-
Medical journals
- Career
The occurrence of Ixodes ricinus ticks and important tick-borne pathogens in areas with high tick-borne encephalitis prevalence in different altitudinal levels of the Czech Republic
Part II. Ixodes ricinus ticks and genospecies of Borrelia burgdorferi sensu lato complex
Authors: M. Daniel 1; N. Rudenko 2; M. Golovchenko 2; V. Danielová 1; A. Fialová 1; B. Kříž 1,3; M. Malý 1
Authors‘ workplace: National Institute of Public Health, Prague 1; Institute of Parasitology, Biology Centre of the Czech Academy of Science, České Budějovice 2; rd Faculty of Medicine, Charles University, Prague 33
Published in: Epidemiol. Mikrobiol. Imunol. 65, 2016, č. 3, s. 182-192
Category: Original Papers
Overview
Study objective:
Three years long research study (2011–2013) on population density of Ixodes ricinus and the infection rate of the pathogens that they transmit was conducted in four topographically distant areas in the Czech Republic. In the previous decade (2001–2010) thirteen loci with increased incidence of tick borne encephalitis cases were defined, suggesting the permanent interaction of human population with ticks and indicating the landmarks for study of the presence of other tick borne pathogens. The work program included the identification of existing spectrum of spirochetes from Borrelia burgdorferi sensu lato complex and the conditions of their occurrence and distribution.Material and methods:
In the areas of the Ústí nad Labem Region, Olomouc Region, South Bohemian Region, and Highlands Region, 600 m2 plots were selected in the local optimal I. ricinus habitats where tick flagging was performed every year in the spring-summer and autumn seasons of the tick questing activity. Collected adult ticks (1369 males and 1404 females) were individually screened for B. burgdorferi s. l. spirochets.Results:
Spirochetes from B. burgdorferi s.l. complex were detected in all 13 studies sites in all altitudes from 280 to 1030 meters a. s. l. The total rate of infection was determined as 11.4% (males 10.4%, females 12.4%) with range limits from 1.4% (Ústí nad Labem in 2011) to 19.7% (South Bohemian Region, 2012).Genospecies were detected in various proportions and in different combinations:
Borrelia afzelii, B. garinii, B. burgdorferi s. s., B. bavariensis, B. bissettii, B. valaisiana, B. spielmanii and B. lusitaniae. The three-year observation justifies the assumption that the regional differences in infectivity of I. ricinus are based on the character of the local biocenosis of the respective region. The dynamics of its seasonal changes, conditioned by climatic factors, determines the annual differences.Conclusion:
Three of the medically most important Borrelia species formed a core group among all detected genospecies. B. afzelii was a dominated one (115 detections), followed by B. garinii (100) and by B. burgdorferi s.s. (19). Other genospecies were detected sporadically. However, the detection of B. bissettii should be emphasized due to the recently proven pathogenic effects of this genospecies and yet little-known sporadic expansion in the Czech Republic. The medical importance and distribution of other sporadically occurred genospecies is also discussed.Key words:
Ixodes ricinus – Borrelia afzelii – B. garinii – B. burgdorferi s. s. – B. bavariensis – B. valaisiana – B. spielmanii – B. lusitaniae – B. bissettii – distribution – altitude – season – medical importanceINTRODUCTION
The presented work follows the publication of Daniel et al. [1] that evaluated and compared the occurrence and dynamic of Ixodes ricinus population and the infection rate of tick borne encephalitis (TBE) virus in them, defined during the three years long research study (2011–2013) in four topographically distant regions of the Czech Republic. The study sites that had the same common denominator such as the permanent contact of human population with tick I. ricinus and that revealed in previous decade (2001–2010) the increased number of cases of infection with tick borne encephalitis virus, were selected in those 4 regions that differ in the type of landscape [2]. Mentioned way of selection of the study sites allowed the suggestion that those sites will be suitable for research on the other pathogens transmitted by I. ricinus. That lead to identification of existing spectrum of spirochetes from Borrelia burgdorferi sensu lato complex and the conditions of their occurrence. Detailed criteria for selection and the method of determining the study sites are presented in Methods. To be able to compare and generalize the results from each area the design of the study was based on the three years of its duration which then included the entire course of development of at least one generation of I. ricinus. Another main concern of this study was the strict compliance of the work in general, and the technique of sample collection in particular, meaning that sample collection should be done by the same well trained and experienced researchers in the same well characterized loci. The main goal of our study was to compare the occurrence of three majorly distributed species from B. burgdorferi s. l. complex, (B. afzelii, B. garinii and B. burgdorferi s.s.) in tick I. ricinus infection rate in the monitored area, and its annual changes. Another task was to define the spectrum of less distributed or known species of B. burgdorferi s. l. complex, their occurrence in our natural environment and highlight their importance in human pathology.
MATERIAL AND METHODS
1. Selection of areas
In the areas with a high incidence of TBE reported in 2001–2010 [3], superior to the whole-country average, the study plots were selected in northern Bohemia (Ústí nad Labem Region), northern Moravia (Olomouc Region), southern Bohemia (South Bohemian Region), and in the Bohemian-Moravian Highlands (Highlands Region) according to the following key: in each area, two high prevalence municipalities (three municipalities in the South Bohemian Region) were identified. In the cadastres of these municipalities, the monitoring plots representing optimal habitats of I. ricinus ticks were selected in the closest vicinity of the built-up area. In addition, in each area, a plot located at a higher altitude than the surrounding landscape and providing a suitable habitat for I. ricinus ticks was selected. The middle of each plot was determined by the geographic coordinates. Large urban units (regional cities) were not included among the selected municipalities because the direct contact of TBE cases with the surrounding green spaces is not commonplace.
2. Localities
South Bohemian Region
Stožec Mt. 48°52´22˝ N, 13°49´41˝ E; 910 – 920 m a.s.l.
Strakonice – Starý Dražejov 49°16´31˝N, 13°52´46˝ E; 480 – 490 m a.s.l.
Netolice – 49°02´30˝ N, 14°10´51˝ E; 460 – 470 m a.s.l.
Zliv – 49°04´24˝ N, 14°22´11˝ E; 405 – 410 m a.s.l.
Ústí nad Labem Region
Povrly – 50°40´38˝ N, 14°08´31˝ E; 280 – 300 m a.s.l.
Benešov nad Ploučnicí – 50°43´56˝ N, 14°18´58˝ E; 280 – 300 m a.s.l.
Děčínský Sněžník – Medvědí louka – 50°48´03˝ N, 14°05´50 ˝ E; 550 – 570 m a.s.l.
Olomouc Region
Červenohorské sedlo – 50°07´20˝ N, 17°09´08˝ E; 1020 – 1030 m a.s.l.
Jeseník – Křížový vrch – 50°13´37˝ N, 17°13´08˝ E; 530 – 550 m a.s.l.
Šumperk – Holubí vrch – 49°58´49˝ N, 16°59´08˝ E; 410 – 420 m a.s.l.
Highlands Region
Baliny (Velké Meziříčí) – 49°20´19˝ N, 15°58´04˝ E; 450 – 470 m a.s.l.
Bystřice nad Pernštejnem – 49°32´41˝ N, 16°17´23˝E; 400 – 450 m a.s.l.
Nedvědice – 49°27´59˝ N, 16°20´01˝ E; 330 – 370 m a.s.l.
The detail characteristic of study habitats is given in [1].
3. Collection of Ixodes ricinus ticks
Ticks were collected by the standard flagging technique [4] on the defined plots. The flag (50 x 70 cm) was made of white fabric with a slight nap (flannel). The ticks’ collection was carried out by one person for three hours. Based on long-term experience, three hours of work performed by a skilled person at the average abundance of ticks in the area correspond to 600 m2 of the area monitored. This approach makes it possible to focus in detail on the habitat and micro-relief of the area where ticks are actually present. Collected ticks were transported to the laboratory, identified to the species level, and stored at -80 °C until further processed. The presence of larvae was recorded only.
4. Tick DNA isolation
The collected ticks were separated by developmental stage, gender, collection area, and collection date. All male and female ticks were analyzed individually. Ticks were homogenized in 100 µl of PBS using an automatic homogenizer (TissueLyzer II (Qiagen). Pools were made from adult ticks by mixing of 10 µl of 10 adult tick samples originated from the same locality. Such pooled samples were used for RNA isolation and further detection of TBE virus [1]. The rest of the samples were used for further experiments. Isolation of genomic DNA was conducted using DNeasy® Blood & Tissue Kit (Qiagen) according to the manufacturer’s protocol. After adding proteinase K the samples were left for overnight incubation at 56 °C. The DNA was eluted from the column by 50 µl of H2O.
5. DNA purification, PCR amplification, and sequencing
Tick DNA samples were selectively controlled (10 samples out of each 100) for the efficiency of tick DNA isolation using PCR primers Ixri-F (GGAAATCCCGTCGCACG) and Ixri-R (CAAACGCGCCAACGAAC) that target a 150 bp fragment of 5.8S rRNA gene [5].
Detection of B. burgdorferi s.l. infection was performed using total tick DNA as template. The MasterTaq kit (Eppendorf, Germany) was used for amplification of fragment of chromosome localized flagellin gene using the gene-specific primers (Fla out F-5′-AARGAATTGGCAGTTCAATC-3′ and Fla out R-5′-GCATTTTCWATTTTAGCAAGTGATG-3′ [6] that produce specific 496 nt fragment. B. burgdorferi s.s. DNA was used as the positive control, and double-distilled water was used as the negative control in each PCR run.
The PCR products were separated by electrophoresis on 1.5% agarose gel and visualized under UV light. PCR products of the expected size were cut off the gel, purified, and submitted for direct sequencing. Sequences were identified using NCBI BLAST similarity search.
The presence of multiple infection was detected by multiplex PCR using GI, GII and GIII primers [7] designed on a basis of ospA sequences of B. burgdorferi s.s. (GI primer set), B. garinii (GII primer set) and B. afzelii (GIII primer set) that produced the 544 bp, 345 bp and 189 bp fragments, respectively. Positive samples that revealed the presence of other Borrelia species were double checked with previously described ospC primers F-5′-AAAGAATACATTAAGTGCGATATT-3′ and R-5′-GGGCTTGTAAGCTCTTTAACTG-3′ [8]. Amplified fragments were sequenced to detect possible co-infection.
6. Statistical analysis
The prevalence of infection Borrelia burgdorferi s.l. in ticks was calculated. To test the significance of differences in prevalence Pearson's Chi-squared test or Fisher's exact test were used. The statistical significance level was set to 0.05. The data were processed by the R software (R Core Team, 2014, version 3.1.2).
RESULTS
1. The detection of Borrelia burgdorferi sensu lato in adult Ixodes ricinus ticks
Two thousands seven hundreds and seventy three (2,773) adult Ixodes ricinus ticks were individually analyzed for the presence of Borrelia burgdorferi s.l. with positive finding in 11.4% (316 out of 2,773). Both tick genders in the examined group were represented about equally, and corresponded to a similar positive findings (F - 12.4%, M -10.4%). The overall result has threshold values, contingent to both ticks region of origin and year of ticks’ collection, from 1.4% (Ústí nad Labem, 2011) to 19.7% (South Bohemian Region, 2012) of positive ticks.
Results of B. burgdorferi s.l. detection in ticks from various regions are shown in Table 1. High average positivity observed in the South Bohemian Region (15.5%) corresponds to a relatively uniform high positivity observed here in different years (2011–2013, i.e. 11.9%, 19.7%, 13.2%), unlike other regions with some year to year fluctuation. This was most clearly reflected in the Ústí nad Labem Region. The annual changes are documented in Table 2. The finding of an upward trend in overall positivity observed in different years (2011 – 8.7%, 2012 – 11.8%, 2013 – 13.6%) is supported by the fact that during all three compared years approximately equal numbers of adult I. ricinus ticks were examined.
1. Prevalence of <i>Borrelia burgdorferi</i> sensu lato in adult <i>Ixodes ricinus</i> ticks in individual regions (2011–2013) 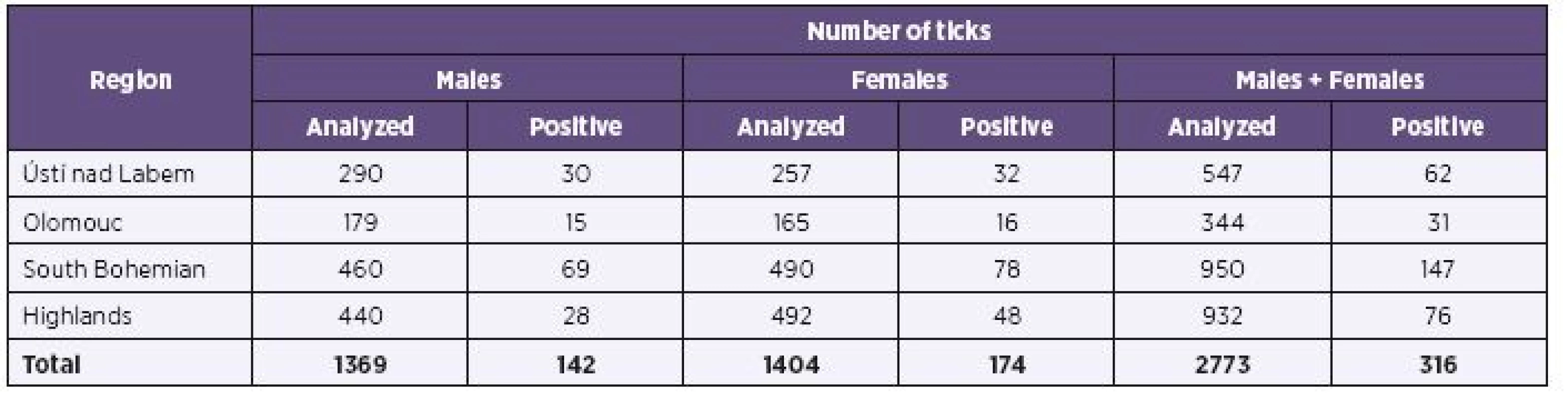
2. Prevalence of <i>Borrelia burgdorferi</i> sensu lato in adult <i>Ixodes ricinus</i> ticks in individual years 2011–2013 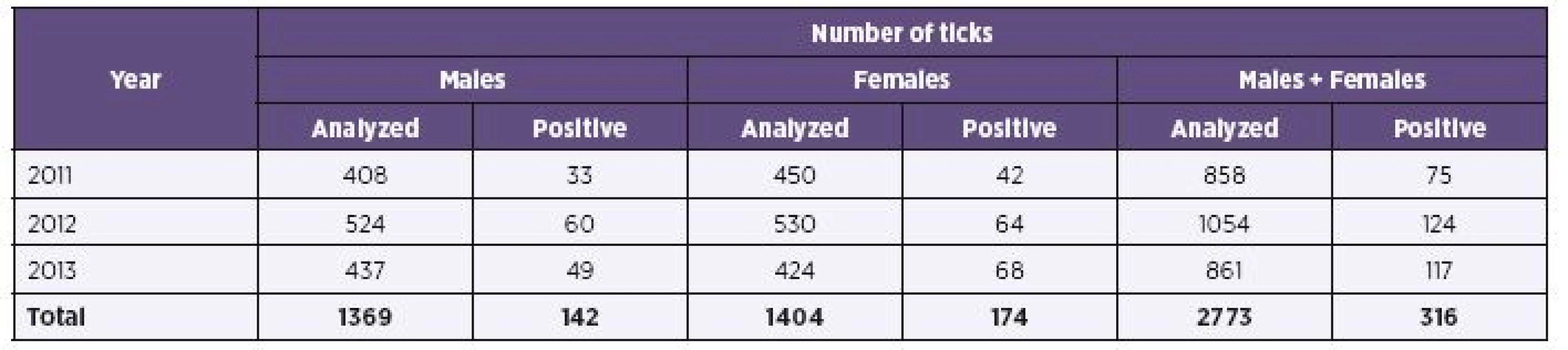
2. Detection of individual genospecies of Borrelia burgdorferi sensu lato complex in adult Ixodes ricinus ticks
The ratios of genospecies found varied with individual locations and habitats. The following single genospecies were detected in various proportions and in different combinations: B. afzelii, B. garinii, B. burgdorferi sensu stricto, B. bavariensis, B. bissettii, B. valaisiana, B. spielmanii and B. lusitaniae (for details see Tables 3a-d). The presence of single genospecies was detected in 244 cases; double infection was found in 33 ticks. In one case, the presence of three spirochete species was determined. Borrelia species in 38 samples could not be identified.
Table 3a. Individual genospecies of <i>Borrelia burgdorferi</i> sensu lato detected in adult <i>Ixodes ricinus</i> ticks in Ústí nad Labem Region 
Table 3b. Individual genospecies of <i>Borrelia burgdorferi</i> sensu lato detected in adult </i>Ixodes ricinus</i> ticks in Olomouc Region 
Table 3c. Individual genospecies of <i>Borrelia burgdorferi</i> sensu lato detected in adult <i> Ixodes ricinus </i> ticks in South Bohemian Region 
Table 3d. Individual genospecies of Borrelia burgdorferi sensu lato detected in adult Ixodes ricinus ticks in Highlands Region 
M (posit) – number of males analyzed(positive) ;F (posit) – number of females analyzed(positive); M + F (posit) – number of males+females analyzed (positive); B.afz – Borrelia afzelii; B. gar – Borrelia garinii; B. bav –Borrelia bavariensis; B. ss – Borrelia burgdorferi sensu stricto; B. biss – Borrelia bissettii; B. val – Borrelia valaisiana; B. spi – Borrelia spielmanii; B. lus – Borrelia lusitaniae; Undet. – Borrelia burgdorferi s. l. genospecies not identified Three of the medically most important Borrelia species formed a core group among all detected genospecies. B. afzelii was a dominated one (115 detections), followed by B. garinii (100) and with a substantial gap – by B. burgdorferi s.s. (19). These three genospecies were also most often observed in dual infection. The most common co-infection was B. afzelii + B. garinii (22x), followed by B. afzelii + B. burgdorferi s.s. (6x), and B. garinii + B. burgdorferi s.s. (2x). All other combinations were found rarely (B. afzelii + B. lusitaniae (2x), B. afzelii + unidentified species (1x). One case represented the triple infection of B. afzelii + B. garinii + B. burgdorferi s. s.
B. valaisiana, B. spielmanii, B. lusitaniae, B. bissettii and B. bavariensis were detected sporadically. However, the detection of B. bissettii should be emphasized due to the recently proven pathogenic effects of this genospecies [9, 10, 11, 12] and yet little-known sporadic expansion in the Czech Republic. B. bavariensis was not primarily identified as a separate species (detected twice as a result of sequence analysis) and thus was included among B. garinii. Significant is also the quadruple detection of B. spielmanii. Details about individual genospecies findings are documented in Tables 3a-d. Both qualitative and quantitative regional differences in the spectrum of the detected pathogenic spirochete genospecies were found. This result is even more evident due to the fact that in all cases a similar number of adult I. ricinus ticks were examined.
Concerning the relationship of tick density and B. burgdorferi s.l. prevalence, the direct correlation was observed. In both indicators such as the number of single positive infections and the number of 4 different double and triple combinations South Bohemia Region clearly dominates. On the opposite side of these results is Olomouc Region. The lowest total counts and only 2 genospecies, i.e. B. afzelii (8 detections) and B. garinii (16 detections) were detected in that region. Two cases of double infection with B. afzelii +B. garinii were identified as well. These findings correlate with the results of nymph and adult ticks’ density and their mutual ratio in the same regions [1].
3. Statistical analyses
Prevalence of Borrelia burgdorferi s.l. complex in individual regions reached 11.3 %, in Ústí nad Labem Region, 9.0 %, in Olomouc Region, 15.5 % in South Bohemian Region, and 8.2 % in Highlands Region, and differed significantly among regions (p < 0.001). Differences among regions are also demonstrable from the perspective of most frequently occurring genospecies, B. afzelii, whose prevalence varies between 2.1% (Highlands Region) and 6.2% (South Bohemia Region) (p < 0.001), and B. garinii, whose prevalence varies between 2.1% (Highlands Region) and 5.1% (South Bohemia Region) (p = 0.004).
The prevalence of infection with B. burgdorferi s. l. differs in individual years significantly (p = 0.006). Infection rates are also significantly different depending on altitude of capture areas (p < 0.001). At a height below 400 m a.s.l. prevalence is 9.2 %, at an altitude of 400–600 m a.s.l. prevalence is 13.0 %, and at an altitude above 600 m a.s.l., where only 3 positive ticks from a total of 77 ticks were, prevalence has reached only 3.9 %.
There were no statistically significant differences in the incidence of co-infection among regions, years or altitudes.
DISCUSSION
The detection of tick-borne pathogens in the vast collection of I. ricinus accumulated over a wide area and within the three-year time period brought the results confirming the importance of the volume of the examined materials for determining the real total infection rate of the vector in the surveyed area. In the extensive set of the analyzed material the relatively low average values in comparison with frequently published data were determined. For example, Gern and Humair in their review [13] refer to 17.4% as European average for the infection of adult I. ricinus ticks, which in our case was achieved only exceptionally, and in the assessment of the situation in one locality and within time-limited period. However, analysis of 11,182 ticks from South Bohemia [14], determined 8.5% of total prevalence rate in adult ticks. This finding supports our results and simultaneously underlines the conclusion that the volume of the analyzed material and a sufficiently long period of time of its assembly is an essential criterion for assessing the general validity of the conclusions reached.
The data obtained displayed also high variability among collection sites and the year of tick collection, reaching the highest value of 19.7% (South Bohemian Region, 2012) of positive ticks and extremely low value 1.4% (Ústí nad Labem, 2011). A three-year observation justifies the assumption that the detected regional differences in infectivity of I. ricinus are based on the character of the local biocenosis of the respective region. The dynamics of its seasonal changes, conditioned by climatic factors, determines the annual differences, including differences in epidemiology of diseases transmitted by ticks.
The positive correlation in the relationship of tick density and B. burgdorferi prevalence was found. When combining the number of incidence of both nymphs and adult ticks infected with tick-borne encephalitis virus [1], the Region of South Bohemia clearly dominated. The same region is a predominant in the number of B. burgdorferi s.l. infection. This observation is in coordination with the results of Hönig et al. [14], where the same correlation was found for adult ticks.
The work presents the results of the detection of B. burgdorferi s.l. DNA as well as the presence of different Borrelia genospecies separately for each gender of I. ricinus. Different meanings of these findings should be emphasized based on the fact that male I. ricinus does not suck the blood of the host animal. His role in the development cycle is to fertilize female (which is also a precondition for her full engorgement). To fulfill this function, the power supply obtained in nymphal stage is sufficient. Therefore the male tick has no direct importance both in the epidemiology of human infections and in the cycle of zoonotic pathogens. However, there is speculation about the possibility of transmission of the spirochete from infected males to uninfected female. The act of fertilization in ticks is carried out by inserting a spermatophore using oral appendages of male to female’s genital opening using secreted of saliva as an effective lubricant [15]. This could be an effective method of transmitting the infection. Almost identical ratio of the infection in male and female ticks supports this hypothesis.
The difference in the genospecies ratios was observed in various locations and habitats. This can relate to the different character of the habitats. A difference in the ecosystems in which borreliae circulate influences the composition of hosts for different developmental tick stages [16] and therefore leads to the representing of different Borrelia genospecies which are host-dependent.
The total predominance of B. afzelii followed by B. garinii is concordant with the data on the occurrence of B. burgdorferi s.l. genospecies in Europe [17]. However, in two Regions the slight prevalence or equality of B. garinii were indicated. This can be explained by several reasons. Firstly, the fact that B. bavariensis was not primarily identified as a separate species and thus was included among B. garinii [18]. Secondly, this finding can be also supported by the fact that only adult ticks were checked for the Borrelia presence in this study. Margos et al. [19] indicated that the prevalence of B. garinii and B. valaisiana in adult ticks exceeded significantly the prevalence of these genospecies in nymphal ticks. Moreover, the predominance of B. garinii over B. afzelii in higher altitudes was already demonstrated in our previous study suggesting that small passerine birds moving on the ground are responsible for permanent local populations of I. ricinus in mountain localities with low numbers of small terrestrial mammals [20]. The prevalence of B. garinii over B. afzelii in I. ricinus ticks was also reported from Poland [21]. A higher prevalence of B. afzelii on the other hand may be caused by the increased involvement of rodents as B. afzelii specific hosts in the lower regions [22]. The prevalence of B. burgdorferi s.s. was rather low that is in agreement with other ecological studies from Europe [17, 23]. However, this fact is in discrepancy with our former findings from the different experimental plots of South Bohemia [16] where the frequency of the occurrence of B. burgdorferi s.s. was distinctly high. However, the prevalence of B. burgdorferi s.s. in this particular region is the highest among all the areas under investigation of this study.
Presence of multiple Borrelia genospecies within single tick samples was recorded. The presence of two Borrelia species in one tick was identified in 10.4% out of positive ticks. The most common co-infection was B. afzelii + B. garinii. However, as we did not distinguish B. garinii and B. bavariensis we may hypothesize, that some of the frequent B. afzelii-B. garinii co-infections are in fact B. afzelii and B. bavariensis. Such co-infection would be more likely, because these two genospecies share rodents as the main host species [18]. Interestingly, one female tick was co-infected with three genospecies (0.3%), indicating either co-infection of a single host by all of these genospecies, by sequential acquisition of Borrelia by immature stages or, rather disputable, from different hosts due to interrupted feeding.
Except the three traditional genospecies of Borrelia (B. afzelii, B. garinii and B. burgdorferi s.s.) other genospecies (B. valaisiana, B. spielmanii, B. lusitaniae, B. bissettii and B. bavariensis) were detected. It is worth to mention that in our previous studies the ticks were examined for the presence of Borrelia, targeting predominantly 3 main genospecies of interest. That is why the data confirming the presence of other species are of great interest.
The presence of B. spielmanii in the South Bohemian Region was previously detected [14, 24]. In this study the above mentioned genospecies was confirmed not only in the South Bohemia Region but in the North of the Czech Republic. These results correspond to those obtained from Germany where B. spielmanii was detected in 10.9% of the infected ticks [25]. Földvari and colleagues [26] reported the presence of B. spielmanii in skin biopsy from the patients with erythema migrans (EM) in Hungary. B. spielmanii was repeatedly reported in patients with EM in the Netherlands, Germany, Hungary and Slovenia [27]. Together with previous publications [28, 29], this finding suggests that B. spielmanii has a pathogenic role in human Lyme borreliosis (LB). Although B. spielmanii is distributed more focally than other species of the B. burgdorferi s.l. complex [30], it occurs all over Europe from the Netherlands through Germany and Czech Republic to Hungary [24, 28, 29, 30].
B. valaisiana and B. lusitaniae were previously reported both as single infections or in the combination with other genospecies from the Czech Republic [14] [20] as well as from the other European countries, such as Slovakia [22, 32, 33], Greece [34], Germany [25], Sweden [35], Portugal [36], Switzerland [37]. The presence of these genospecies in ticks from the Czech Republic is of importance as both B. valaisiana and B. lusitaniae were already detected in human samples: B. valaisiana was detected in patients with LB symptoms in Switzerland and Greece [34, 38]. The first isolation of B. lusitaniae from human sample was reported in Portugal [39] followed by other reports that confirmed the association of human LB with B. lusitaniae [40, 41], indicating the potential involvement of these Borrelia genospecies in human LB.
Of particular interest could be the identification of B. bissettii in tick from the South Bohemia Region. Although widely distributed in the United States, this Borrelia genospecies is of limited sporadic expansion in the Old World. Previously detected in human patients with LB from the Czech Republic [9, 10] those results provide strong evidence of involved of B. bissettii in human LB in Europe. Hovewer, B. bissettii has never been reported in ticks in this highly endemic region. Only recently B. bissettii was detected in ticks in Europe. A single I. ricinus tick from Slovakia was found to be reactive with probes specific for B. bissettii [22]. However, the fact than this tick was also reactive with probes for two other genospecies of B. burgdorferi s.l. complex complicated the specific identification of the spirochetes. Later, B. bissettii-like DNA was identified in tick from the Czech Republic [42]. But already in 2014 Tappe and colleagues [25] while checking B. burgdorferi s.l. infections in I. ricinus in the city of Hanover (Germany) detected B. bissettii as a single infection already in ten ticks with the total prevalence of 2.1%. They also detected B. bissettii in 7 ticks in the co-infection with another Borrelia. Our finding of B. bissettii DNA in a female tick from South Bohemia confirms the fact that this Borrelia species is becoming more „popular“ in the European samples both from hosts and vectors and the integration of this species into the complex of traditionally recognized European Borrelia is only the question of time. History has shown that when a pathogen is introduced into a new region and new ecosystem, one should expect the unexpected. New vectors may be involved in the transmission cycle and diseases in vertebrates with which the pathogen did not evolve, may be more severe than occurs in endemic regions. This could be the reason why in the last few years researchers and physicians have reported more unusual characteristics of LB [11, 12].
Acknowledgements
Supported by Ministry of Health, Czech Republic – conceptual development of research organization („The National Institute of Public Health – NIPH, 75010330“).
Do redakce došlo dne 8. 3. 2016.
Adresa pro korespondenci:
RNDr. Milan Daniel, DrSc.
Státní zdravotní ústav
Šrobárova 48
100 42 Praha 10
e-mail: midaniel@seznam.cz
Sources
1. Daniel M, Danielová V, Kříž B, et al. The occurrence of Ixodes ricinus ticks and important tick-borne pathogens in areas with high tick-borne encephalitis incidence in different altitudinal levels of the Czech Republic. Part I. Ixodes ricinus ticks and tick-borne encephalitis virus. Epidemiol Mikrobiol Imunol, 2016; 65(2): 118–128.
2. Regional phytogeographical division of the Czech Republic.(Map) Prague: Academia Publ. House; 1987.
3. Kříž B, Beneš Č, Daniel M. et al. Incidence onemocnění klíšťovou encefalitidou v České republice v letech 2001–2011 v jednotlivých krajích a obcích s rozšířenou působností. (Incidence of tick-borne encephalitis in the Czech Republic in 2001–2011 in different administrative regions and municipalities with extended power.) Epidemiol Mikrobiol Imunol, 2013; 62(1): 9–18. (In Czech.)
4. Wilson ML. Population ecology of ticks vectors: interaction, measurement, and analysis. In Sonenshine DE, Mather TN. (Eds.), Ecological dynamics of tick-borne zoonoses. Oxford Univ. Press, New York--Oxford; 1994. pp. 20–44.
5. Paulauskas A, Radzijevskaja J, Ambrasiene D, et al. Detection of tick-borne pathogens by molecular methods. Biologija 2008; 54 : 92–197.
6. Clark K, Hendricks A, Burge D. Molecular identification and analysis of Borrelia burgdorferi sensu lato in lizards in the southeastern United States. Appl Environ Microbiol, 2005; 71 : 2616–2625.
7. Demaerschalck I, Ben Messaoud A, De Kesel M, et al. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J Clin Microbiol, 1995; 33 : 602–608.
8. Wang IN, Dykhuizen DE, Qiu W, et al. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics, 1999; 151 : 15–30.
9. Rudenko N, Golovchenko M, Mokráček A, et al. Detection of Borrelia bissettii in cardiac valve tissue of a patient with endocarditis and aortic valve stenosis in the Czech Republic. J Clin Microbiol, 2008; 46 : 3540–3543.
10. Rudenko N, Golovchenko M, Růžek D, et al. Molecular detection of Borrelia bissettii DNA in serum samples from patients in the Czech Republic with suspected borreliosis. FEMS Microbiol Lett, 2009; 292 : 274–281.
11. Golovchenko M, Vancová M, Clark K, et al. A divergent strain isolated from a resident of the southeastern United States was identified by MLST analysis as Borrelia bissettii. Parasites and Vectors, 2016; 9 : 68. doi 10.1186/s13071-016-1.
12. Rudenko N, Golovchenko M, Vancova M, et al. Isolation of live Borrelia burgdorferi sensu lato spirochetes from patients with undefined disorders and symptoms not typical for Lyme diseases. Clin Microbiol Infect, 2016; 22, 267.e9–267.e15. doi:10.1016/j.cmi.2015.11.009.
13. Gern L, Humair P-F. Ecology of Borrelia burgdorferi sensu lato in Europe. In Gray J, Kahl O, Lane RS et al. (Eds), Lyme Borreliosis Biology, Epidemiology and Control. New York, CABI Publishing 2002; pp.149–174.
14. Hönig V, Švec P, Halas P, et al. Ticks and tick-borne pathogens in South Bohemia (Czech Republic) – Spatial variability in Ixodes ricinus abundance, Borrelia burgdorferi and tick-borne encephalitis virus prevalence. Ticks Tick Borne Dis, 2015; 6 : 559–567.
15. Kaufman W R. Factors that determine sperm precedence in ticks, spiders and insects: a comparative study. In: Bowman AS, Nuttall PA. (Eds). Ticks biology, disease and control. Cambridge: Cambridge University Press; 2008 : 164–185.
16. Danielová V, Daniel M, Rudenko N, et al. Prevalence of Borrelia burgdorferi sensu lato genospecies in host-seeking Ixodes ricinus ticks in selected South Bohemian locations (Czech Republic). Cent Eur J Public Health, 2004; 12 : 151–156.
17. Rauter C, Hartung T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl Environ Microbiol, 2005; 71 : 7203–7216.
18. Margos G, Vollmer SA, Cornet M, et al. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl Environ Microbiol, 2009; 75 : 5410–5416.
19. Margos G, Wilske B, Sing A, et al. Borrelia bavariensis sp. nov. is widely distributed in Europe and Asia. Int J Syst Evol Microbiol, 2013; 63 : 4284–4288.
20. Danielová V, Daniel M, Schwarzová L, et al. Integration of a tick-borne encephalitis virus and Borrelia burgdorferi sensu lato into mountain ecosystems, following a shift in the altitudinal limit of distribution of their vector, Ixodes ricinus (Krkonoše mountains, Czech Republic). Vector Borne Zoonotic Dis, 2010; 10 : 223–230.
21. Lenčáková D, Hizo-Teufel C, Peťko B, et al. Prevalence of Borrelia burgdorferi s.l. OspA types in Ixodes ricinus ticks from selected localities in Slovakia and Poland. Int J Med Microbiol, 2006; 296 Suppl 40 : 108–118.
22. Hanincová K, Schäfer SM, Etti S, et al. Association of Borrelia afzelii with rodents in Europe. Parasitology, 2003; 126 (Pt 1):11–20.
23. Hubálek Z, Halouzka J. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur J Epidemiol, 1997; 13 : 951–957.
24. Derdáková M, Beati L, Peťko B. Genetic variability within Borrelia burgdorferi sensu lato genospecies established by PCR-single-strand conformation polymorphism analysis of the rrfA-rrlB intergenic spacer in Ixodes ricinus ticks from the Czech Republic. Appl Environ Microbiol, 2003; 69 : 509–516.
25. Tappe J, Jordan D, Janecek E, et al. Revisited: Borrelia burgdorferi sensu lato infections in hard ticks (Ixodes ricinus) in the city of Hanover (Germany). Parasit Vectors, 2014; 7 : 441.
26. Földvári G, Farkas R, Lakos A. Borrelia spielmanii Erythema Migrans, Hungary. Emerg Infect Dis, 2005; 11 : 1794–1795.
27. Maraspin V, Ruzic-Sabljic E, Strle F. Lyme borreliosis and Borrelia spielmanii. Emerg Infect Dis, 2006; 12 : 1177.
28. Wang G, van Dam AP, Dankert J. Phenotypic and genetic characterization of a novel Borrelia burgdorferi sensu lato isolate from a patient with Lyme borreliosis. J Clin Microbiol, 1999; 37 : 3025–3028.
29. Fingerle V, Michel H, Schulte-Spechtel U, et al. A14S – a new Borrelia burgdorferi s.l. genospecies as relevant cause of human disease [abstract]. Int J Med Microbiol, 2004; 294 (Suppl 1): 207.
30. Richter D, Schlee DB, Allgöwer R, et al. Relationships of a novel Lyme disease spirochete, Borrelia spielmani sp. nov., with its hosts in central Europe. Appl Environ Microbiol, 2004; 70 : 6414–6419.
31. Michel HB, Wilske B, Hettche G, et al. An ospA-polymerase chain reaction/restriction fragment length polymorphism – based method for sensitive detection and reliable differentiation of all European Borrelia burgdorferi sensu lato species and OspA types. Med Microbiol Immunol (Berl), 2003; 193 : 219–226.
32. Majláthová V, Majláth I, Derdáková M, et al. Borrelia lusitaniae and green lizards (Lacerta viridis), Karst Region, Slovakia. Emerg Infect Dis, 2006; 12 : 1895–1901.
33. Tarageľová VR, Mahríková L, Selyemová D, et al. Natural foci of Borrelia lusitaniae in a mountain region of Central Europe. Ticks Tick Borne Dis, 2016; 7(2): 350–356.
34. Diza E, Papa A, Vezyri E, et al. Borrelia valaisiana in cerebrospinal fluid. Emerg Infect Dis, 2004; 10 : 1692–01693.
35. Fraenkel CJ, Garpmo U, Berglund J. Determination of novel Borrelia genospecies in Swedish Ixodes ricinus ticks. J Clin Microbiol, 2002; 40 : 3308–3312.
36. De Michelis S, Sewell HS, Collares-Pereira M, et al. Genetic diversity of Borrelia burgdorferi sensu lato in ticks from mainland Portugal. J Clin Microbiol, 2000; 38 : 2128–2133.
37. Lommano E, Bertaiola L, Dupasquier C, et al. Infections and co-infections of questing Ixodes ricinus ticks by emerging zoonotic pathogens in Western Switzerland. Appl Environ Microbiol, 2012; 78 : 4606–4612.
38. Ryffel K, Péter O, Rutti B, et al. Scored antibody reactivity determined by immunoblotting shows an association between clinical manifestations and presence of Borrelia burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana in humans. J Clin Microbiol, 1999; 37 : 4086–4092.
39. Collares-Pereira M, Couceiro S, Franca I, et al. First isolation of Borrelia lusitaniae from a human patient. J Clin Microbiol, 2004; 42(3): 1316–1318.
40. da Franca I, Santos L, Mesquita T, et al. Lyme borreliosis in Portugal caused by Borrelia lusitaniae? Clinical report on the first patient with a positive skin isolate. Wiener klinische Wochenschrift, 2005; 117(11): 429–432.
41. Lopes de Carvalho I, Fonseca JE, Marques JG, et al. Vasculitis-like syndrome associated with Borrelia lusitaniae infection. Clin Rheumatol, 2008; 27(12): 1587–1591.
42. Hulínská D, Votýpka J, Kříž B, et al. Phenotypic and genotypic analysis of Borrelia spp. isolated from Ixodes ricinus ticks by using electrophoretic chips and real-time polymerase chain reaction. Folia Microbiol (Praha), 2007; 52 : 315–324.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2016 Issue 3-
All articles in this issue
- The incidence of viral hepatitis A in the Hradec Králové Region in the Czech Republic in the last decade
- Effect of lipophosphonoxins on inhibition of bacterial colonization of bone cements
- Stenotrophomonas maltophilia as the cause of ventilator-associated pneumonia in a female patient with toxic epidermal necrolysis and Clostridium colitis: time for off-label tigecycline?
- HIV/AIDS epidemics in sub-Saharan regions in the 2010s: Regional analysis of UNAIDS data
- Avidity of selected autoantibodies – usefulness of their determination for clinical purposes
-
The occurrence of Ixodes ricinus ticks and important tick-borne pathogens in areas with high tick-borne encephalitis prevalence in different altitudinal levels of the Czech Republic
Part II. Ixodes ricinus ticks and genospecies of Borrelia burgdorferi sensu lato complex - Campylobacteriosis in the South Bohemian Region – a Recurrent Problem
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Stenotrophomonas maltophilia as the cause of ventilator-associated pneumonia in a female patient with toxic epidermal necrolysis and Clostridium colitis: time for off-label tigecycline?
- Avidity of selected autoantibodies – usefulness of their determination for clinical purposes
-
The occurrence of Ixodes ricinus ticks and important tick-borne pathogens in areas with high tick-borne encephalitis prevalence in different altitudinal levels of the Czech Republic
Part II. Ixodes ricinus ticks and genospecies of Borrelia burgdorferi sensu lato complex - HIV/AIDS epidemics in sub-Saharan regions in the 2010s: Regional analysis of UNAIDS data
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

