and L2 – the number of cases captured by each one of the two systems
m – the number of missing cases from both systems
d – the duplicatesThe total number of measles cases in the population (N) was calculated manually by Chapman’s formula [23]:
Where L1 is the number of cases in the NRL register, L2 is the number of cases reported to ISIN, and d (duplicates) is the number of cases captured by both systems. 95% CIs was calculated using:
The system sensitivity was assessed through estimations of the total cases in the population under surveillance using CRM. Data sources were the ISIN and the NRL register (samples of measles cases sent from regional laboratories to the NRL confirmation testing and genotyping). The data were matched using the UPIN. Based on the UPIN, by name and surname we manually compared number of cases from the ISIN and from the NRL register. The sensitivity of reporting was calculated as the proportion of reported cases to the estimated true number of cases according to the formula: Se (%) = N/NE x 100; where Se = estimated sensitivity, N = number of reported cases, NE = estimated true number of cases calculated by the CRM [24].
RESULTS
Within the period from 1st January 2018 to 30th June 2019 the total of 765 measles cases, including 715 confirmed, 28 probable and 22 possible cases based on the EU case definition were reported in the ISIN. The descriptive analysis of demographic characteristics of the patients with measles revealed that by gender 404 (53%) cases occurred in males while 361 (47%) in females. Age range of cases in the ISIN was 0–75 years. The most affected age groups were adults 30–39 years in 195 (25%) cases and 40–49 years in 196 (25 %) cases, while adults aged 20 years and above accounted for 75% of the cases, 676 (88%) cases were of autochthonous origin while 89 (12%) cases were imported. As a country of import was reported: Ukraine in 57 of 89 imported cases (66%), Vietnam 5 (6%), France 4 (5%), Austria 2 (2%), Greece 2 (2%), Slovakia 2 (2%), Thailand 2 (2%), Egypt 1 (1%), Georgia 1 (1%), Germany 1 (1%), Hungary 1 (1%), India 1 (1%), Israel 1 (1%), Madagascar 1 (1%), Myanmar 1 (1%), Poland 1 (1%), Russia 1 (1%), Serbia 1 (1%), Switzerland 1 (1%) and United Kingdom 1 (1%).
Each report identified UPIN, included date of onset, week of reporting in the ISIN, patient’s age, gender, region of reporting, imported/autochthonous case and in these variables 100% completeness of 765 reported cases was found. Among 2 imported reports (2%) no travel history was mentioned. In 698 (91%) of cases no complications were reported. In 36 cases (5%) other complications (diagnosis B05.8 based on ICD-10), in 16 (2%) pneumonia, in 9 (1%) intestinal complications, 5 (< 1%) otitis media and 1 case (< 1%) meningitis were described. Of total 726 cases with known history of hospitalisation (95% of all cases), 512 (70.5%) were hospitalised at infectious diseases ward and 10 (1%) in other health care facility. 204 (28%) cases were isolated at home. Measles vaccination status was reported in the ISIN as: “yes = vaccinated” in 309 (40%) cases, “no” in 308 (40%) and a missing value was in 148 (20%) cases. Any vaccine dose was reported in 244 (32%) cases in the ISIN (Tab. 1). Date of vaccination was available for 49% (152/309) of vaccinated cases. The cases were reported from all regions of the CZ. The regional distribution in the ISIN was as follows: City of Prague (78 cases), Pardubice Region (61), Moravian-Silesian Region (54), Hradec Králové Region (53), Central Bohemia Region (48), Pilsen Region (36), South Bohemian Region (17), Southern Moravia Region (11), Liberec Region (5), The Ústi Region (4), Olomouc Region (4), Vysočina Region (3), Zlín Region (2) and Karlovy Vary Region (1).
1. Measles cases in the Czech Republic, age group and vaccination status, ISIN, January 1, 2018 until June 30, 2019 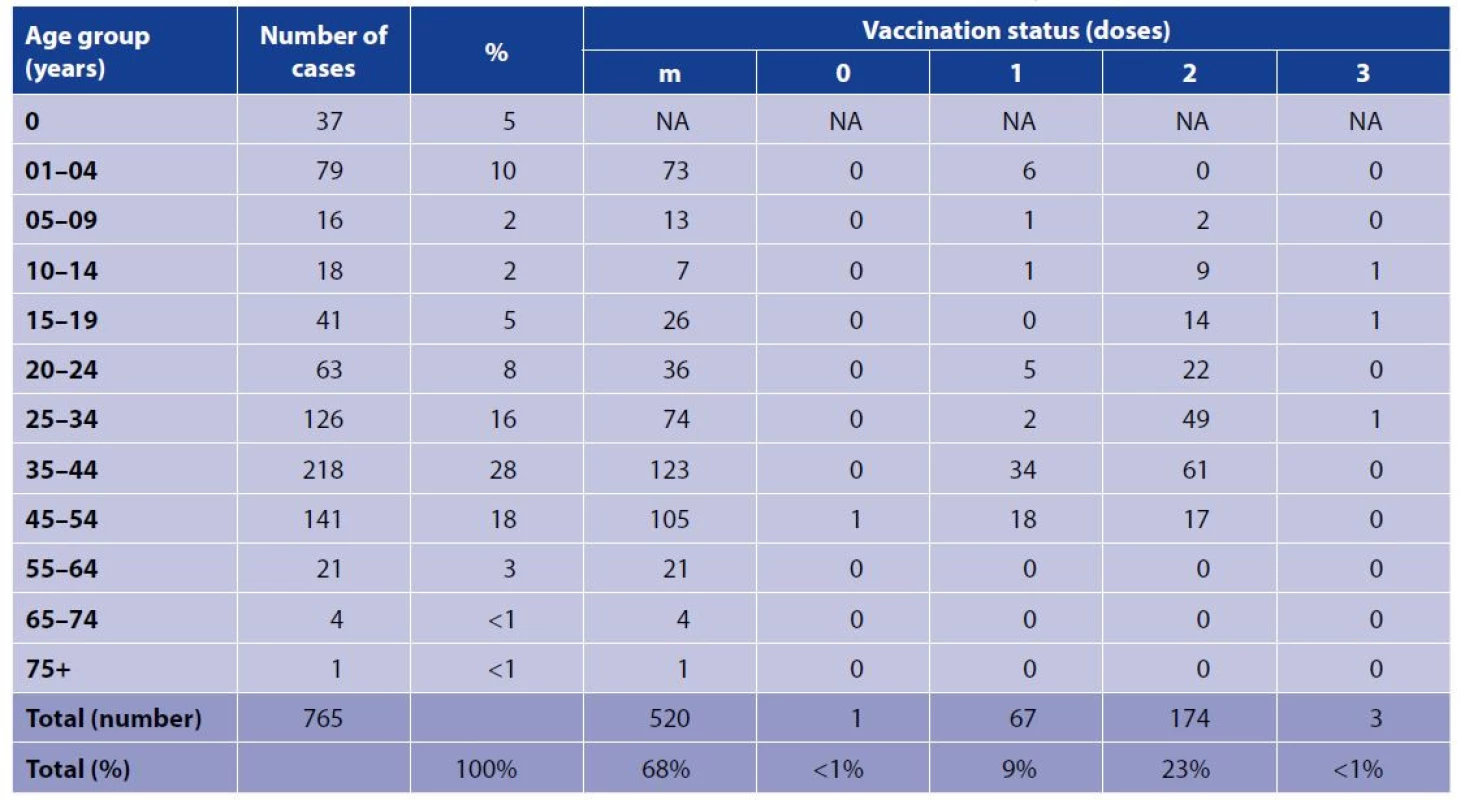
m = missing data
NA = not applicable
ISIN = Information system of infectious diseasesDate of laboratory testing was filled in the ISIN in 752 (98%) cases. Most tested laboratory specimen were serum in 309 cases (41.5%, n = 744) and nasopharyngeal swab in 216 cases (29%, n = 744). Any laboratory method was reported in the ISIN in 706 measles cases (92%). The cases were tested mainly by the Polymerase Chain Reaction (PCR) method in 282 (40%) and Enzyme-Linked ImmunoSorbent Assay (ELISA) in 272 (38.5%). Total of 375 cases (50%, n = 752) were confirmed in the NRL, 377 cases (50%, n = 752) were confirmed in regional laboratories from all regions in the Czech Republic and in 13 cases the information was missing. The results of serologic testing were positive in 668 cases (94%, n = 712), borderline in 4 cases (< 1%), negative in 28 cases (4%) inconclusive in 12 cases (2%) and missing in 53 cases (7%), however, all these cases were reported in the ISIN. The most missing data were on IgM or IgG serology (55% and 56% respectively). In total, 387 outbreak related cases (originating from five outbreaks) and 378 sporadic cases were reported in the ISIN (Tab. 2). Information about genotypes D8 and B3 are mentioned based on data available in the ISIN and in the NRL register.
2. Number of reported measles cases by reported source, genotype, region and epidemiological settings in the Czech Republic, January 1, 2018 until June 30, 2019 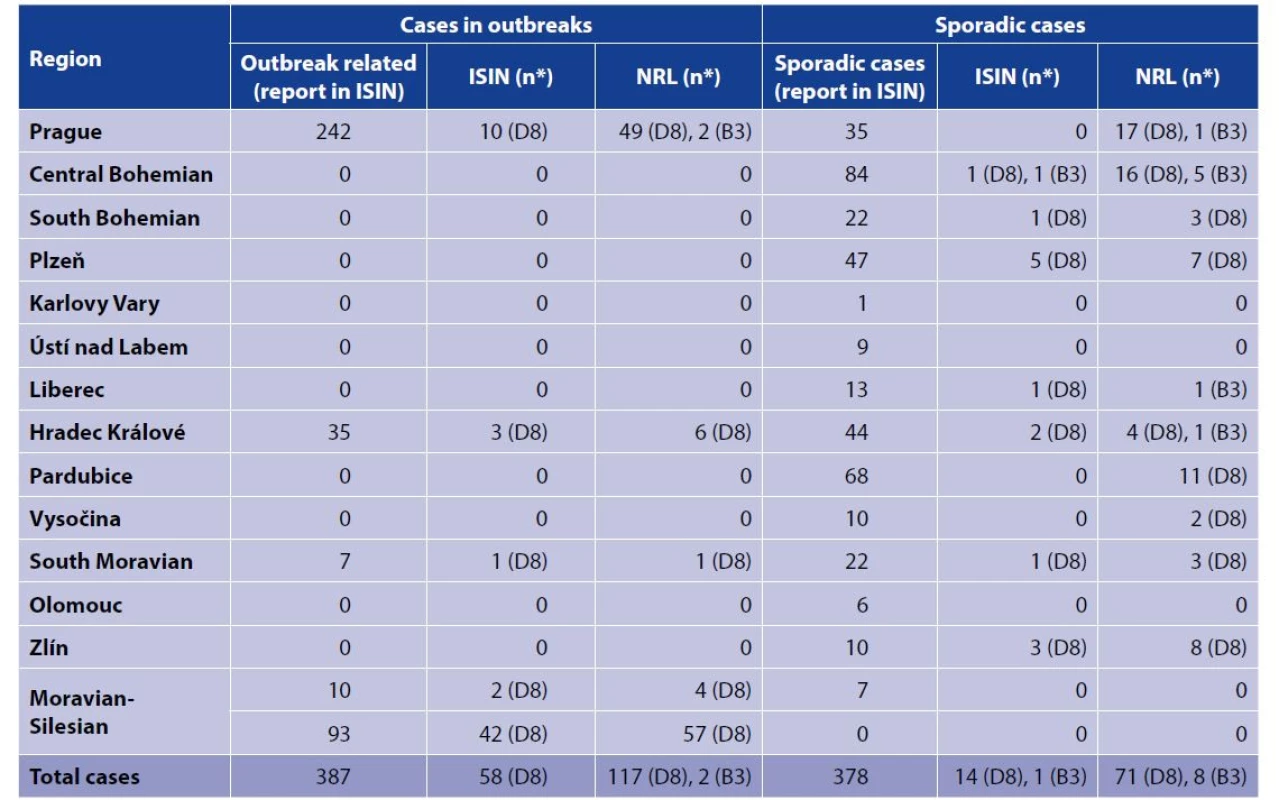
(*) = genotype in brackets
ISIN = Information system of infectious diseases
NRL = National Reference LaboratoryIn total, 653 (L1) measles cases (samples) were sent to the NRL and 765 (L2) was the number of measles cases reported to the ISIN. The number of matched cases was 612 (d). In 2018, 207 measles cases were reported to the ISIN, of which 31 (15%) were not tested in the NRL. From January to June 2019, 558 cases of measles were reported to the ISIN, of which 122 (22%) cases were not tested in the NRL (Tab. 3). Using CRM we estimated 816 (95% CI: 809-823) as the true number of measles cases. Actual number of reported cases was 806. The overall estimated sensitivity of surveillance of measles cases was 98.8%. Five percent (n = 41) of cases tested positively in the NRL were not reported to the ISIN.
3. Number of reported and estimated measles cases in the Czech Republic according to reporting source (ISIN, NRL), January 1, 2018 until June 30, 2019 [22] ![Number of reported and estimated measles cases in the Czech Republic according to reporting source (ISIN, NRL), January
1, 2018 until June 30, 2019 [22]](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image_pdf/373309e7b010c39dff1ff33ce9a8c743.jpg)
(+) captured by this source
(–) not captured by this source
ISIN = Information system of infectious diseases
NRL = National Reference LaboratoryDISCUSSION
The main purpose of public health surveillance is to provide complete data regarding the disease burden in the respective population, create alerts and warnings and to show, where the public health response is highly appreciated. The assessment of the data should be performed at the timely manner. The conclusions and recommendations derived should be evidence-based and as such be presented to stakeholders and decision makers. Based on such data, decisions towards improving public health and their jurisdiction shall be made [25, 26]. Due to administrative problems in legislation process, the EU case definition 2012 and the EU case definition 2018 have not been implemented in the Czech Republic yet. Therefore, in the Czech Republic is still valid legislation based on EU case definition 2008.
ECDC reports that the age-specific notification rates decreased with increasing age, with unvaccinated children < 1 year and aged 1–4 years most affected, while adults aged 20 years and above accounted for 36% of cases [27]. Our study showed different findings, because adults aged 20 years and above accounted for 75% of cases. This is a very typical picture in populations with good vaccine coverage. Any vaccine dose was reported in 244 (32%) cases in the ISIN. In ECDC report, 11% of cases were vaccinated with one dose of measles, mumps and rubella (MMR) vaccine, vs. 9% in our study; 7% with two doses vs. 23% in our study and < 1% had been vaccinated with an unknown number of doses [27]. Our records are based on case report, so maybe prone to error (compared to a vaccination register). In our study in 21% (65/309) of vaccinated cases the number of vaccine doses was not reported. It is important to note that surveillance systems as well as the proportion of laboratory confirmed cases in EU/EEA Member States are heterogeneous, and direct comparisons between countries should be made with caution.
Completeness of the reporting is an important attribute to achieve this objective. In the real world however, it is a very challenging task to capture all cases of a disease in the population; therefore, evaluation of completeness is required on a regular basis [24, 26]. Percentage of measles routine surveillance reports submitted from subnational to national level for countries in European region reporting case-based surveillance data monthly that meet surveillance indicator completeness has been improved from 75% in year 2009 to 100% in year 2018 [28]. In time 1. 1.–30. 6. 2019 in ISIN in total were reported 558 measles cases. We declare that 590 measles cases were reported in the Czech Republic in the year 2019. It means that 94% (558/590) of measles cases reported in ISIN in the year 2019 were notified during our period. Data completeness in our study was similar to results of Italian study where completeness was >98% for mandatory variables, the incomplete variables were 67% for ‘laboratory results of serologic test’ and 76% for ‘sampling date’ [29]. Authors in South Africa compared laboratory surveillance of measles with the notifiable diseases surveillance system; fewer cases were notified than confirmed in the laboratory [30]. Completeness for the laboratory system for measles was (63% vs. 47%) [30]. In our study the data completeness was on a high level for tested variables, except for vaccination status data, laboratory methods and results of IgM and IgG serology, where is potential for improvement.
Some discrepancies were noticed in laboratory methods which had incorrect information uploaded in the ISIN, e.g.: PCR is about the same as proof of RNA virus; the complement-fixation reaction should belong to serology method. In six cases was written “other laboratory method”, including a one case “CLIA” (chemiluminescent immunoassay), two cases serology method and three cases antibodies against measles. In three cases laboratory method was filled in as unknown, but for two cases in result section in the ISIN was written IgM and IgG positive and for the last case “laboratory-confirmed measles disease”. Due to the fact laboratories do not have direct access to the ISIN, these discrepancies in the ISIN may have occurred due to the rewriting of laboratory data. Moreover, a laboratory, including regional ones, was mentioned several times with different names or abbreviations. Room for improvement remains for using standardised laboratory register to implement to the ISIN. It is easier to use a laboratory name register (as a default set up) than a free text option, which complicates the data analysis process.
Capture-recapture method is based on the amount of overlap in two or more samples (or lists) of the target population; the greater the overlap of unique individuals in multiple samples, the smaller the unobserved population [31]. For using two lists capture-recapture method to obtain a valid population estimate, three conditions are assumed: for each list, the probability a case is recorded on the list is equal for all cases; the probability of being recorded on a list is independent of the probability of being recorded on the other list; and there is no immigration or emigration to the population during the study period. Even if these assumptions are violated, as in our study, Hook and Regal suggest the estimates may still be valid [32]. To our knowledge, this is the first CRM study dealing with measles reporting system completeness and sensitivity performed in the Czech Republic. Five percent of measles cases which were tested in the NRL have not been reported in the ISIN. Using the CRM, we did not detect significant underreporting. Difference between reported versus estimated cases was found only in the period January to June 2019 (Tab. 3). We have no scientific evidence of what could have caused this difference. It could be caused by the highest number of cases reported in this period, including outbreak cases. In the CRM in the analysis of a measles epidemic authors in Romania revealed that due to the careful pairing of the cases, it is unlikely that there were errors of overestimation [33]. In an addition this method demonstrates the possibility of having unreported cases through the measles surveillance system, which in the study in Romania identified only a third out of all estimated existing cases [33]. The overall estimated sensitivity of surveillance of measles cases in the Czech Republic was 98.8%. This result is satisfying and was higher than demonstrated the study in Italy when evaluation of the integrated measles and surveillance system sensitivity was 82% [29] and 50% in South African study [30].
Limitations. Two‐list CRM is simple analytical method, which is vulnerable to serious errors depending on extent of independence of used lists. If the lists are positively dependent (largely overlap), then results might underestimate real problem size. Conversely, negatively dependent lists (with very little overlap) may end up with overestimation of results. The sources used in this study were dependent on each other, therefore bias toward underestimation might be present. The NRL might have some data on the possible and probable cases that were not confirmed. Cases reported to the ISIN might more likely be found in the NRL register than otherwise. Further, amount of samples from regional laboratories not sent to the NRL was unknown as zero reporting is not set up. Serology was expected to be performed in approximately 60 regional laboratories based on the External Quality Assessment (EQA) done by the NRL. In some clinical cases of measles, paired sera were not available in the NRL. Regional laboratories are not obliged to send negative sera for confirmation therefore some information might be missed. The surveillance data are generally subject to further limitations. Surveillance underestimate actual disease incidence as not all patients seek a medical care [34]. In agreement with German study [35], our results confirm that measles are under-reported (5%) even in mandatory surveillance system, particularly when measles cases are sporadic. Another important application for this method is in epidemiology for estimating prevalence of a particular disease and estimating the completeness of ascertainment of disease registers. However, the CRM can principally be applied to any situation where there are two or even more incomplete lists [36]. In some of cases in the ISIN were reported that case was vaccinated but there was not more information about the vaccination. The vaccination data for patients with unknown vaccination status could not be further verified. There is no vaccination register established in the Czech Republic. Depending on the date of birth, we can only assume that those who were born in the period 1969–1989 were vaccinated according to at that time valid vaccination calendar, but these older data on vaccination are not reflected in the ISIN.
CONCLUSIONS AND RECOMMENDATIONS
Surveillance systems require ongoing maintenance and evaluation if the data that result from them are to be accurately interpreted. Efforts should be made to improve incomplete variables, in order to comply with the WHO’s Plan for measles and rubella elimination goal. The CRM represents the cost-effective way to estimate the real number of measles cases in the population, in a short time frame and without necessity of using many resources [33]. Additionally, it allows estimating number of unreported cases within the surveillance system, which was in our study identified as 1.2%. True number of measles cases in the population was estimated using the CRM in this study.
Maintaining high level of data quality within surveillance system is one of the crucial points to achieve the global goal of eliminating measles. Physicians can play a key role in two ways: stress the importance of measles vaccination while communicating with parents and by fair and thorough reporting of all measles cases to the appropriate health authority [35]. RPHAs are the main key stakeholders to provide first data quality checks, data entry and regular evaluation at the regional level. We recommend set up quarterly report reminders from the NIPH to regional epidemiologists to inform about the current measles epidemiological situation and needs, similar as is already done within invasive pneumococcal diseases surveillance in the Czech Republic [24]. A major challenge is to raise awareness of the importance of data completeness, further improving of the data quality, as proposed by Italy [29]. Regional laboratories participating on measles reporting shall be informed about the necessity to include zero measles reporting to the RPHAs and then to the NIPH, similarly as it is performed for zero polio reporting in the Czech Republic. This proposal shall be discussed with stakeholders and approved by the MoH of the Czech Republic (the main decision maker). The WHO recommend submitting measles surveillance reports, including “zero” reports at least on monthly basis [37, 38]. We suggest that regional laboratories should be provided with access to enter the results into the ISIN. This proposal shall be discussed with stakeholders and approved by the MoH of the Czech Republic.
As health-care services and information technology continue to evolve, the possibility exists for numerous changes in conducting routine public health surveillance. Technical changes of the inner mechanism in the electronic system ISIN must ensure higher data completeness (e. g. more mandatory fields or more logical syntax to check the data). Ongoing evaluation of disease-reporting completeness will continue to be a necessary part of public health surveillance, enabling more accurate interpretation of surveillance information for disease control and prevention [25].
Conflict of interest: The authors declare that they have no competing interests.
Funding: None.
Ethical approval: Not applicable.
Authors contributions
ML conceived designed the study in cooperation with VP and SB. ML and SR performed comparing two data sources and RL supplied genotyping data. ML analysed data including all calculations and interpretations and drafted the initial version of the text. MS participated in all aspects of preparing the paper. HO and JC contributed to the text. All authors were involved in revising the manuscript and approved the final version.
Acknowledgements
We would like to acknowledge Barbara Schimmer, EPIET Scientific Coordinator based at RIVM, the Netherlands for the revision of the manuscript.
Do redakce došlo dne 21. 9. 2021.
Adresa pro korespondenci:
MUDr. Michaela Špačková, Ph.D.
Oddělení epidemiologie infekčních nemocí, CEM
Státní zdravotní ústav Praha
Šrobárova 49/48
100 00 Praha 10
e-mail: michaela.spackova@szu.cz
Sources
1. World Health Organization. Measles [online]. 2019-12-05 [cit. 2022-01-25]. Available from:<https://www.who.int/newsroom/fact-sheets/detail/measles>.
2. O‘Connor P, Jankovic D, Muscat M, et al. Measles and rubella elimination in the WHO Region for Europe: progress and challenges. Clin Microbiol Infect, 2017;23(8):504–510.
3. European Centre for Disease Prevention and Control. Measles cases in the EU treble in 2017, outbreaks still ongoing [online]. 2018 [cit. 2021-04-14]. Available from: https://www.ecdc.europa. eu/en/news-events/measles-cases-eu-treble-2017-outbreaks - still-ongoing.
4. The National Institute of Public Health. Cases of selected infectious diseases in the Czech Republic, January-December 2020 compared with the corresponding period of preceding years 2011-2019 (number of cases) [online]. 2020 [cit. 2021-04-22]. Available from: http://www.szu.cz/uploads/documents/szu/infekce/ 2020/tabulka_leden_prosinec_2020.pdf.
5. European Centre for Disease Prevention and Control. Measles – Annual epidemiological report for 2019 [online]. 2020 [cit. 2021-05-06]. Available from: https://www.ecdc.europa. eu/en/publications-data/measles-annual-epidemiological-report - 2019.
6. Trmal J, Limberková R. Vyhodnocení epidemie spalniček v Ústeckém kraji [Report on a measles epidemic in the Usti nad Labem Region]. Epidemiol Mikrobiol Imunol, 2015;64(3):139 – 145. [in Czech]
7. Trmal J, Kupcova J, Dvořáková L, et al. Návrat spalniček do Ústeckého kraje. [Measles re-emerging in the Usti Region]. Epidemiol Mikrobiol Imunol, 2014;63(2):154–159. [in Czech]
8. World Health Organization. Report of the 8th meeting of the European regional verification commission for measles and rubella elimination (RVC) (2019) [online]. 2019 [cit. 2020-05-28]. Available from: http://www.euro.who.int/en/ health-topics/communicable-diseases/measles-and-rubella/ publications/2019/8th-meeting-of-the-european-regional - verification-commission-for-measles-and-rubella-elimination - rvc-2019.
9. World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2019 global summary [online]. 2020 [cit. 2021-06-12]. Available from: https://apps.who.int/immunization_ monitoring/globalsummary/countries?countrycriteria% 5Bcountry%5D%5B%5D=CZE&commit=OK.
10. European Centre for Disease Prevention and Control. Measles Rapid Risk Asessment Country Profiles 2019 [online]. 2020 [cit. 2021-04-01]. Available from: https://www.ecdc.europa.eu/sites/ default/files/documents/Measles-RRA-country-profiles-2019. pdf.
11. European Centre for Disease Prevention and Control. Risk assessment: Who is at risk of measles in the EU/EEA? [online]. 2019 [cit. 2021-04-24]. Available from: https://www.ecdc.europa.eu/ sites/default/files/documents/RRA-Measles-EU-EEA-May-2019. pdf.
12. Dáňová J, Šálek J, Kocourková A, et al. Factors associated with parental refusal of routine vaccination in the Czech Republic. Cent Eur J Public Health, 2015;23(4):321–323.
13. Danova J, Gopfertova D, Bobak M. Rates of contraindications and use of alternative vaccines in routine immunisation of children: A population based study in the Czech Republic. Vaccine, 2007;25(19):3890–3895.
14. Larson H, de Figueiredo A, Karafillakis E, et al. State of vaccine confidence in the EU 2018. Luxembourg: Publications Office of the European Union, 2018;10 : 1–77. ISBN 978-92-79-96560-9.
15. Smetana J, Chlibek R, Hanovcova I, et al. Decreasing seroprevalence of measles antibodies after vaccination – possible gap in measles protection in adults in the Czech Republic. PloS One, 2017;12(1):e0170257.
16. Šejda J. Control of measles in Czechoslovakia (CSSR). Rev Infect Dis, 1983;5(3):564–567.
17. Sejda J. Evaluation of the eight-year period of compulsory measles vaccination in the Czech Socialist Republic (CSR). J Hyg Epidemiol Microbiol Immunol, 1979;23(3):273–283.
18. Ministry of Health of the Czech Republic. Decree No. 537/2006 Coll. on Immunisation against Infectious Diseases [online]. 2006 [cit. 2021-04-24]. Available from: https://www.zakonyprolidi.cz/ cs/2006-537/zneni-20211211.
19. The Commission of the European Communities. Commission Decision of 28/IV/2008 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council [online]. 2008 [cit. 2021-04-26]. Available from: https://www.ec.europa.eu.
20. Ministry of Health of the Czech Republic. Decree of the MoH of the Czech Republic No 275/2010 Coll. Amending the Decree No 473/2008 Coll. on Surveillance System for Selected Infectious Diseases [online]. 2008 [cit. 2021-04-26]. Available from: https:// www.zakonyprolidi.cz/cs/2008-473.
21. European Centre for Disease Prevention and Control. Data quality monitoring and surveillance system evaluation: A handbook of methods and applications [online]. 2014 [cit. 2021-05-11]. Available from: https://www.ecdc.europa.eu/ en/publications-data/data-quality-monitoring-and-surveillance - system-evaluation-handbook-methods-and#no-link.
22. European Centre for Disease Prevention and Control. Long-term surveillance strategy 2014–2020 (revised) [online]. 2018 [cit. 2022-01-25]. Available from: https://www.ecdc.europa.eu/sites/ default/files/documents/LTSS-revised_0.pdf.
23. Chapman DG. Some properties of hyper-geometric distribution with application to zoological census. University of California Publications Statistics, 1951;1 : 131–160.
24. Stock NK, Maly M, Sebestova H, et al. The Czech surveillance system for invasive pneumococcal disease, 2008-2013: a follow - up assessment and sensitivity estimation. PLoS One, 2015;10(6):e0131117.
25. Doyle TJ, Glynn MK, Groseclose SL. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. Am J Epidemiol, 2002;155(9):866–874.
26. Mellou K, Sideroglou T, Kallimani A, et al. Evaluation of underreporting of salmonellosis and shigellosis hospitalised cases in Greece, 2011: results of a capture-recapture study and a hospital registry review. BMC Public Health, 2013;13 : 875.
27. European Centre for Disease Prevention and Control. Measles In: ECDC. Annual epidemiological report for 2018 [online]. 2020 [cit. 2021-06-14]. Available from: https://www.ecdc.europa. eu/en/publications-data/measles-annual-epidemiological-report - 2018.
28. Zimmerman LA, Muscat M, Singh S, et al. Progress toward measles elimination – European region, 2009–2018. MMWR Morb Mortal Wkly Rep, 2019;68(17):396–401.
29. Turiac I, Fortunato F, Cappelli M, et al. Evaluation of measles and rubella integrated surveillance system in Apulia region, Italy, 3 years after its introduction. Epidemiol Infect, 2018;146(5):594 – 599.
30. Benson F, Musekiwa A, Blumberg L, et al. Comparing laboratory surveillance with the notifiable diseases surveillance system in South Africa. Int J Infect Dis, 2017;59 : 141–147.
31. Wesson P, Lechtenberg R, Reingold A, et al. Evaluating the completeness of HIV surveillance using capture–recapture models, Alameda County, California. AIDS Behav, 2018;22(7):2248–2257.
32. Cadwell BL, Smith PJ, Baughman AL. Methods for Capture-Recapture Analysis When Cases May Not Be Identified Uniquely. Stat Med, 2005;24(13):2041–2051.
33. Brumboiu I, Mootien CP. The Capture-Recapture Method in the Analysis of a Measles Epidemic in the County of Cluj, Romania. Appl Med Inform, 2019;41(4):140–146.
34. Tomášková H, Zelená H, Kloudová A, et al. Serological survey of measles immunity in the Czech Republic, 2013. Cent Eur J Public Health, 2018;26(1):22–27.
35. Mette A, Reuss AM, Feig M, et al. Under-Reporting of Measles: An Evaluation Based on Data From North Rhine–Westphalia. Dtsch Arztebl Int, 2011;108(12):191–196.
36. Poorolajal J, Haghdoost AA, Mahmoodi M, et al. Capture-recapture method for assessing publication bias. J Res Med Sci, 2010;15(2):107–115.
37. World Health Organization. Framework for verifying elimination of measles and rubella. Wkly Epidemiol Rec, 2013;88(9):89–99.
38. World Health Organization. Framework for the verification process in the WHO European Region [online]. 2014 [cit. 2021-06 - 15]. Available from: http://www.euro.who.int/__data/assets/ pdf_file/0009/247356/Eliminating-measles-and-rubella-Framework - for-the-verification-process-in-the-WHO-European-Region. pdf.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2022 Issue 2-
All articles in this issue
- Monitoring changes in invasive disease caused by Haemophilus influenzae in the Czech Republic between 1999 and 2020
- Norovirus infections in the Czech Republic in 2008–2020
- Incidence of tuberculosis among HIV-positive persons in the Czech Republic between 2000 and 2020
- Genetic diversity of human strains of Listeria monocytogenes in the Czech Republic in 2016–2020
- Candida glabrata – basic characteristics, virulence, treatment, and resistance
- First evaluation of completeness and sensitivity of the measles surveillance system in the Czech Republic, January 1, 2018 until June 30, 2019
- Colistin resistance and heteroresistance in Klebsiella pneumoniae & Escherichia coli clinical isolates from intensive care units
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Candida glabrata – basic characteristics, virulence, treatment, and resistance
- Incidence of tuberculosis among HIV-positive persons in the Czech Republic between 2000 and 2020
- Norovirus infections in the Czech Republic in 2008–2020
- Monitoring changes in invasive disease caused by Haemophilus influenzae in the Czech Republic between 1999 and 2020
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career



