-
Medical journals
- Career
Detection of antigen-specific T cells in patients with neuroborreliosis
Authors: D. Pícha; L. Moravcová; D. Smíšková
Authors‘ workplace: Department of Infectious Diseases, 2nd Faculty of Medicine, Charles University and Na Bulovce Hospital, Prague, Czech Republic
Published in: Epidemiol. Mikrobiol. Imunol. 66, 2017, č. 4, s. 210-212
Category: Short Communication
Overview
There is a lack of laboratory tests in clinical practice that can detect the activity of borrelial infection. This was the reason for testing an antigen-specific T-cell detection method in patients with neuroborreliosis: the ELISPOT method, which is capable of detecting antigen-specific T lymphocytes in clinical conditions. A group of 32 patients (20 diagnosed with neuroborreliosis; NB) was examined using this commercial method (LymeSpot intrerferon-γ Assay Kit®). Of these 20 NB patients, 10 were found to be positive and 10 negative; four of the five persons tested prior to the antibiotic treatment were positive. Eight patients served as the control group, giving four positive and four negative results. The results achieved so far appear to be unequivocal; still, the test could be expected:
a) in non-specific clinical symptoms with borderline or negative proof of antibodies;
b) in eraly stage of the disease;
c) in the case of seropositivity and unequivocal clinical picture.
The basic prerequisite for the clinical utilization of the method, however, is that it be thoroughly tested.Keywords:
neuroborreliosis – immune response – T cells – interferon-γINTRODUCTION
Lyme borreliosis (LB) is a spirochetal disease affecting a number of organs, most frequently the nervous system, skin and joints. The clinical symptoms associated with LB vary considerably and serology therefore provides the main diagnostic support. Frequent nonspecific reactions necessitate the second step – confirmation by WB. Unfortunately, the sensitivity of many serological tests hardly exceeds 50% in early forms, irrespective of whether native antigens, a mixture of recombinant antigens or specific peptides are used [1]. On the contrary, antibodies can persist for months or years after successful antibiotic treatment [2] and seronegative forms of LB have also been described [3, 4]. The other factors make the antibody proof diagnostically inferior. A positive serological test in itself is not confirmation of an active infection [5]; some cross-reactive antigen reactions do exist among bacterial pathogens (Treponema pallidum e.g.), and isolated IgM positivity can be a symptom of polyclonal immune response activation (Epstein-Barr virus infection) or rheumatic diseases. The solitary detection of specific antibodies in all of these cases is not sufficient for the diagnosis of LB and antibiotic treatment.
Consequently, other methods are tested to improve the laboratory LB diagnostic. T lymphocytes are necessary for cell-mediated immunity to function properly. Shortly after the initiation of the infectious process, antigen-specific T lymphocytes are stimulated for proliferation, differentiation and cytokine secretion. These changes lead to the synthesis of B lymphocytes and the production of specific antibodies. After the introduction or start of the immune reactions, the intensity of antigen stimulation decreases and, hand in hand with this, there is a rapid decrease in the number of activated T-cells and cytokine levels. For this reason, the monitoring of the activated T-cells is tested as an additional laboratory method to support standard serological laboratory tests. Compared with the latter, the great advantage of cellular immune response tests is that they provide more accurate information on recent immunologic response. Some studies describing borrelia-specific T-cells tests have already been published [for instance 6, 7, 8] and their results suggest they could be helpful for early LB diagnostics. However, this experience has not been systemized or fully evaluated. Our first cases of proving antigen-specific T-cells in LB are published in this article.
PATIENTS GROUP AND METHODS
Thirty patients hospitalized with a suspected neuroinfectious disease were included in this prospective study. CSF was examined in all these cases; two additional patients had other infections (bacterial pneumonia, pulmonary TBC). The group studied was divided into three parts. Group A – 20 patients with NB, all these patients were positive for intrathecal synthesis of antiborrelial antibodies (positive AIBb), and in 14 of them the CSF lymphocytic pleocytosis was proven. Group B – 4 patients with CSF and serum LB antibody positivity, AIBb negative, one with CSF pleocytosis; Group C – 8 control patients, 6 patients with aseptic CNS infection (3 with tick-borne encephalitis – TBE, 3 with unknown etiology), 1 with pneumonia, 1 with pulmonary TBC. Seven out of 20 patients with positive AIBb were examined before the antibiotic treatment was initiated, and the rest during therapy.
Antiborrelial antibodies were detected using the commercial diagnostic kit ELISA-Viditest anti-borrelia recombinant IgG VlsE/IgM (VIDIA s. r. o., ČR) and the results were confirmed by Western blotting (Blot-Line Borrelia HGAIgM/HGAIgG TestLine, Clinical diagnostic s. r. o., ČR).
Intrathecal synthesis of antiborrelial antibodies (AIBb) was calculated using the VidiTab computer program (Vidia s.r.o., ČR); the value AIBb ≥ 1.5 was considered positive.
A borrelia-specific T lymphocytes examination was performed using the commercial LymeSpot Assay Interferon-γ Kit (GenID GmbH, Germany, imported into the Czech Republic by Medisko Praha, s. r. o.) at the manufacturer’s recommendation. Peripheral blood lymphocytes were isolated from fresh blood in gradient Ficoll-PaqueTM PLUS and the concentration was adjusted to 2 x 106 cells/mL. This suspension was added to wells coated with an anti-human interferon-γ monoclonal antibody. The specific borrelial antigen Borrelia burgdorferi B31 lysate and the mixture of recombinant OspC and OspA with p18 peptide were added and then incubated for 24 hours in a CO2 incubator. During this period, the synthesized interferon-γ was bound by the monoclonal antibody. After removing the cell suspension, the secondary anti-human interferon-γ alkaline phosphatase labelled antibody was added. After the following washing steps the interferon-γ production was visualized by the addition of the substrate solution (BCI/NBT) and the colour reaction was read as a spot at the bottom of the well. One spot was identified as one cell producing interferon-γ and the results were counted using the EliSpot automatic reader (AOD iSPOT, AID, Germany). The stimulation index (SI) is used for interpretation. SI expresses the number of the spots from the culture with the antigen mixtures/number of spots from the negative control. The value of the SI > 5 was interpreted as positive.
RESULTS AND DISCUSSION
The results of examination of the antigen-specific effector TH cells are summarized in Table 1. In group A, the result was positive in 10 patients; 10 results were negative. However, one difference was found when the results were assessed and compared with the beginning of the antibiotic treatment. Of the five patients with the positive AI and CSF pleocytosis examined before treatment, four were positive and only one negative. On the contrary, when they were tested during antibiotic treatment (9 patients), seven were negative and only two positive. Of the 6 patients with the positive AIBb without CSF pleocytosis, 4 were positive and 2 negative. In group B (4 patients; AIBb negative), no positive result was found. An interesting result was discovered in the control group (C) – four positives (one patient suffered from TBE and three from aseptic meningoencephalitis. Two had no circumstance evidence leading to suspicion of NB, one was treated by ceftriaxone due to IgM seropositivity for LB discover later. No later IgG seroconversion was observed.
1. Results of LymeSpot interferon-γ Assay Kit 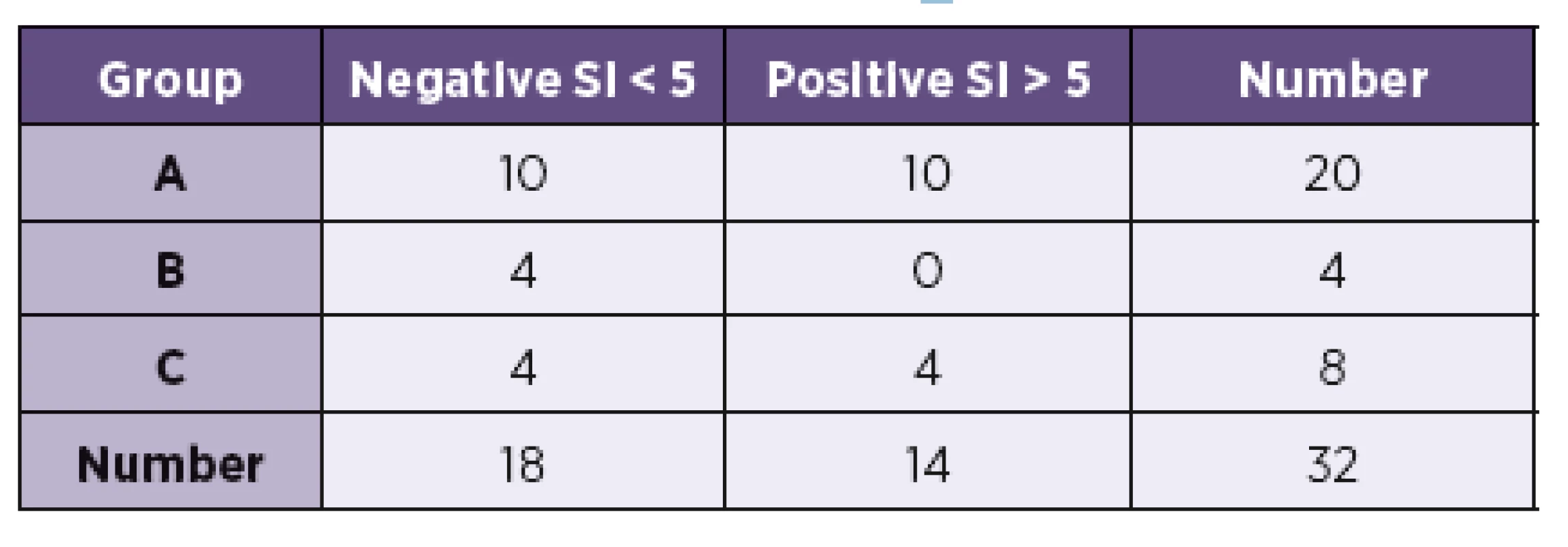
It is evident that the number of patients examined is far from sufficient for a detailed assessment or statistical analysis. The results presented herein may indicate that future testing should first be targeted at a more homogenous cohort, although a study carried out with a bigger group could certainly provide some very interesting results as well. The positive results in the group C can serve as an example. Moreover, the time factor seems to be important. It should be explained whether the higher number of positive results before treatment is an accidental fluctuation or an important phenomenon, while the latter has already been published [6, 8]. But it is necessary to verify whether ELISPOT positivity – proof of interferon-γ synthesis after the stimulation of the bor-relial antigens – is only specific to the borrelial infection. The control group should comprise healthy patients and patients with other infectious diseases in an acute or delayed phase of the disease as well, especially those whose clinical symptoms share clinical manifestations of LB.
Laboratory tests based on the proof of cytokines are rarely a part of routine laboratory diagnostic either in LB or other infections (exceptions exist, e.g. the QuantiFERON test, T-SPOT TB) [9]. The advantage of cellular tests based on the immunochemical principle (such as ELISPOT) is that they save time – 24 hours with ELISPOT, 6 days with proliferative radionuclide-based methods. Moreover, these methods are relatively risky due to the use of radionuclide material and the possible limits on repetition. The tests results based on the examination of the whole blood (like QuantiFERON test) can be influenced by the production of cytokines other than interferon-γ that are secreted by other cells (natural killer cells, monocytes), which results in false positivity. The disadvantage of ELISPOT tests is that their standardization can sometimes be limited as some methodological factors can interfere – e.g. the taking and preparation of lymphocytes (temperature, transportation, treatment etc.), which to some extent complicate routine clinical practice.
Despite the aforementioned problems, we consider the LymeSpotAssay interferon-γ Kit a possible contribution for additional LB diagnostic especially in specialized centres when:
- i) clinical symptoms are not diagnostic and a serological test is borderline or negative,
- ii) in long-term diseases with a non-specific clinical picture and seropositivity,
- iii) in the early phase of infection (2–6 weeks).
Besides the experimental utilization of the method, a necessary but not a sine qua non prerequisite is careful clinical testing, a condition which, it seems, has not so far been fulfilled.
Adresa pro korespondenci:
doc. MUDr. Dušan Pícha, CSc.
Klinika infekčních, parazitárních a tropických nemocí
Nemocnice Na Bulovce
Budínova 67/2
180 81 Praha 8-Libeň
e-mail: dusan.picha@bulovka.cz
Sources
1. Leeflang MMG, Ang CW, Berkhout J, et al. The diagnostic accurancy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis, 2016; 16 : 140. doi: 10.1186/s12879-016-1468-4.
2. Kalish RA, McHugh G, Granquist J, et al. Persistence of immunoglobulin M or immunoglobulin G responses to Borrelia burgdorferi 10-20 years after active Lyme disease. Clin Infect Dis, 2001;33(6): 780–785.
3. Dejmkova H, Hulinska D, Tegyova D, et al. Seronegative Lyme arthritis caused by Borrelia garinii. Clin Rheumatol, 2002;21(4): 330–334.
4. Dattwyler RJ, Volkman DJ, Luft BJ. Seronegative Lyme disease. Dissociation of specific T - and B-lymphocyte responses to Borrelia burgdorferi. N Engl J Med, 1988;319(22): 1441–1446.
5. Aguero-Rosenfeld ME, Wang G, Schwartz I, et al. Diagnosis of Lyme borreliosis. Clin Microbiol Rev, 2005;18(3): 484–509.
6. von Baehr V, Doebis C, Volk HD, et al. The lymphocyte transformation test for Borrelia detects active Lyme borreliosis and verifies effective antibiotic treatment. Open Neurl J, 2012;6 : 104–112. doi: 10.2174/1874205X01206010104.
7. Jin C, Roen DR, Lehmann PV, et al. An enhanced ELISPOT assay for sensitive detection of antigen-specific T cell responses to Borrelia burgdorferi. Cells, 2013;2(3): 207–620.
8. Callister SM, Jobe DA, Stuparic-Stancic A, et al. Detection of INF-γ secretion by T cells collected before and after successful treatment of early Lyme disease. Clin Infect Dis, 2016;62(10): 1235–1241.
9. Connel TG, Rangaka MX, Curtis N, et al. QuantiFERON-TB Gold: state of the art for the diagnosis of tuberculosis infection? Expert Rev Mol Diagn, 2006;6(5): 663–677.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2017 Issue 4-
All articles in this issue
- The prevalence, incidence, persistence and transmission ways of human papillomavirus infection (HPV)
- Flow cytometry in microbiology
- Crohn’s disease and ulcerative colitis – current view on genetic determination, immunopathogenesis and biologic therapy
- Mycological diagnosis of pulmonary Aspergillus infections with a focus on serological methods
- Human alveolar echinococcosis and an overview of the occurrence of Echinococcus multilocularis in animals in the Czech Republic
- Detection of the etiological agents of hospital-acquired pneumonia – validity and comparison of different types of biological sample collection: a prospective, observational study in intensive care patients
- Detection of antigen-specific T cells in patients with neuroborreliosis
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Human alveolar echinococcosis and an overview of the occurrence of Echinococcus multilocularis in animals in the Czech Republic
- Crohn’s disease and ulcerative colitis – current view on genetic determination, immunopathogenesis and biologic therapy
- Flow cytometry in microbiology
- Mycological diagnosis of pulmonary Aspergillus infections with a focus on serological methods
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

