-
Medical journals
- Career
Rotavirus gastroenteritis in the Czech Republic before the start of vaccination
Authors: Pazdiora Petr; Beneš Čestmír
Authors‘ workplace: Unit of Biostatistics and Informatics, National Institute of Public Health, Prague, Czech Republic ; Department of Epidemiology, Charles University in Prague, Faculty of Medicine, Pilsen, Czech Republic
Published in: Epidemiol. Mikrobiol. Imunol. 62, 2013, č. 4, s. 131-137
Overview
Background:
The primary objective of our study was to investigate rotavirus gastroenteritis (RVGE) retrospectively in the Czech Republic (CzR) and try to estimate its significance in the most affected age groups in the prerotavirus vaccine era.Methods:
To analyze the epidemiological data on RVGE in the CzR, two databases were used retrospectively. The first database consisted of regular yearly reports from the laboratories diagnosing rotavirus infections from 1998 to 2006. The second database used was EPIDAT (the official notification system of the hygiene service). The data from 1998 to 2006 was analysed.Results:
From 1998 to 2006, the laboratories reported 1,430 to 4,815 cases of RVGE per year. By extrapolation, in the CR in 2006, there were an estimated 4,076 rotavirus-related hospitalizations (696.7/100,000 in the age group < 5 years). The most commonly applied detection systems in 2006 were immunochromatography and latex agglutination. Of the RVGE cases recorded in the Epidat database between 1998–2006, 76.0–89.2% were for children aged less than five years. Seasonality was observed with the highest incidence rates between January and May with most cases usually occurring in March. Over nine years, there were six deaths linked directly to RVGE – three deaths reported in children under two and three deaths reported in elderly people whose deaths were related to the epidemics in retirement homes.Conclusions:
The estimated incidence rates of RVGE hospitalization in 2006 was higher in the CzR than that reported in other industrialized European countries. Our findings might verify the need for rotavirus vaccine implementation in the Czech Republic and reinforce the importance of rotavirus gastroenteritis surveillance.Keywords:
Czech Republic – gastroenteritis – rotavirus – seasonalityRotaviruses were discovered in animals in the 1960s. The virus was first described in humans when it was found by electron microscopy in duodenal biopsies from children with acute gastroenteritis. Seven rotavirus serogroups (serogroups A to G) have been described. Most human pathogens belong to groups A, B, and C. Group A rotaviruses are the most important from a public health point of view [1, 2].
The symptoms of rotavirus gastroenteritis (RVGE) include diarrhea and vomiting which may lead to severe dehydration and even death unless rehydration therapy is promptly initiated. Every year, an estimated 611,000 children die worldwide of rotavirus disease, mainly in low-income countries. Studies between 2000–2004 indicate that rotavirus causes approximately 39% of childhood diarrhea hospitalizations worldwide [3]. By the age of five, nearly every child will have had an episode of rotavirus gastroenteritis, one in five will visit a clinic, one in 50 will be hospitalized, and approximately one in 205 will die. Although severity and mortality rates associated with RVGE may differ, disease rates among children in developed and developing countries are similar [2, 4–6].
In the European Union, the annual burden of rotavirus disease is estimated at 231 deaths and almost 700,000 outpatient visits for children under five. Rotavirus is a significant cause of hospitalization. One in 54 children < 5 years of age with RVGE are hospitalized causing almost 87,000 hospital admissions in the European Union [7]. Due to the registration of two vaccines against RVGE in 2006, there has been growing interest in surveillance of this infection and in obtaining medical and economic data in order to support the launch of the vaccination in individual countries. A number of studies conducted in European countries have described RVGE hospitalization rates and the impact of RVGE on primary care centers [8–19].
The main aim of our study was to investigate RVGE in the Czech Republic (CzR) retrospectively and to try and estimate its significance in the most affected age groups before the start of vaccination.
METHODS
To analyze the epidemiological data on RVGE before the start of vaccination against rotavirus infection in the CzR, two databases were used retrospectively.
The Department of Epidemiology of the Medical Faculty of Charles University in Pilsen has been receiving regular yearly reports from all Czech laboratories diagnosing rotavirus infections since 1998. Although in the 1990s there were effectively only regional laboratories, in recent years, local district laboratories have also taken up the diagnosing and reporting. The following data was reported from 1998–2006: the number of tests, the total number of positive tests, the number of positive tests in children under three (from 2004 also under 5’s), the number of positive tests per month and area (district) covered by the tests, the methods, and the diagnostic kit applied. The lab report rate was considered to be equivalent to the hospitalization rate. Co-operation was on a voluntary basis. Due to the fact that the co-operating diagnosing laboratories did not perform the tests for all districts in the CzR, the collected data was recalculated according to the population of those districts diagnostically covered.
The second database used was EPIDAT. It is the official mandatory notification system of the hygiene service and it is driven by clinician notification and/or laboratory notification. Rotavirus infections have been reported to EPIDAT since 1993. The data from 1998 to 2006 was analysed. The reported infections were divided into two subgroups – sporadic (without temporal and topical association) and epidemic (at least three cases with these associations).
To estimate the burden of rotavirus disease in the CzR, we used the method previously applied by M Soriano-Gabarro et al. [7]. According to their model for EU countries, 370 hospitalizations due to rotavirus infection were calculated per 100,000 children under five, while for each inpatient there are eight outpatients and for each outpatient there are four home-treated cases.
RESULTS
Between 1998 and 2006, in the CzR there were 96,000–105,000 newborns per year and altogether there were 445,974–486,327 children under the age of five depending on the particular year. In the period in question there were 5,300 beds in pediatric and infection wards all over the country.
Table 1 presents the epidemiological data obtained from the Department of Epidemiology of the Medical Faculty of Charles University in Pilsen including the number of laboratories, number of confirmed rotavirus infections, and the calculated incidence rates per 100,000. The number of co-operating laboratories rose by 142%, from 33 in 1998 to 80 in 2006. While the number of confirmed rotavirus infections was 1,430 in 1998; it increased by 237% to 4,815 in 2006. Both incidence rates per 100,000 inhabitants and incidence rates for children under three increased by 242% and 253% respectively.
1. Laboratory results of rotavirus diagnostics (CzR, 1998–2006) Tabulka 1. Výsledky laboratorní diagnostiky rotavirů (ČR, 1998–2006) 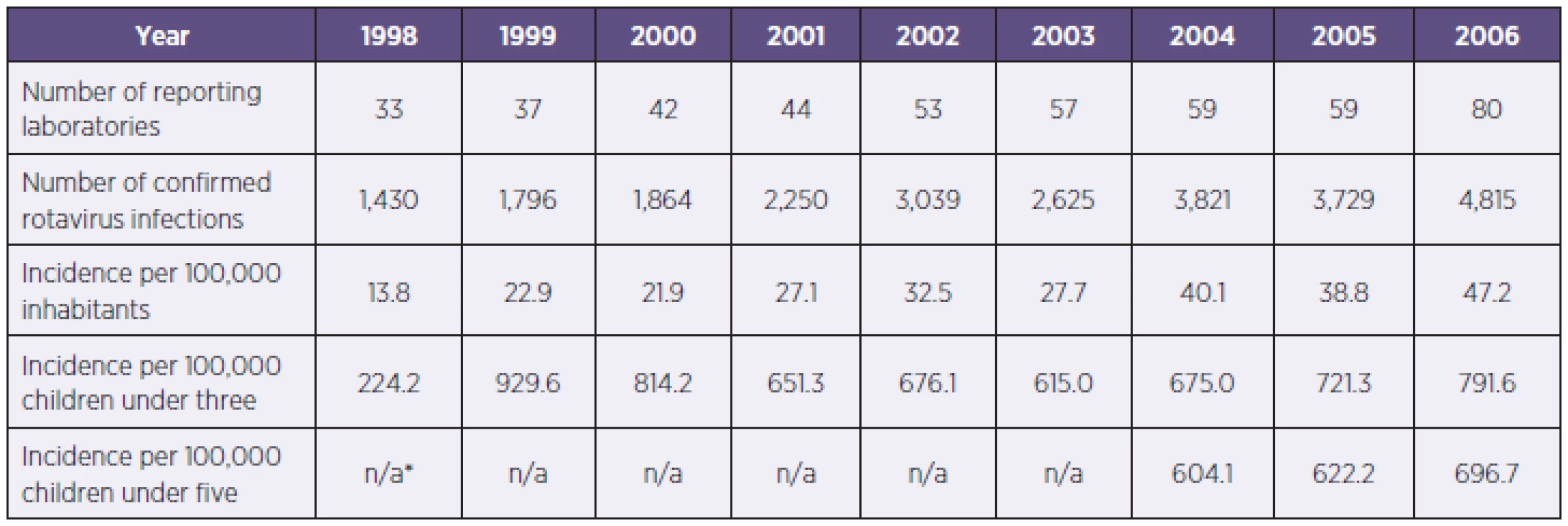
*n/a: not available until 2004. In 1998, the most commonly used detection system was latex agglutination – 28 times (84.8%), while in 2006 the most commonly applied detection systems were immunochromatography (55.0%) and latex agglutination (42.5%) amongst all 80 laboratories – only five laboratories used ELISA tests or electron microscopy (once).
Table 2 presents selected EPIDAT data. It shows both sporadic and confirmed epidemic infections. The number of RVGE officially reported during the observed period increased from 423 in 1998 to 3,657 cases in 2006, and total reported laboratory-confirmed sporadic infections was 12,184 for the whole observed period. 52.8% of the total of all reported sporadic infections occurred in boys/men. Although a slight decrease was observed in the proportion over the nine-year period, children under three was the most affected age group in all observed years. In 2006, this group made up 58.9% of the reported infections. Over nine years, there were six deaths linked to RVGE – three deaths reported in children under two and three deaths reported in elderly people (aged 82, 94, and 96) whose deaths were related to the epidemics in retirement homes. During the observed period, the percentage of laboratory confirmed infections reported to EPIDAT rose from 19.5 to 62.8%.
2. Reported rotavirus infections (EPIDAT, CzR 1998–2006) Tabulka 2. Hlášené rotavirové infekce (EPIDAT, ČR, 1998–2006) 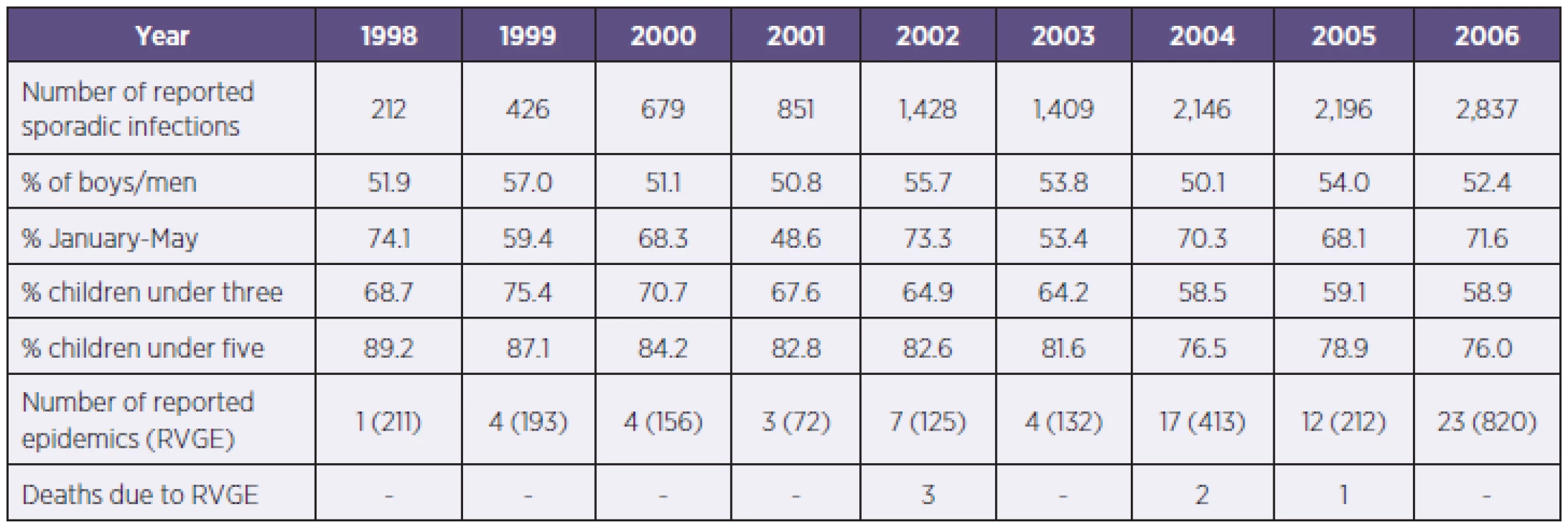
From the EPIDAT data the highest incidence rates of rotavirus infections were observed between January and May with the annual percentage of diagnosed sporadic cases ranging from 48.6 to 74.1% (Fig. 1). Over the observed period, 17.5 and 16.4% of cases occurred in March and April respectively, while September and October made up only 3.0% of the infections. We found a seasonal pattern in rotavirus infections and over the nine observed years the highest incidence occurred five times in March.
Fig.1 Seasonality of rotavirus infections in selected age-groups (CzR, 1998–2006) Obr. 1 Sezonnost rotavirových infekcí ve vybraných věkových skupinách (ČR, 1998–2006) 
To estimate the burden of rotavirus disease in the CzR, we applied the previously mentioned model by M Soriano-Gabarro et al. [7]. According to the national Czech database in 2006 there were 486,327 children under the age of five. Table 3 represents two different estimates based on different initial incidence rates. The first estimate was made with the initial incidence rate at 370/100,000 according to M Soriano-Gabarro’s study [7], and the estimated number of inpatients was found to be 2,165. The second estimate was made with the incidence rate at 696.7/100,000 based on the 2006 laboratory observation. Applying the same pattern, the number of inpatients totaled 4,076.
3. Estimated annual incidence of rotavirus infections in children under five in the CzR (2006) Tabulka 3. Odhadovaná roční incidence rotavirových infekcí v ČR u dětí do 5 let (2006) 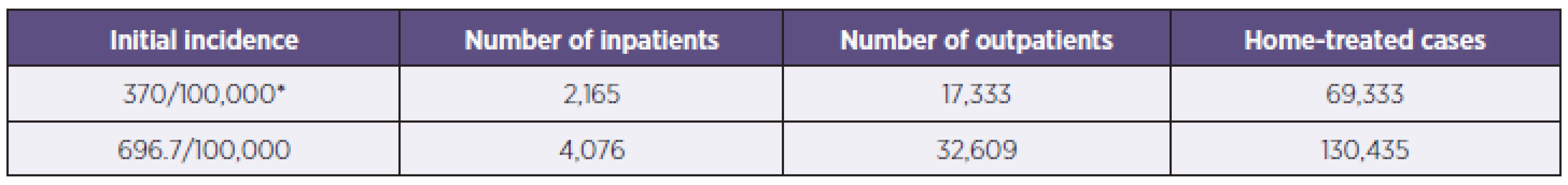
*Ref [7] DISCUSSION
The diagnosing of rotavirus infections commenced in the CzR as early as the late 1970s. The testing became widely accessible in the course of the 1990s when regional, and then later district laboratories as well, were increasingly in demand [15]. The gradual expansion of testing led to a subsequent increase in reported infections – from 53 in 1993, to 3,657 in 2006. This development is also reflected in the results of the presented study from the period 1998–2006. The collected data must still be considered basic and for a variety of reasons incomplete. Specifically, surveillance data are representative of cases for which a biological specimen was tested, not necessarily all rotavirus infections. It may be estimated that the number of tests performed and infections confirmed will continue growing as the diagnostics become incorporated into all children’s wards in the country. But this will make up only a fraction of cases occurring in the community as only a minority seek medical care, and of this small number, only a part will undergo testing of stool specimens. The patterns of disease in adults are less likely to be represented in these data – most laboratories (but also clinicians) do not routinely test patients other than children.
Both sources of data used have their limits set by the development of diagnostic methods and their accessibility and also the methods of data collection. Currently, laboratories provide more precise basic data on the incidence and trends in the CzR. Despite a certain possible distortion (repeated tests, nosocomial infection detection or occasional detection of outbreaks among outpatients), the data equals the number of hospitalized cases with rotavirus infection in its respective parts of the country. The collected data is close to the results of studies based on laboratory data and details of hospitalized cases in other European countries.
Over the years in question, the number of reported RVGE in both databases soared. With regard to laboratory surveillance, the reported RVGE episodes went up by 237% from the original figure of 1,430 cases. The increase in laboratory-reported RVGE was influenced not only by the greater number of diagnosing laboratories, but also largely by the higher frequency of testing in child patients with gastroenteritis, and partially by the higher sensitivity of the test used. The interest in the virological diagnostics of gastroenteritis spread gradually from the regional infection wards to district hospital children’s wards. The growing willingness of many laboratories with smaller numbers of tests performed i.e. positive confirmations also played a partial role. Similarly, there was an increase in RVGE that was officially reported to the EPIDAT database, rising from 423 cases in 1998 to 3,657 in 2006. This change is related both to a higher quality of laboratory reporting and to the increasing interest of epidemiologists all over the country in gathering more objective data. The surveillance span was also indirectly influenced by a gradually increase in data on the high RVGE incidence in other countries and the real availability of vaccination.
The incidence of 600–700 per 100,000 children under five equals, for example, the results from periods before the start of vaccination from Austria or France [9,14], while it is lower than results from Ireland or data collected for the REVEAL Study in Belgium, France and Sweden (12.0–7.7/1,000) [10, 17]. Data from other European countries for this age group are lower [8, 11, 13, 18, 19]. When evaluating the differences among these countries, not only the variety of detecting methods used, but also different hospitalization policies and the approach to health care of parents should be considered. Hence country-specific epidemiologic data is the most important tool for understanding the burden of rotavirus infection locally.
Due to the fact that in the CzR gastroenteritis is predominantly tested in hospitalized patients and nosocomial diarrhoea is rarely explained virologically a possible statistical distortion caused by nosocomial and outpatient treated diarrhoea cases has been ignored.
More precise data may only be collected from prospective studies with an active approach to diagnostics. At present, this problem has only attracted the attention of two institutions in the Czech Republic, in Pilsen and Prague [15, 16, 20–22]. While routinely examining all children admitted for hospitalization to wards in Pilsen in 2006, the incidence of rotavirus infections in Pilsen children under three was confirmed 2,100/100,000 cases.
Data obtained from the information system of the CzR hygiene service may serve to provide more detailed analysis as to the age, sex, nationality, residence, clinical significance, seasonality, epidemic frequency, community etc. An essential precondition for the validity of the EPIDAT data is better quality of reporting; during the observed period, the percentage of the reported laboratory confirmed infections rose (from 20 to 63%). Therefore, currently, even such a data source may be considered representative.
When evaluating epidemiological situations, it is essential to consider the differences in the number of laboratories and their accessibility, and also the sensitivity of diagnostic kits, especially when performing the tests with a certain delay after the first symptoms. The fact that Czech laboratories use a variety of methods is primarily down to finance, but also, the number of tests performed. The methods with the highest sensitivity (ELISA, electron microscopy) are used particularly by the laboratories with a high number of tests for regional health centers.
In the case of occasional tests for smaller childrens’ wards most laboratories logically prefer cheaper methods which may be used immediately after receiving a sample. However, in such cases, a lower sensitivity must be taken into consideration. Widespread use of the most sensitive tests logically results in more rotavirus infection confirmations and consequently in a higher incidence. Such differences also appear during routine examinations in other countries [21, 23–25].
In the CzR, rotavirus infections are present all year round with the highest figures regularly being from January to May and the lowest number of infections diagnosed in September and October. The nine-year period confirmed certain variations in seasonality with the peak most commonly coming in March (five times). The year 2001 was exceptional, when the number of cases remained higher even in the second half of the year. The seasonality affects all age groups. This does not differ from the epidemiological situation in most European countries. It is interesting to compare the data on seasonality with neighboring countries, in Austria from November to May with a peak in March, in Hungary December to February [14, 26]; from Czech long-term records the season starts as early as January, peaking in March and April. Similar differences were observed before the start of universal vaccination in Western and Northern Europe [17, 27].
The more studies performed from a variety of European countries the more differences in the incidence of rotavirus infections appear not only to be between the countries, but also within the territories of individual countries [28–30]. Due to the spread in diagnostics of rotavirus serotypes and genotypes, it will be possible to evaluate the relationship of the types to the variety of incidence, seasonality etc. [31, 32]. The first data mapping the serotype representation in the CzR indicate certain differences between various Central European countries [20, 22, 33, 34].
As in other countries, most rotavirus infections were confirmed in the youngest age groups. In the CzR, infections of children under three comprise 58–75% and under five between 76–89% of all infections reported. According to the results of the Pilsen and Prague institutions, most hospitalized children with rotavirus infection fall within the age group from 7 to 36 months. Also as in other countries, the infection more often affects boys or men. Although the problem of rotavirus infection in older age groups rarely receives special attention, it is necessary to point out their significance not only in relation to nosocomial transmission but also to transmission within families, psychiatric hospitals, retirement homes and other such institutions. It is confirmed by three reported lethal cases of the infection in elderly people. The numbers of these cases in older age groups are certainly underreported.
In developed countries, deaths resulting from rotavirus infections at an early age are rather exceptional. The number of deaths is closely related to the standard of living and income [7]. It is estimated that every year, 231 children under five die in the European Union. When analyzing the situation in Central and Eastern Europe, the number of deaths is estimated at 12 per year, and 52 per year including Russia [12]. Over the nine years of this study, the CzR reported three deaths of small children (between 1 and 18 months). Every new local study may contribute to greater precision in the figures currently estimated, which are based on the results of studies carried out in only a limited number of countries. This is also confirmed by different estimates of the number of rotavirus infections based on our own long-term data. Deaths of infants associated with rotavirus gastroenteritis are not acceptable in developed countries, especially when they are preventable by vaccination.
CONCLUSIONS
Our findings might verify the need for universal rotavirus vaccine implementation in the CzR and reinforce the importance of RVGE surveillance including routine testing. The vaccination against rotavirus infections started in 2007 in the CzR, but the coverage is still very low – by in the end of 2012 standing at only 16% among children born in this year . We also plan to analyse data from the period after the start of vaccination against these infections.
Acknowledgements: We thank all the laboratories and epidemiologists who participated in the study for their contribution. We thank Jaroslava Čechová from the Department of Epidemiology at the Medical Faculty in Pilsen for her technical assistance.
The study was supported by a financial grant from the Ministry od Education, Youth and Sports, the Czech Republic (VZ MSM 0021620819).
Conflict of interest
The authors declare that there is no conflict of interest.
Do redakce došlo dne 9. 7. 2013.
Corresponding author:
Prof. Petr Pazdiora, M.D., Ph.D.
Dr. E. Beneše 13
305 99 Plzeň
Česká republika (Czech Republic)
e-mail: pazdiora@fnplzen.cz
Sources
1. Bishop, R. F., Davidson, G. P., Holmes, I. H., Ruck, B. J. Virus particle in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet, 1973, roč. 2, s. 1281–1283.
2. Dennehy, P. H. Rotavirus vaccines: an overview. Clin. Microbiol. Rev., 2008, roč. 21, s. 198–208.
3. Parashar, U. D., Gibson, C. J., Bresee, J. S., Glass, R. I. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis., 2006, roč. 12, s. 304–306.
4. Glass, R. I., Bresee, J. S., Jiang, B., Parashar, U. Rotavirus and rotavirus vaccines. Adv. Exp. Med. Biol., 2006, roč. 582, s. 45–54.
5. Parashar, U. D., Hummelman, E. G., Bresee, J. S., Miller, M. A. et al. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis., 2003, roč. 9, s. 565–572.
6. Giaquinto, C., Van Damme, P., Huet, F., Gothefors, L. et al. Clinical consequences of rotavirus acute gastroenteritis in Europe, 2004-2005: The REVEAL study. J. Infect. Dis., 2007, roč. 195, s. 26–35.
7. Soriano-Gabarró, M., Mrukowicz, J., Vesikari, T., Verstraeten, T. Burden of rotavirus disease in European Union Countries. Pediatr. Infect.Dis. J., 2006, roč. 25, s. 7–11.
8. Fischer, T. K. Incidence of hospitalizations due to rotavirus gastroenteritis in Denmark. Acta Paediatr., 2001, roč. 90, s. 1073–1075.
9. Fourquet, F., Desenclos, J.C., Maurage, C., Baron, S. Acute gastro-enteritis in children in France: estimates of disease burden through national hospital discharge data. A. Pediatr., 2003, roč. 10, s. 861–868.
10. Handysides, S. Surveillance of rotavirus infection in Ireland 1997–1999. Eurosurveillance, 2001, roč. 5, s. 1804.
11. Luquero, A. F. J., Eiros, B. J. M., Rubio, A. P., Bachiller, L. M. R. et al. Gastroenteritis by rotavirus in Spanish children. Analysis of the disease burden. Eur. J. Pediatr., 2008, roč. 167, s. 549–555.
12. Mészner, Z., Balogh, A., Bányai, K., Kovacs, J. et al. The clinical burden of rotavirus disease: Retrospective analysis of infant and childhood gastroenteritis in seven countries in Central and Eastern Europe. Pediatr. Infect. Dis. J., 2008, roč. 27, s. 33–41.
13. Mrukowicz, J. Z., Krobicka, B., Duplagaski, J., Szajewska, H. et al. Epidemiology and impact of rotavirus diarrhoea in Poland. Acta Paediatr., suppl., 1999, roč. 88, s. 53–60.
14. Rendi-Wagner, P., Kundi, M., Mikolasek, A., Mutz, I. et al. Active hospital-based surveillance of rotavirus diarrhea in Austrian children, period 1997 to 2003. Wien. Klin. Wochenschr., 2006, roč. 118, s. 280–285.
15. Pazdiora, P. Diagnosis of rotavirus infections in 2004, major characteristics in 1998–2004 in the Czech Republic. Epidemiol. Mikrobiol. Imunol., 2006, roč. 55, s. 32–36.
16. Ambrožová, H., Schramlová, J. Viral gastroenteritis in children. Klin. Mikrobiol. Inf. Lék., 2005, roč. 1, s. 83–91.
17. Van Damme, P., Giaquinto, C., Huet, F., Gothefors, L. et al. Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004–2005: The REVEAL Study. J. Infect. Dis., 2007, roč. 195, s. 4–16.
18. Vesikari, T., Rautanen, T., Von Bonsdorff, Ch. Rotavirus gastroenteritis in Finland: burden of disease and epidemiological features. Acta Paediatr., suppl., 1999, roč. 88, s. 24–30.
19. Ryan, M. J., Ramsay, M., Brown, D., Gay, N. J. et al. Hospital admissions attributable to rotavirus infection in England and Wales. J. Infect. Dis., 1996, roč. 174, s. 12–18.
20. Pazdiora, P., Švecová, M. G-serotypes of group A rotaviruses in Pilsen region (Czechia). Folia Microbiol. (Praha), 2006, roč. 21, s. 133–135.
21. Arientova, S., Schramlova, J., Ambrozova, H., Maresova, V. et al. Electron microscopy in the diagnosis of viral gastroenteritis in hospitalised children in the Czech Republic. Folia Microbiol. (Praha), 2012, roč. 57, s. 177–182.
22. Diez-Domingo, J., Baldo, J. M., Patrzalek, M., Pazdiora, P. et al. Primary care-based surveillance to estimate the burden of rotavirus gastroenteritis among children aged less than 5 years in six European countries. Eur. J. Pediatr., 2011, roč. 170, s. 213–222.
23. Dewar, J., de Beer, M., Elliott, E., Monaisa, P. et al. Rapid detection of rotaviruses—are laboratories underestimating infection in infants? S. Afr. Med. J., 2005, roč. 95, s. 494–495.
24. Gunson, R. N., Miller, J., Leonard, A., Carman, W. F. Importance of PCR in the diagnosis and understanding of rotavirus illness in the community. Commun. Dis. Public Health, 2006, roč. 6, s. 63–65.
25. Atchison, C. J., Lopman, B. A., Harris, C. J., Tam, C. C. et al. Clinical laboratory practices for the detection of rotavirus in England and Wales: can surveillance based on routine laboratory testing data be used to evaluate the impact of vaccination? Euro Surveill., 2009, roč. 14, s. 1–6.
26. Szücs, G., Uj, M., Mihály, I., Deák, J. Burden of human rotavirus-associated hospitalizations in three geographic regions of Hungary. Acta Paediatr., suppl., 1999, roč. 88, s. 61–65.
27. Koopmans, M., Brown, D. Seasonality and diversity of group A rotaviruses in Netherland. Acta Paediatr., suppl., 1999, roč. 88, s. 31–37.
28. Carlin, J. B., Chondros, P., Masendycz, P., Bugg, H. Rotavirus infection and rates of hospitalisation for acute gastroenteritis in young children in Australia, 1993–996. Med. J. Aust., 1998, roč. 169, s. 252–256.
29. Desenclos, J. C, Rebiere, I., Letrillard, L., Flafault, A. et al. Diarrhoea-related morbidity and rotavirus infection in France. Acta Paediatr., suppl., 1999, roč. 88, s. 42–47.
30. Frühwirth, M., Heininger, U., Ehlken, B., Petersen, G. et al. International variation in diaease burden of rotavirus gastroenteritis in children with community - and nosocomially acquired infection. Pediatr. Infect. Dis. J., 2001, roč. 20, s. 784–791.
31. Santos, N., Hoshino, Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol., 2005, roč. 15, s. 29–56.
32. Van Damme, P., Giaquinto, C., Maxwell, M., Todd, P. et al. Distribution of rotavirus genotypes in Europe, 2004-2005: The REVEAL Study. J. Infect. Dis., 2007, roč. 195, s. 17–25.
33. Ehlken, B., Laubereau, B, Karmaus, W., Petersen, G. Prospective population-based study on rotavirus disease in Germany. Acta Paediatr., 2002, roč. 91, s. 769–775.
34. Tcheremenskaia, O., Marucci, G., De Petris, S., Ruggeri, F. M. et al. Molecular epidemiology of rotavirus in Central and Southeastern Europe. J. Clin. Microbiol., 2007, roč. 45, s. 2197–2204.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2013 Issue 4-
All articles in this issue
- Invasive meningococcal disease in the Czech Republic – analysis of the epidemiological situation and vaccination strategy recommendations
- Molecular biological and epidemiological characteristics of the varicella-zoster virus (VZV)
- Bacterial contamination of the indoor air in a transplant unit
- Rotavirus gastroenteritis in the Czech Republic before the start of vaccination
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Bacterial contamination of the indoor air in a transplant unit
- Molecular biological and epidemiological characteristics of the varicella-zoster virus (VZV)
- Invasive meningococcal disease in the Czech Republic – analysis of the epidemiological situation and vaccination strategy recommendations
- Rotavirus gastroenteritis in the Czech Republic before the start of vaccination
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

