-
Medical journals
- Career
Update on urinary bladder pathology
Authors: Eva Compérat
Authors‘ workplace: La Pitié-Salpêtrière, Université Pierre et Marie Curie, Paris VI
Published in: Čes.-slov. Patol., 50, 2014, No. 4, p. 129-136
Category: Review Articles - Uropathology
Overview
The clinical significance of different histological subtypes of bladder cancer is challenging. The presence of variant architecture identifies mostly a high-risk population with a worse prognosis and suited for complementary treatment. This review outlines the histological variants of bladder cancer and the diagnostic problems.
Keywords:
urothelial carcinoma - histology - variant histology - nested carcinoma - micropapillary carcinoma - inverted growth
Urothelial carcinomas (UC) present in numerous and very different aspects. Therefore, it is exceedingly important to remain familiar not only with the WHO classification, but also to keep updated with newly described entities (1). Furthermore, several studies have demonstrated that specific tumor subtypes show different clinical outcomes and might also respond differently to treatment (i.e. squamous cell carcinomas or small cell carcinomas). However, this fact is often hard to prove, as many of those subtypes are rare, some variants are difficult to diagnose and confirm, especially when there is no muscle invasive bladder cancer (MIBC). In these cases it is important to recognize and interpret the histological features correctly. The most challenging and probably the most difficult are the nested UC and the large nested variant. But also other entities might be problematic such as the micropapillary variant or the inverted growth pattern as well as poorly differentiated variants such as the plasmacytoid variant or the lymphoepithelial one.
The first part of the paper will briefly describe the well know entities and the difficulties in their diagnosis, the second part will treat the newly described or less known entities, and the last part will handle immunohistochemistry and the issue of the metastatic poorly differentiated lesions. This review will not consider the small cell and neuroendocrine bladder carcinomas.
WELL KNOWN SUBTYPES OF UROTHELIAL CARCINOMAS
Invasive micropapillary carcinoma
Invasive micropapillary carcinoma (IMPC), as described by Amin in 1994, is now a well know entity, and it is globally agreed that this subtype shows an aggressive behavior. There is a propensity for lymphovascular invasion and lymph node metastasis. A high stage presentation at the time of diagnosis is frequent. Nevertheless, there still exist several points, which have not been explored adequately. One major problem is the cut-off, when pathologists have to consider an UC as an IMPC. Amin did not specify in his first description a clear cut-off and several reports have suggested different percentages as a cut-off since then (2-5). Johanson, Samaratunga and Compérat suggested diagnosing IMPC even if the micropapillary component is less than 10 %. Similarly, Kamat et al. proposed rendering the diagnosis even when a minor component less than 5 % is present (6). Recent studies demonstrated that the clinical outcome of IMPC is related to the percentage. Most of these tumors are muscle invasive at the time of diagnosis. In case of TURB (transurethral resection and biopsy) with IMPC and no detrusor muscle present, a new TURB has to be perform. Kamat et al. reported upstaging in 52.7 % of cases, Compérat et al. in 79 % of cases after cystectomy (5,6). Vascular invasion (VI) might be difficult to establish, because of the hollow spaces around the nests, nevertheless VI is very common in the IMPC and one of the reasons for its aggressiveness (Fig. 1). VI can easily be confirmed by immunohistochemistry (IHC). The main problem is to distinguish IMPC of the bladder from other micropapillary adenocarcinomas such as carcinomas from the ovary, colon, pancreas, peritoneum or breast. The imaging studies to exclude any other distant primary lesion is a key to confirm the diagnosis.
1. IMPC, hollow spaces around the micropapillae. No fibrovascular cores in the papillae. 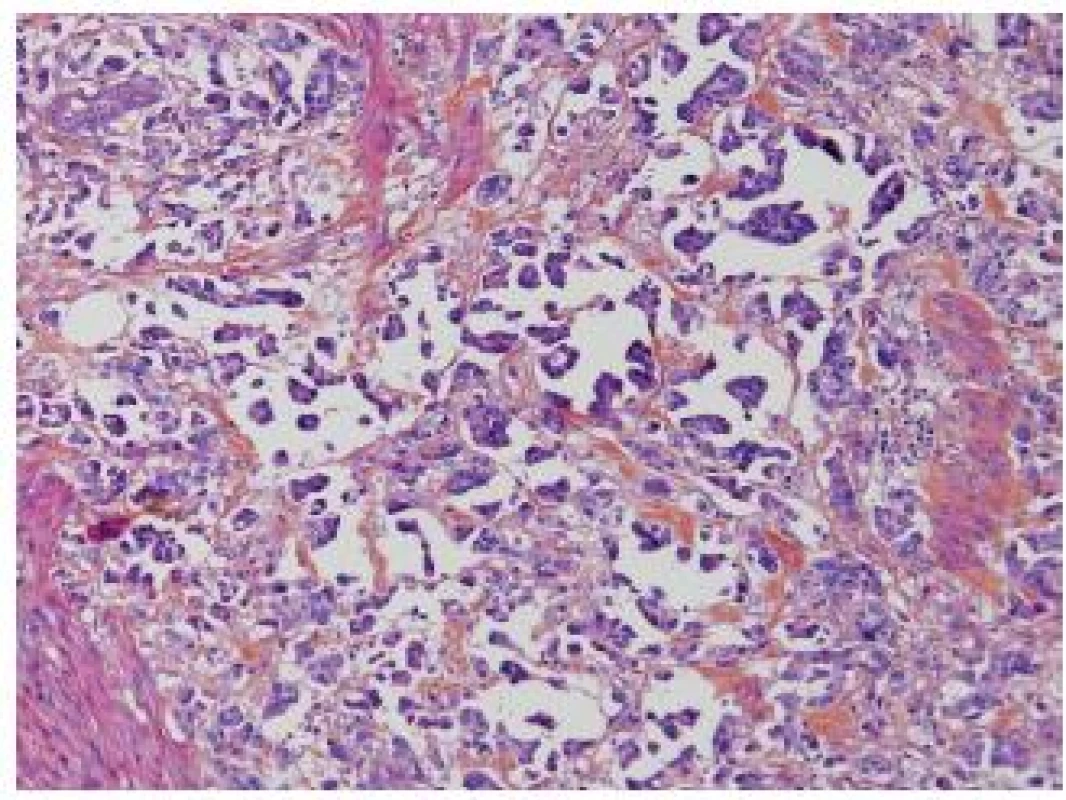
Furthermore, there is a spectrum of heterogeneity in morphology, and the difference between invasive UC with stromal reaction and IMPC is not always evident. Sangoi et al. demonstrated in a recent paper that the reproducibility of the diagnosis of IMPC had kappa value 0.54 (moderate reproducibility), although in the classic forms IMPC was well recognized with a 93% agreement. The highest association with IMPC diagnosis had multiple nests within the same retracted lacunar spaces, extensive retraction and back-to back lacunae, but morphologic features such as intracytoplasmic vacuolization, epithelial ring forms, and peripheral nuclei were also recorded as features of IMPC. Another criterion was the nest width, which in case of < 4.5 nuclei is a good argument in favor of IMPC. On the other hand, micropapillae described as elongated nests or processes were only moderately sensitive/specific (7). Although there is an overall agreement that recognizing IMPC is important, more consensus is required to define this entity.
Plasmacytoid carcinoma
The plasmacytoid UC is a very rare variant of the UC characterized by tumor cells, which resemble plasma cells. Tumor cells are arranged as single cells in a loose stroma, they have similarly to plasma cells abundant eosinophilic cytoplasm and are poorly differentiated (8) (Fig. 2A). The tumor might show other components such as sarcomatoid or high grade UC, but it presents quite frequently in its pure form (1). The plasmacytoid variant is rare and the published series are small. The incidence in the bladder is less than 1%, and in the majority of cases plasmacytoid carcinoma concerns men. This variant has a very particular aspect of discohesive single cells, with more or less dense eosinophilic cytoplasm, which might contain small vacuoles. The stroma is loose, edematous and sometimes myxoid. The tumor cells can be arranged in small nests, cords, or relatively frequently in sheets infiltrating diffusely the underlying tissue. A mixture with other UC types and carcinoma in situ (CIS) can be present and might be most helpful to make the right diagnosis of urothelial origin. As this tumor has a diffuse growth, staging can be difficult without using immunohistochemistry. The plasmacytoid variant is known to be aggressive in the bladder and the overall survival is poor. The cases of plasmacytoid UC present very often in an advanced stage with lymph node metastasis and even with an intraperitoneal spread (9). In a recent paper, Hartmann et al. demonstrated in a series of 21 patients that the increased nuclear E-cadherin expression and the loss of membrane expression increased risk of death 2 fold (p = 0.04) and the overall survival of these patients was 27.4 months (10). They suggest the cystectomy as a treatment option with adjuvant cisplatin based chemotherapy (11). It is important to recognize this entity as urothelial of origin, and not to misdiagnose it as a metastasis of a distant adenocarcinoma, melanoma or lymphoma.
2. A: Poorly differentiated UC with plasmocytoid features, the cells are discohesive, stroma is poor. B: Poorly differentiated UC with rhabdoid features. Pseudosarcomatous stroma in between the tumor cells. 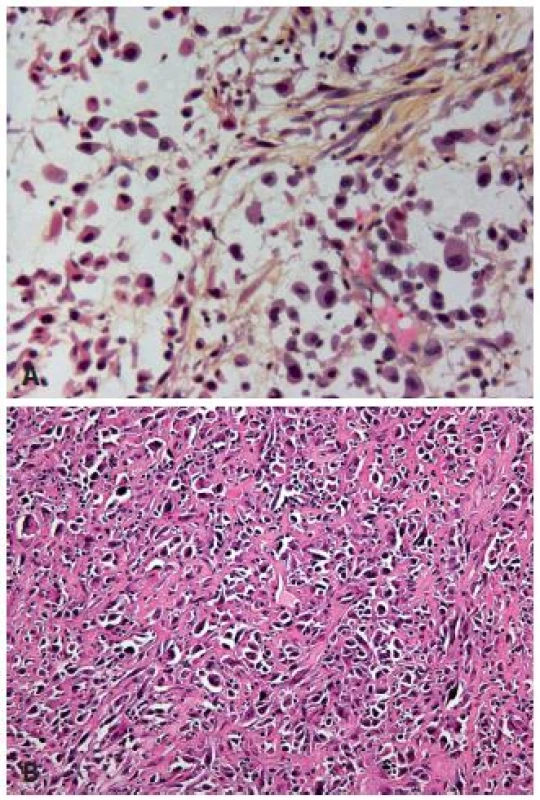
Rhabdoid features
UC can display focal rhabdoid features, which are characterized by large discohesive cells with distinctive cell borders (Fig. 2B). These carcinomas have to be considered as poorly differentiated lesions, and they are highly aggressive. The loss of INI-1 expression will distinguish malignant extra-renal rhabdoid tumor or renal medullary carcinoma from UC with rhabdoid features (12).
Lymphoepithelioma-like carcinoma
Another rare poorly differentiated entity difficult to recognize is the lymphoepithelioma-like UC, which resembles undifferentiated carcinomas as seen in the nasopharynx. The tumor displays male predominance and appears to have a better outcome than some of the other UC types. The neoplastic cells are large and are arranged in sheets, cords and trabeculae. Inflammatory infiltrate is predominantly lymphoid, but other inflammatory cells might also be present and they can completely obscure the epithelial tumor cells (Fig. 3). The presence of an associated CIS might be helpful to establish the diagnosis. As this type of UC is sometimes poorly circumscribed, the invasion might be difficult to evaluate and thus immunohistochemistry is very helpful to define the depth of infiltration. This tumor can occur in a pure form or in mixed forms with other types of UC (1,8). None of the cases is EBV-related, in contrast to its nasopharyngeal counterpart (13). The treatment of choice is the resection, and chemotherapy can be considered in case of localized tumors. Nevertheless, this entity should not be misdiagnosed as a lymphoma - epithelial markers help to guide pathologists to the correct diagnosis.
3. Obscuring inflammation in lymphoepithelioma-like carcinoma; the epithelial cells are not easily recognizable. 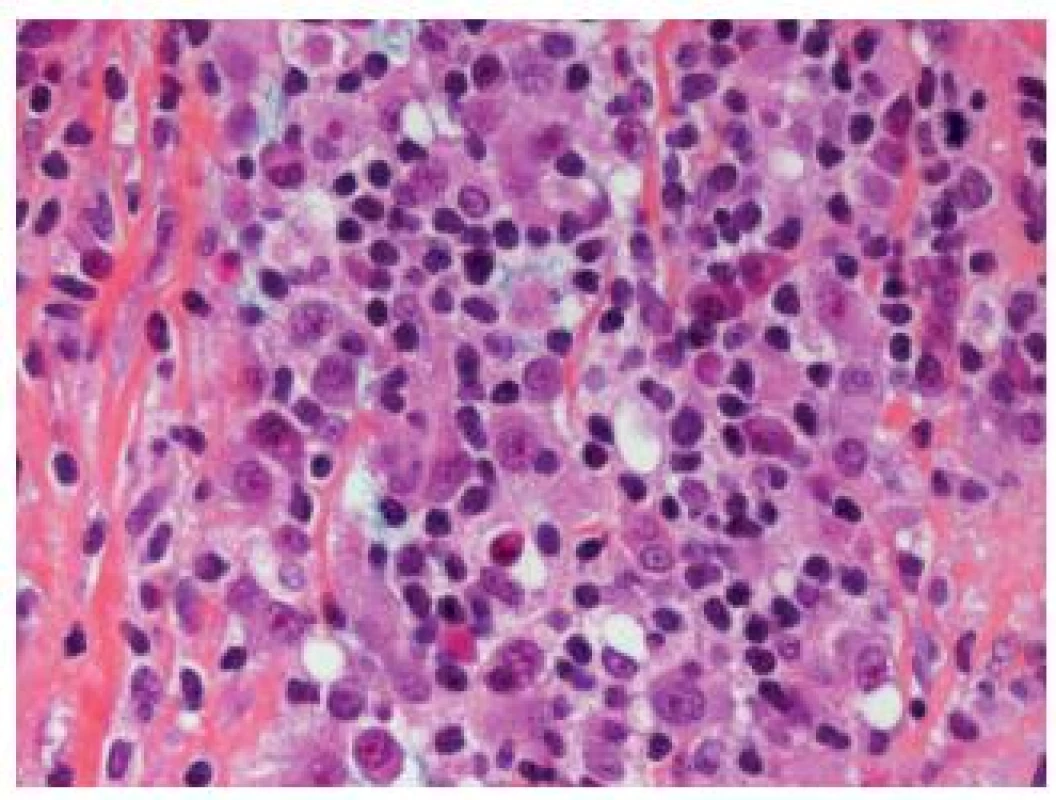
Clear cell UC
This entity is also called glycogen rich-type. The clear cell pattern is rarely predominant. More frequently, focal clearing of the cells can be observed in UC. Only few cases have been documented in the literature. Typically, this carcinoma is of a high grade and invasive, mostly with the solid growth. The cell membranes are well defined, cytoplasm is clear and abundant, nuclei are placed centrally and have pleomorphic aspects. This entity should not be misdiagnosed as a clear cell adenocarcinoma or a metastasis of a clear cell carcinoma (8,14).
Lipoid (“lipid-rich”) UC
This variant of UC displays large cells with an optically empty, mostly multivacuolated cytoplasm (Fig. 4). To call this entity, the pathologist should find at least 10 – 50 % of these cells (1,15). Ultrastructural studies could show that the cells contain lipids rather than mucin. The immunostains are the same as in a conventional UC.The outcome is poor.
4. Lipid-rich variant, this UC is poorly differentiated. 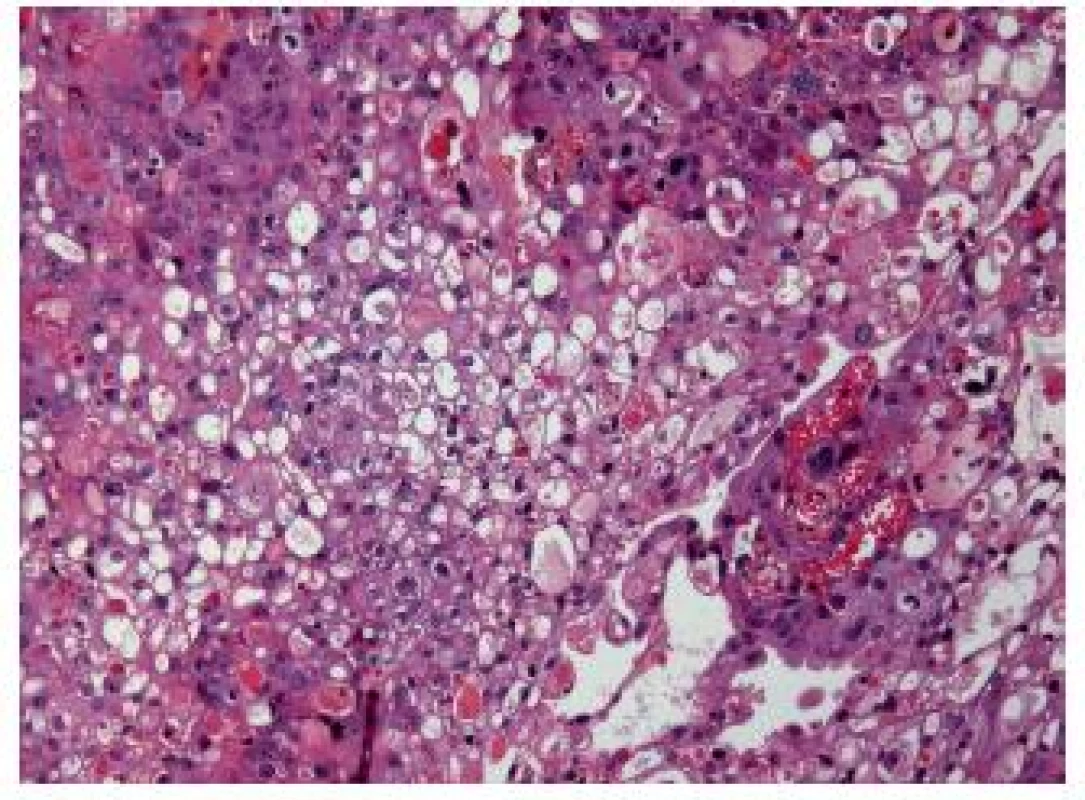
Nested carcinoma and closely related subtypes
The nested variant of UC is probably one the most difficult to recognize. This variant, described by Stern in 1979, but not recognized as a malignant lesion, was considered only as an infiltrating UC until 1989 (16,17). This entity can easily be under-recognized. The superficial component of this tumor is composed of small clusters of well delimited nests, with few or no atypia (Fig. 5A). Mitoses are rare, the immunohistochemical profile is close to the conventional UC (18). Therefore, the distinction from von Brunn’s nest is not easy. When the nested variant was compared to classical high grade UC, it presented more often as an invasive disease, especially on TURBs (70 % versus 31 % respectively), with more frequent extravesical involvement (83 % vs. 33 % respectively) and metastases (67 % vs. 19 % respectively). Even with therapy, 70 % of patients died of the disease within 4 - 40 months (17,19). Nevertheless, with the improved recognition of these lesions the patients seem to be treated in a better way, and recent studies demonstrated that despite the aggressive presentation, it had not a worse recurrence ratio or outcome than the classical UC (20) (Fig. 5).
5. A. Superficial infiltrating nested carcinoma. In case when no muscle is present, new TURB has to be done. B: Easily recognizable nested carcinoma with dense infiltration of the lamina propria. 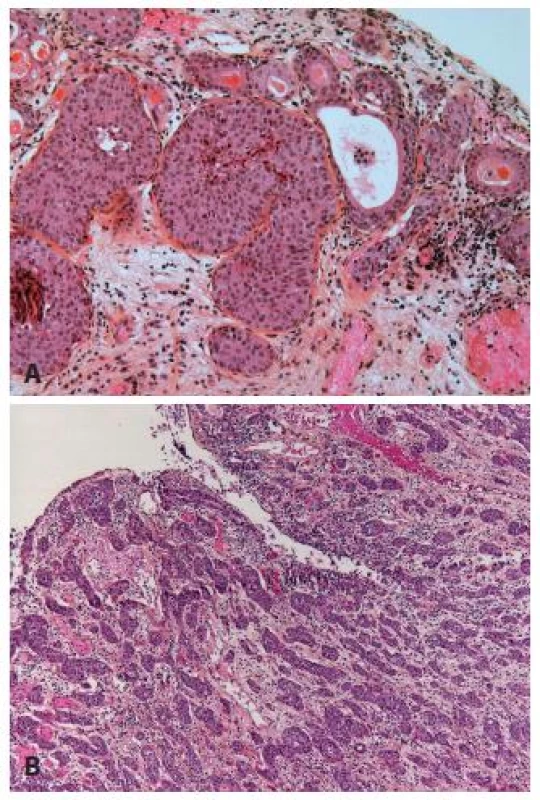
Small tubular type UC
Small tubular type UC is a histological subtype very closely related to the nested variant. This carcinoma is made of small to medium sized round elongated tubules, which might be misdiagnosed as a nephrogenic adenoma or cystitis glandularis (Fig. 6). Cytology of these tumors is low grade, nevertheless they have to be recognized as an invasive tumors. In case of muscle invasion the problem is quickly resolved. But, just like the nested variant, it might be very difficult to confirm the pT1 stage of these carcinomas, if they show only superficial invasion. The distinction from the extension of a prostatic carcinoma can be challenging, but immunohistochemistry helps to resolve the problem (8).
6. Small tubular type with few atypia, the stroma is edematous. 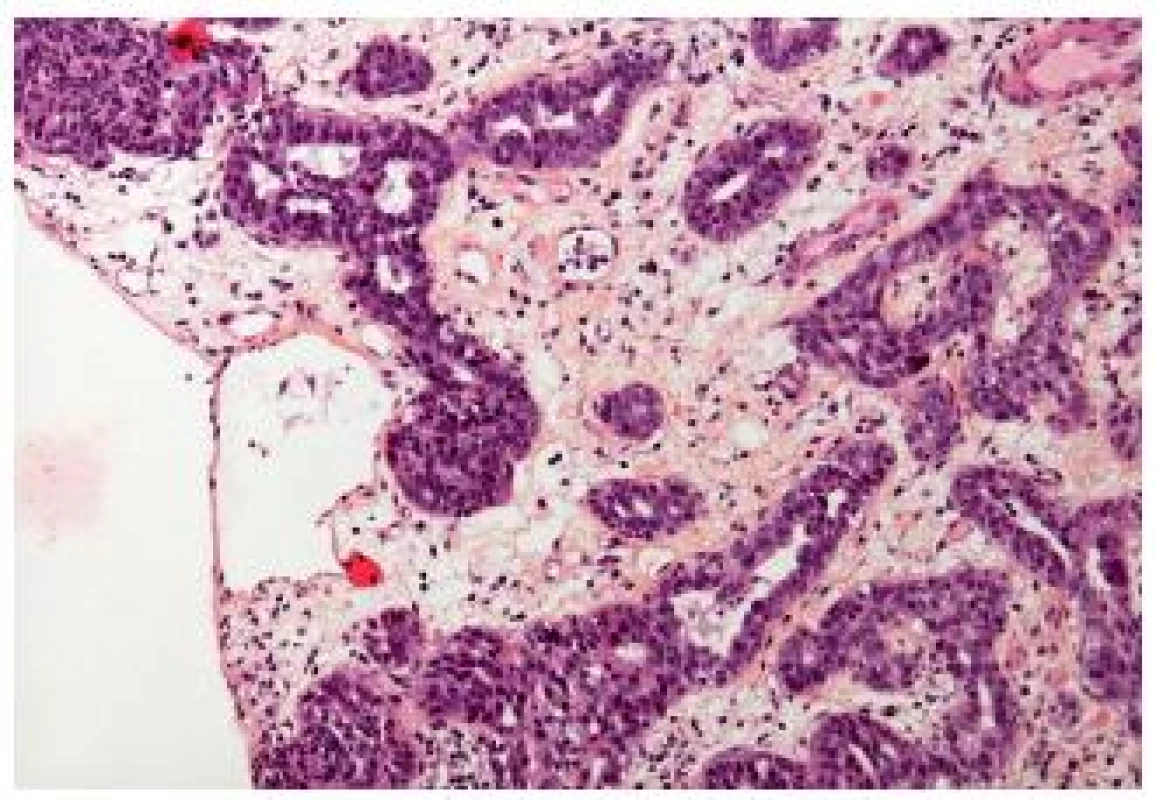
Microcystic carcinoma
The microcystic UC also belongs into the group of the deceptively “benign” carcinomas. There exist marked variations of these nested aspects and some authors consider that microcystic carcinomas represent a subtype of nested UC. Typically, pathologists observe numerous microcysts, nest of UC and UC with glandular differentiation (Fig. 7). Nevertheless, in case of few nests, it might be very difficult to confirm the invasive disease, and sometimes only at pT2 stage the diagnosis of invasive UC can be given with certitude. On the other hand, the nested/microcystic UC should not be mistaken and cystis cystitica or nephrogenic adenoma can be taken for invasive carcinoma, if florid. Especially in superficial biopsies or resection specimen, this variant is very challenging. A recent paper demonstrated variable positivity for cytokeratin 7 and 20, MUC1, MUC5AC, p63 and GATA-3 (21).
7. Microcystic variant of UC, well delimited carcinomatous nests infiltrating into the lamina propria. 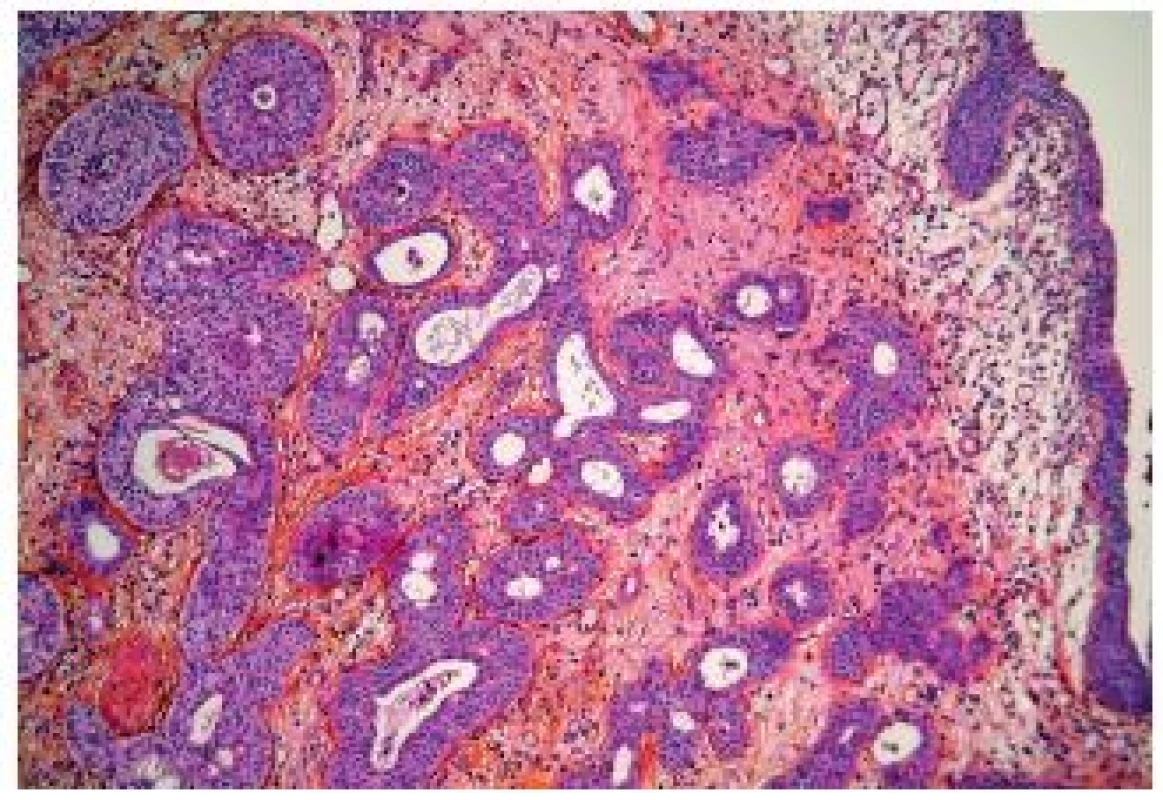
These cysts are of a small size (1 - 2 mm), mostly round or oval, and might contain secretion. Like all the other above mentioned subtypes of bland appearance, they do not have much atypia or mitoses. The growth is often infiltrative into the bladder wall. Nevertheless, the prognosis does not seem to be worse than in classical UC (21).
NEWLY DESCRIBED AND LESS KNOWN ENTITIES
Large cell tumors
This recently described subtype is characterized by the sheets of large polygonal or round cells with moderate to abundant cytoplasm and distinct cell borders. In more than half of the cases the tumor exists in a pure form (Fig. 8). The architecture varies from an infiltrating patterns to solid expansive nests. Focally discohesive patterns have been observed as well. Most patients present in an advanced stage with lymph node metastasis, the overall survival is poor (5 - 26 months), but only few data documented that. Nevertheless, this entity has to be recognized as an aggressive and advanced stage of UC (22).
8. Large cell UC, with severe atypia and poor differentiation. This type can be problematic to be recognized as a primary UC. 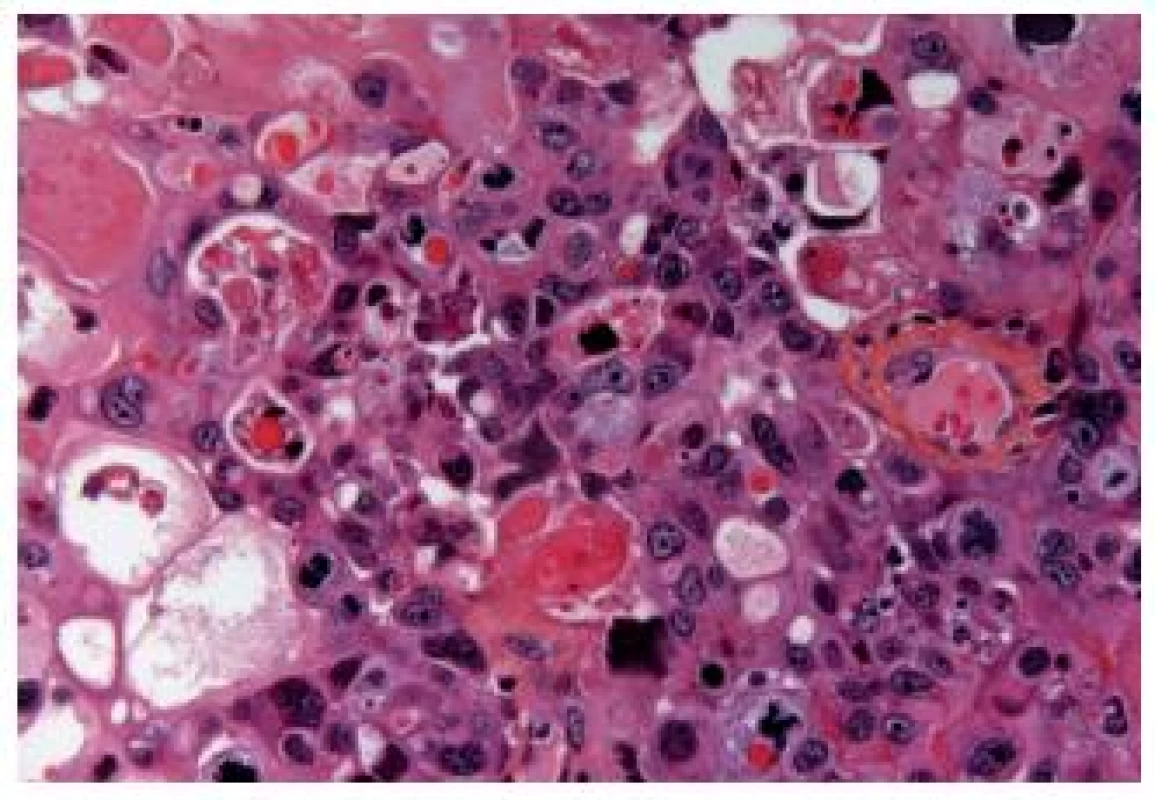
Large nested cell variant of urothelial carcinoma
Another newly described entity represents the large nested (LN) variant UC. These are invasive carcinomas, consisting of large nests of cells. These nests have a bland appearance, but they are frequently invasive into the lamina propria. They share features of the nested and papillary pattern as well as of the inverted papilloma-like growth. Therefore, it can be very difficult to confirm the invasiveness of this carcinoma. Cox and Epstein reported the largest series with 23 cases, nevertheless, this entity in a non-pure form might be more frequent. This variant, as all muscle invasive UCs, has to be considered as an aggressive disease with a metastatic potential. This entity might mimic von Brunn’s proliferation and inverted papilloma. Even in case of muscle invasion, the diagnosis can be difficult, because the well delimited nests can easily be mistaken as tangential cutting; in pT1 stage this issue becomes even worse (23) (Fig. 9).
9. Large nested cell variant. Well delimited large nests in the middle of the detrusor muscle. 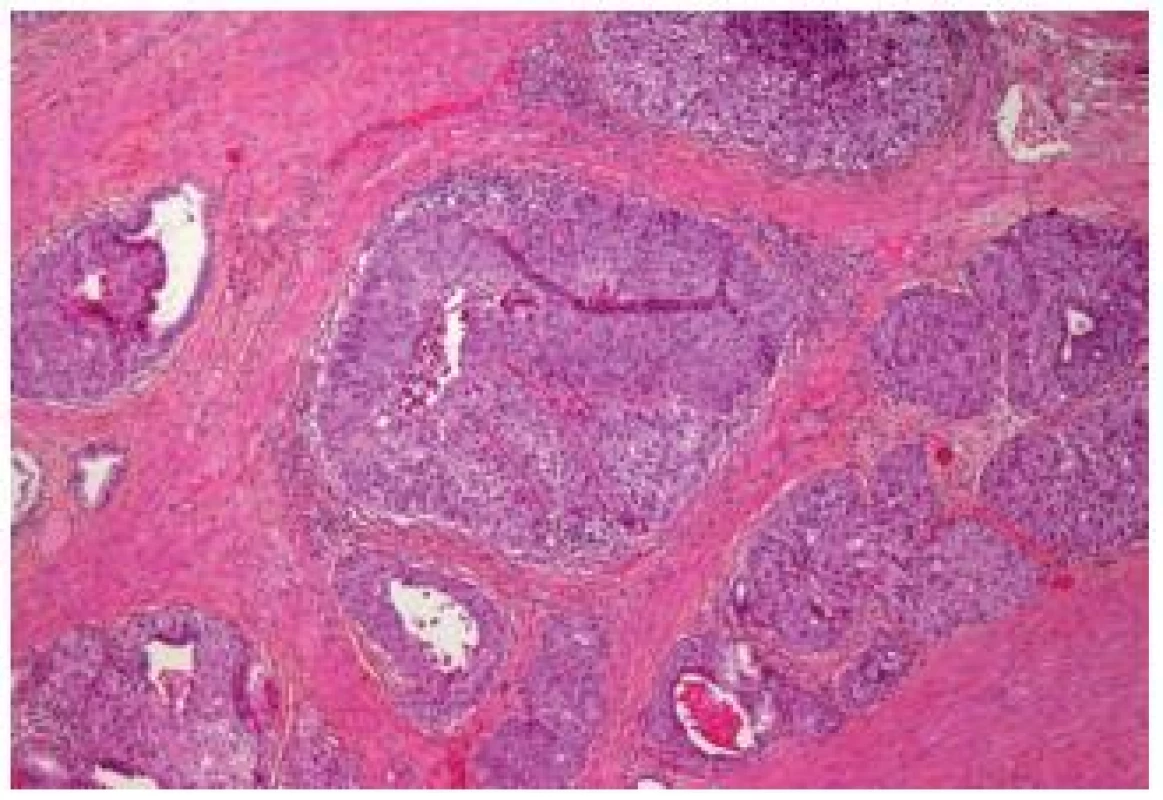
Inverted papilloma-like growth
This entity is a part of the benign looking, but nevertheless aggressive UC. It displays an inverted papilloma-like growth pattern. This entity does not figure in the WHO 2004 classification, but it has to be mentioned, especially because of the difficulties in its staging.
Major differential diagnosis is the inverted papilloma, and it might be very difficult to make the correct diagnosis even for experienced pathologists. Furthermore, it is complicated to establish the depth of invasion in these tumors (Fig. 10). UC with inverted growth has a tendency to have thicker cords and columns with irregularity and mitoses. Solid areas can be also observed. In contrast to the inverted papillomas, cytologic atypia is frequent as well as mitoses. On the other hand, necrosis is rarely seen; the peripheral spindling and palisading like in his benign counterpart is also more or less absent. This type of tumor shows varying pushing borders and the destruction of the bladder wall by an invasion is often not evident. Like other UCs, this tumor has to be graded, especially if not invasive (24).
10. Inverted UC infiltrating deeply into the lamina propria. 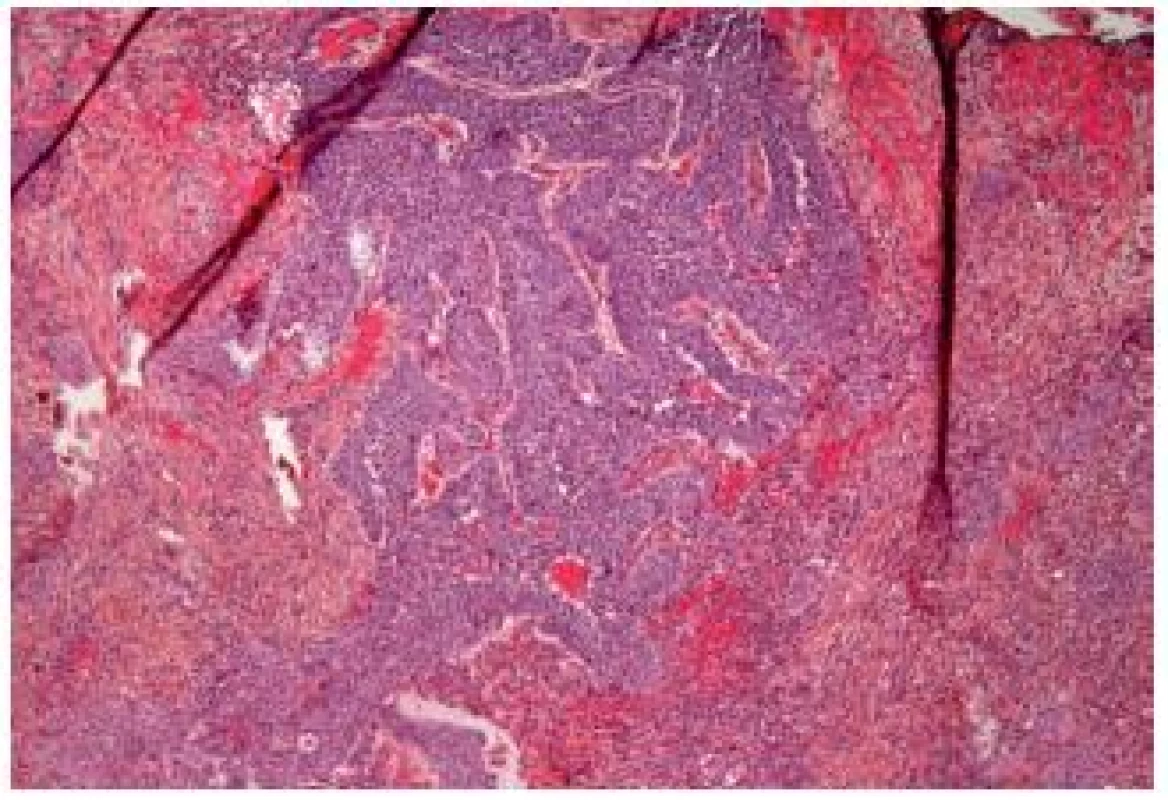
Poorly differentiated carcinomas
Sarcomatoid carcinoma of the urinary bladder
Sarcomatoid carcinoma includes the carcinosarcoma of the bladder. This entity is relatively rare, representing 0.6 % of bladder carcinomas. Both entities can be lumped together as molecular studies demonstrated a common clonal origin for the carcinomatous and sarcomatous components (Fig. 11). Furthermore, the outcome with or without heterologous elements is similar (25). The sarcomatous areas can be mixed with UC, but also with other less classical subtypes such as squamous cell carcinoma, adenocarcinoma or small cell carcinoma. Varying differentiations of the sarcoma component can be present in the same lesion, such as osteo-, lipo - or rhabdomyosarcoma. The coexistence with carcinoma in situ is frequent and helpful to confirm the urothelial origin. The differential diagnosis can include benign lesions such as inflammatory myofibroblastic tumors, but also carcinomas or sarcomas. Immunohistochemistry is very helpful in this type of tumor - the inflammatory myofibroblastic tumors will express smooth muscle actin, desmin, pan-cytokeratin, and ALK-1 (26). Sarcomatous bladder carcinomas mostly present as a high stage disease with frequent lymph node invasion and distant metastasis.
11. Sarcomatoid carcinoma. Poorly differentiated appearance, the tumor cells are fusiform. 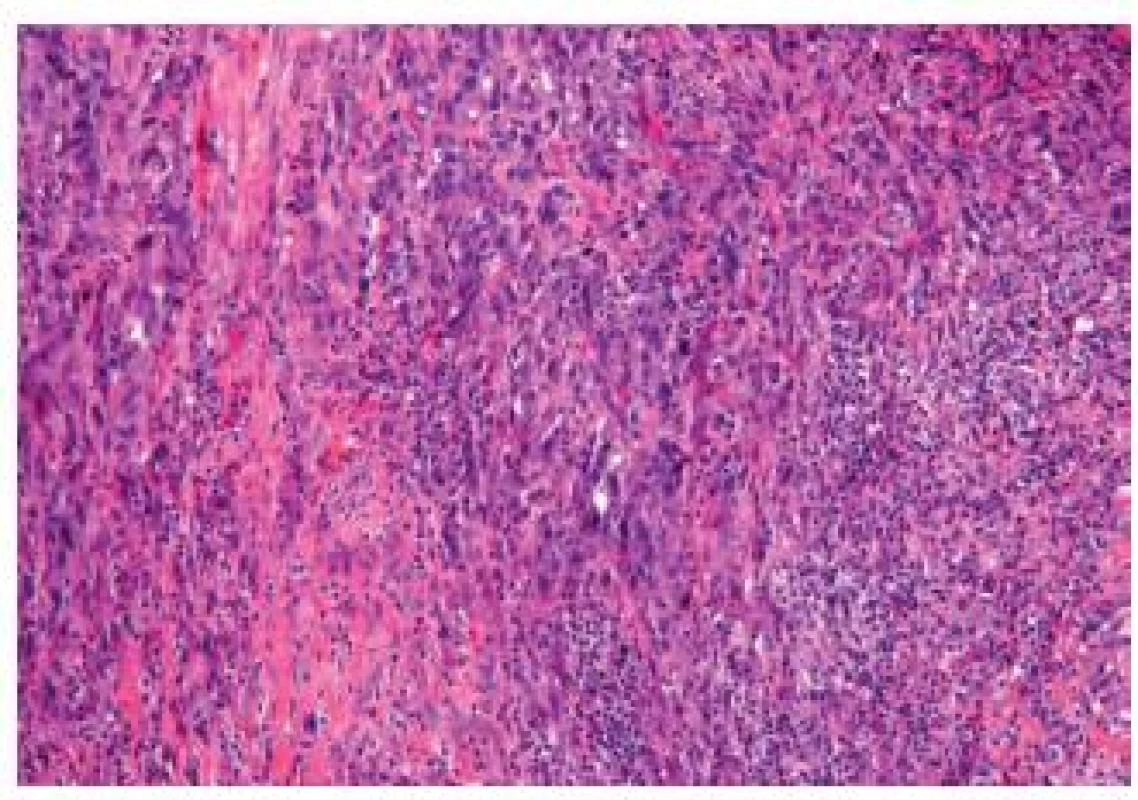
UC with pseudosarcomatous stromal reaction should not be mistaken for sarcomatoid carcinoma. Superficial very atypical cells can be observed, with an abundant cytoplasm and often hyperchromatic and atypical nuclei. Osseous and cartilaginous metaplasia can also be present, and although these findings are rare, they should not be mistaken for a heterologous differentiation. Osteoclast-like-giant cells have also been described in these lesions and should not be mistaken for a giant-cell rich bladder carcinoma.
Undifferentiated urothelial carcinoma with osteoclastic giant cells (OGC) and giant cell (GC) carcinomas
The OGC entity closely resembles osteoclastic giant cell tumors in the soft parts and bones. This entity has been more frequently described in the upper urinary tract. The cells are oval, plump, of a big size, the stroma is richly vascularized and the osteoclast-like giant cells express CD68, CD51 and CD54 (27) (Fig. 12). The urothelial component expresses the usual UC markers. The lymphovascular invasion and metastases are frequent. The GC variant is very rare and the most challenging issue is to make a diagnosis of a primary UC and not to mistake it as a metastasis (Fig. 13). GC UC are undifferentiated neoplasms with a high degree of nuclear anaplasia. The overall survival, like in other poorly/undifferentiated UC variants, is poor (8).
12. Undifferentiated carcinoma admixed with giant cells. 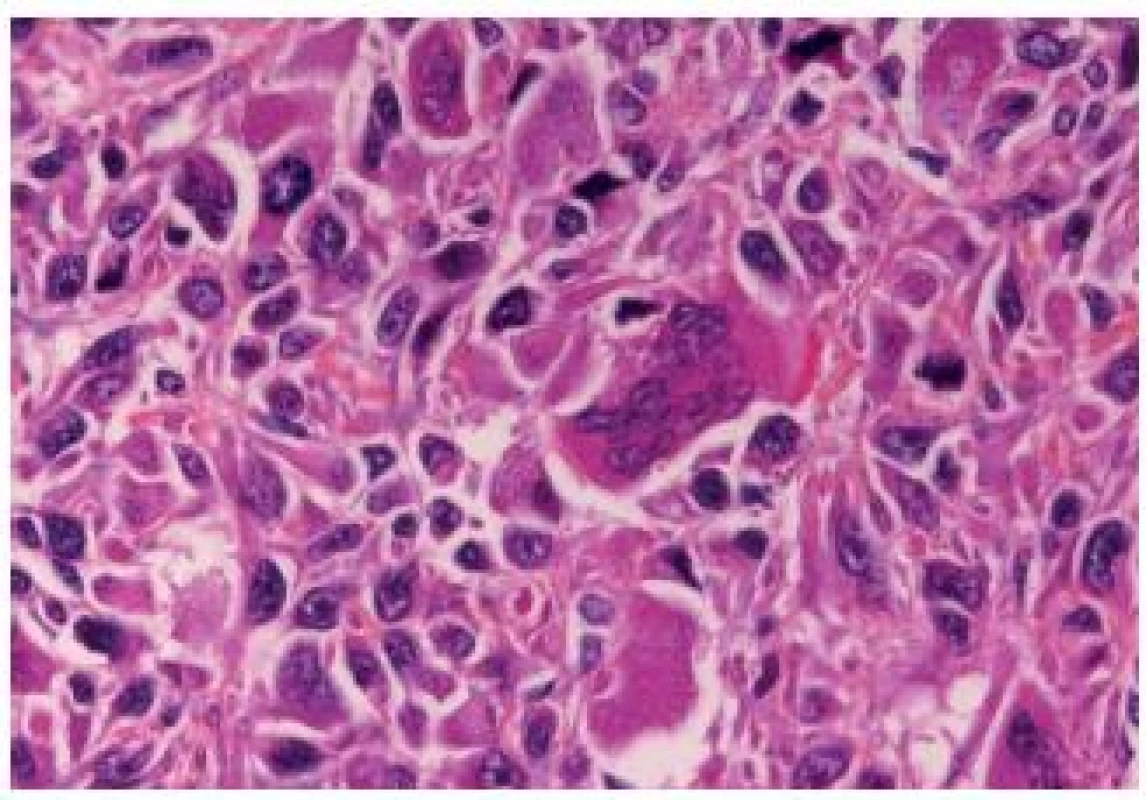
13. Undifferentiated giant cell UC, tumor cells have eosinophilic abundant cytoplasm. 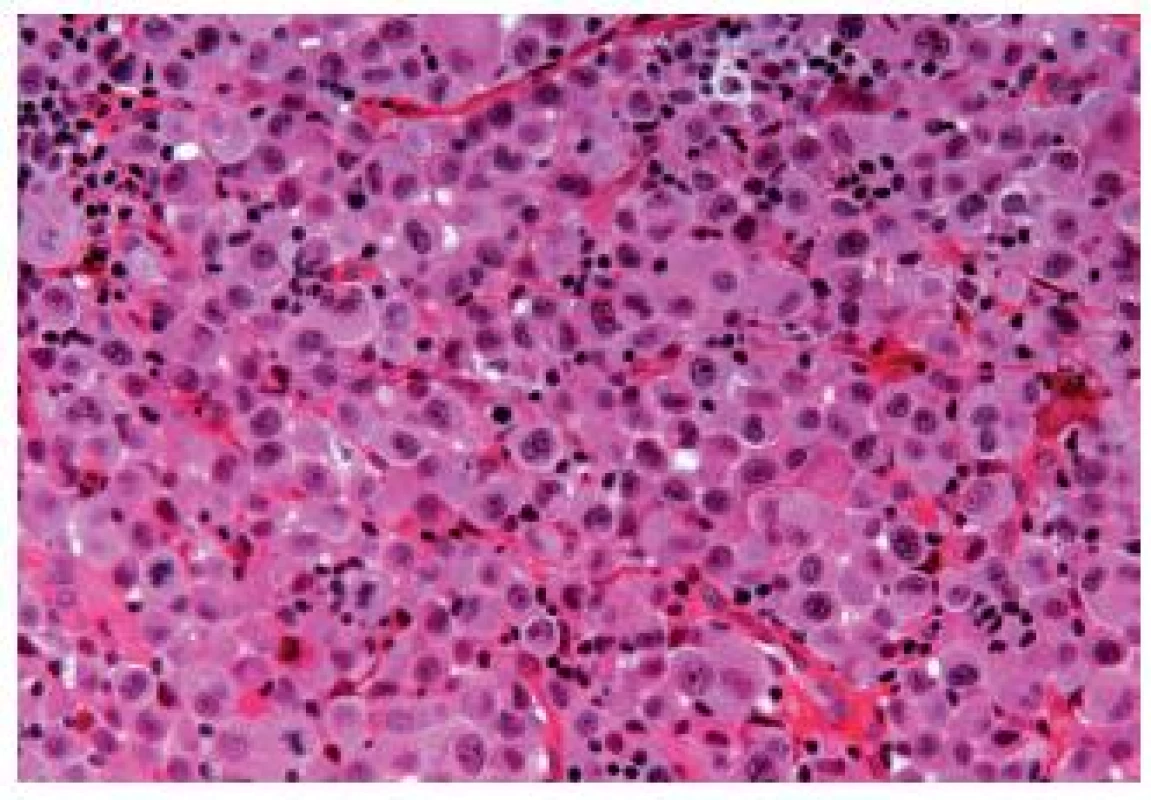
Squamous cell carcinoma (SCC)
According to the WHO 2004 classification, a tumor can only be called squamous if it consists at least of 95 % of (keratin forming) squamous cells carcinoma. SCC represents 5 % of all neoplasms in the bladder; with the better management of schistosomiasis, the incidence in the endemic regions like Egypt decreased (28,29).
These tumors present as an infiltrating masses, frequently with inflammatory and necrotic areas. The squamous differentiation is characterized by sheets of cells with well-defined cell borders, eosinophilic cytoplasm and focal keratin pearls formation. The association with a squamous metaplasia, hyperkeratosis or squamous CIS is frequent. Immunohistochemistry is positive for p63, high molecular weight cytokeratins (HMW-CKs) and AE1/3.
Differential diagnosis includes the UC with squamous differentiation, or a metastasis. Secondary involvement from the anus or cervix can occur. SCC has its prognosis close to the classical urothelial carcinomas.
Verrucous carcinomas are rare well-differentiated neoplasms, presenting mostly with pushing borders, which should not be taken for inverted growth pattern (1).
Adenocarcinoma
The biggest problem when diagnosing a bladder adenocarcinoma is to make sure that it is a primary malignancy and not a metastasis. Most adenocarcinomas share morphological patterns with colonic adenocarcinomas. Villous adenomas, acinar, cribriform or solid carcinomas have been reported. Neuroendocrine differentiation can also be observed. Mucinous, tubulovillous and colloid differentiation have been described recently in some reports (30). In mucinous tumors, individual detached cells within the lakes of mucin, like in the colonic counterpart, appear. Sometimes, there might be a component with signet ring cells (Fig. 14). A metaplasia in association with the adenocarcinoma is a frequent finding. CIS admixed with adenocarcinoma can be helpful to establish the diagnosis of a primary tumor. The differential diagnosis includes all forms of metastatic adenocarcinoma of various origins. The primary localizations can be the gastrointestinal tract as well as prostate. Some primary signet cell carcinomas are able to mimic metastatic gastrointestinal tumors. They might also resemble lobular carcinoma of the breast. The use of ER, PR and GCDFP-15 immunostains should lead to the correct diagnosis.
14. Signet ring-cell component in an UC. 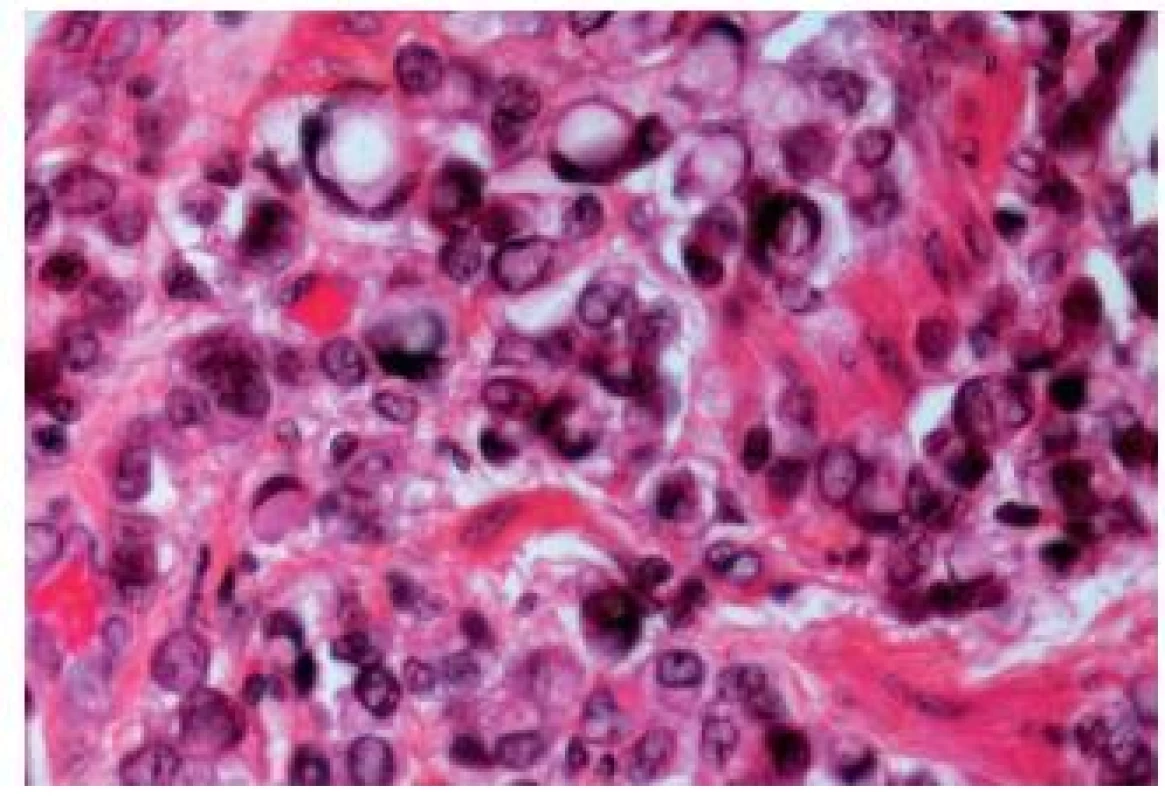
PAS and Alcian blue staining can be positive, especially in case of mucinous or signet ring cell carcinomas. There is no specific immunoprofile of adenocarciomas in the bladder. These carcinomas display CK7 in 75 %, CK20 in nearly 100 %, MUC-2 in 100 % and CDX-2 in 50 % (31). CEA can be positive and β-catenin stains positively in the cytoplasm. The latter finding is of importance, especially if there is a doubt about a metastasis from the gastro-intestinal tract, because some colonic tumors display nuclear positivity for β-catenin. The survival depends on the subtype of adenocarcinoma. Mucinous tumors are associated with a survival rate of 55 %; the signet ring cell adenocarcinomas have a very poor outcome (27 %) (32).
Urachal carcinomas
Urachal carcinomas are malignant tumors arising in urachal remnants. They represent uncommon malignancies, localized at the dome or anterior bladder wall. Most of these carcinomas are adenocarcinomas, but other types and variants also occur commonly (Fig. 15). The outcome is often poor, because of the advanced stage at the time of their discovery (33); they represent 22 - 35% of urinary bladder adenocarcinomas (34).
15. Urachal adenocarcinoma with relatively well differentiated appearance. 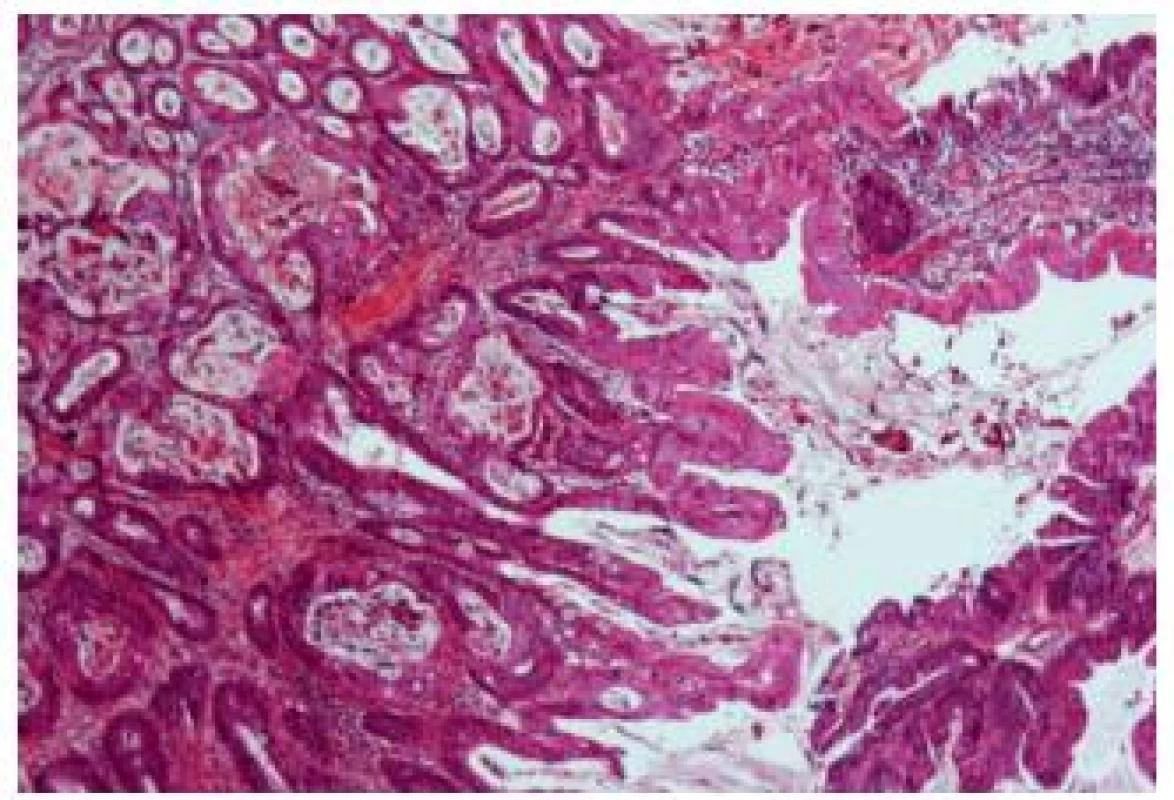
Several staging systems have been proposed recently (35,36). Most follow the system proposed by Sheldon et al. (36): pT1 represents no invasion beyond the urachal mucosa, pT2 - invasion is confined to the urachus, pT3 - local extension to the (a) bladder or (b) abdominal wall or (c) viscera other than the bladder, and pT4 metastasis to (a) regional lymph nodes and (b) distant sites.
There are conflicting data concerning prognosis and survival compared to “classical” bladder carcinomas. Goplalan et al. demonstrated that the pathologic stage is an important prognostic factor in urachal carcinomas (34). Herr et al. noted in their study of urachal carcinomas staged according to the Sheldon classification, that the patients with bladder and urachus confined disease had better outcome than those with higher stage (37).
IMMUNOHISTOCHEMISTRY AND THE ISSUE OF POORLY DIFFERENTIATED LESIONS METASTASIS
Several immunohistochemical makers are considered as being helpful and specific and are used in daily practice. The most commonly used are CK7, CK20, p63 and HMW-CKs. More recently described markers are GATA-3 and S-100P, or Uroplakin II.
A recent paper explored the immunohistochemical expression of the most common and also recently described antibodies in different subtypes of urothelial carcinoma (38). The results are presented in the Table 1.
1. Immunohistochemical expression of markers in different subtypes of urothelial carcinoma. 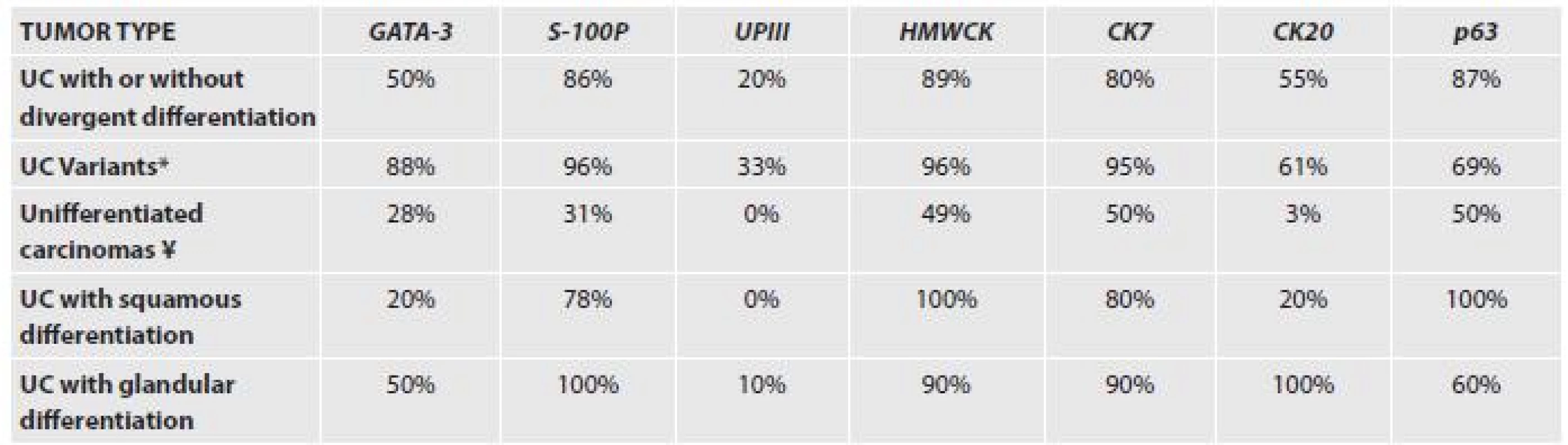
UPIII – uroplakin III *IMPC, plasmocytoid, nested, clear cell and microcystic ¥ lymphoepithelioma- like carcinoma, small cell carcinoma, sarcomatoid carcinoma with rhabdoid and giant cells In difficult cases, several markers will have to be employed. Moreover, tissue specific markers for other organs such as PSA, p504s, CDX-2 and many others can be useful. In case of neuroendocrine tumors of the bladder, one should be aware of the positivity of markers such as TTF-1 in 30% of UC cases.
In conclusion, pathologists have to be aware of the numerous aspects of UCs, with their varying morphological features. Especially in metastatic sites, it might be exceedingly difficult to make the correct diagnosis.
✉ Correspondence address:
Eva Compérat, M.D.
Department of Pathology, Hôpital La Pitié-Salpétrière
Assistance-Publique Hôpitaux de Paris,
Université Pierre et Marie Curie University Paris VI,
47-83, Bd de l’Hôpital, 75013 Paris, France
email: ecomperat@yahoo.fr
Sources
1. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, (eds). Pathology and genetics of tumours of the urinary system and male genital organs. World Health Organization Classification of Tumours. Lyon: IARC Press; 2004 : 250-257.
2. Amin MB, Ro JY, el-Sharkawy T, Lee K. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol 1994; 18(12): 1224-1232.
3. Johansson SL, Borghede G, Holmäng S. Micropapillary bladder carcinoma: a clinicopathological study of 20 cases. J Urol 1999; 161(6): 1798-1802.
4. Samaratunga H, Khoo K. Micropapillary variant of urothelial carcinoma of the urinary bladder; a clinicopathological and immunohistochemical study. Histopathology 2004; 45(1): 55-64.
5. Compérat E, Roupret M, Yaxley J, et al. Micropapillary urothelial carcinoma of the urinary bladder: a clinicopathological analysis of 72 cases. Pathology 2010; 42(7): 650-654.
6. Kamat AM, Dinney CP, Gee JR, et al. Micropapillary bladder cancer: a review of the University of Texas M. D. Anderson Cancer Center experience with 100 consecutive patients. Cancer 2007; 110(1): 62-67.
7. Sangoi AR, Beck AH, Amin MB, et al. Interobserver reproducibility in the diagnosis of invasive micropapillary carcinoma of the urinary tract among urologic pathologists. Am J Surg Pathol 2010; 34(9): 1367-1376.
8. Amin MB. Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol 2009; 22 Suppl 2: S96-S118.
9. Ricardo-Gonzalez RR, Nguyen M, Gokden N, Sangoi AR, Presti JC Jr, McKenney JK. Plasmacytoid carcinoma of the bladder: a urothelial carcinoma variant with a predilection for intraperitoneal spread. J Urol 2012; 187(3): 852-855.
10. Keck B, Wach S, Kunath F, et al. Nuclear E-cadherin expression is associated with the loss of membranous E-cadherin, plasmacytoiddifferentiation and reduced overall survival in urothelial carcinoma of the bladder. Ann Surg Oncol 2013; 20(7): 2440-2445.
11. Keck B, Wach S, Stoehr R, et al. Plasmacytoid variant of bladder cancer defines patients with poor prognosis if treated with cystectomy and adjuvant cisplatin-based chemotherapy. BMC Cancer 2013; 13 : 71.
12. Carvalho JC, Thomas DG, McHugh JB, Shah RB, Kunju LP. p63, CK7, PAX8 and INI-1: an optimal immunohistochemical panel to distinguish poorly differentiated urothelial cell carcinoma from high-grade tumours of the renal collecting system. Histopathology 2012; 60(4): 597-608.
13. Tamas EF, Nielsen ME, Schoenberg MP, Epstein JI. Lymphoepithelioma-like carcinoma of the urinary tract: a clinicopathological study of 30 pure and mixed cases. Mod Pathol 2007; 20(8): 828-834.
14. Oliva E, Amin MB, Jimenez R, Young RH. Clear cell carcinoma of the urinary bladder: a report and comparison of four tumors of mullerian origin and nine of probable urothelial origin with discussion of histogenesis and diagnostic problems. Am J Surg Pathol 2002; 26(2): 190-197.
15. Leroy X, Gonzalez S, Zini L, Aubert S. Lipoid-cell variant of urothelial carcinoma: a clinicopathologic and immunohistochemical study of five cases. Am J Surg Pathol 2007; 31(5): 770-773.
16. Stern JB. Unusual benign bladder tumor of Brunn nest origin. Urology 1979; 14(3): 288-289.
17. Talbert ML, Young RH. Carcinomas of the urinary bladder with deceptively benign-appearing foci. A report of three cases. Am J Surg Pathol 1989; 13(5): 374-381.
18. Beltran AL, Cheng L, Montironi R, Blanca A, et al. Clinicopathological characteristics and outcome of nested carcinoma of the urinary bladder. Virchows Arch. In press 2014.
19. Holmäng S, Johansson SL. The nested variant of transitional cell carcinoma--a rare neoplasm with poor prognosis. Scand J Urol Nephrol 2001; 35(2): 102-105.
20. Linder BJ, Frank I, Cheville JC, et al. Outcomes following radical cystectomy for nested variant of urothelial carcinoma: a matched cohort analysis. J Urol 2013; 189(5): 1670-1675.
21. Lopez Beltran A, Montironi R, Cheng L. Microcystic urothelial carcinoma: morphology, immunohistochemistry and clinical behaviour. Histopathology 2014; 64(6): 872-879.
22. Lopez-Beltran A, Cheng L, Comperat E, et al. Large cell undifferentiated carcinoma of the urinary bladder. Pathology 2010; 42(4): 364-368.
23. Cox R, Epstein JI. Large nested variant of urothelial carcinoma: 23 cases mimicking von Brunn nests and inverted growth pattern of noninvasive papillary urothelial carcinoma. Am J Surg Pathol 2011; 35(9): 1337-1342.
24. Hodges KB, Lopez-Beltran A, Maclennan GT, Montironi R, Cheng L. Urothelial lesions with inverted growth patterns: histogenesis, molecular genetic findings, differential diagnosis and clinical management. BJU Int 2011; 107(4): 532-537.
25. Armstrong AB, Wang M, Eble JN, et al. TP53 mutational analysis supports monoclonal origin of biphasic sarcomatoid urothelial carcinoma (carcinosarcoma) of the urinary bladder. Mod Pathol 2009; 22(1): 113-118.
26. Kawano H, Tanaka S, Ishii A, Cui D, Eguchi S, Hashimoto O, Ikeda E. Osteoclast-rich undifferentiated carcinoma of the urinary bladder: an immunohistochemical study. Pathol Res Pract 2011; 207(11): 722-727.
27. Harik LR, Merino C, Coindre JM, Amin MB, Pedeutour F,Weiss SW. Pseudosarcomatous myofibroblastic proliferations of the bladder: a clinicopathologic study of 42 cases. Am J Surg Pathol 2006; 30(7): 787-794.
28. Hansel DE, Zhang Z, Petillo D, Teh BT. Gene profiling suggests a common evolution of bladder cancer subtypes. BMC Med Genomics 2013; 6 : 42.
29. Xylinas E, Rink M, Robinson BD, et al. Impact of histological variants on oncological outcomes of patients with urothelial carcinoma of thebladder treated with radical cystectomy. Eur J Cancer 2013; 49(8): 1889-1897.
30. Makise N, Morikawa T, Takeshima Y, Homma Y, Fukayama M. A case of urinary bladder urothelial carcinoma with squamous, glandular, and plasmacytoid differentiation. Case Rep Oncol 2014; 7(2): 362-368.
31. Chu PG, Chung L, Weiss LM, Lau SK. Determining the site of origin of mucinous adenocarcinoma: an immunohistochemical study of 175 cases. Am J Surg Pathol 2011; 35(12): 1830-1836.
32. Wang J, Wang FW, Kessinger A. The impact of signet-ring cell carcinoma histology on bladder cancer outcome. World J Urol 2012; 30(6): 777-783.
33. Paner GP, Barkan GA, Mehta V, et al. Urachal carcinomas of the nonglandular type: salient features and considerations in pathologic diagnosis. Am J Surg Pathol 2012; 36(3): 432-442.
34. Gopalan A, Sharp DS, Fine SW, et al. Urachal carcinoma: a clinicopathologic analysis of 24 cases with outcome correlation. Am J Surg Pathol 2009; 33(5): 659-668.
35. Molina JR, Quevedo JF, Furth AF, Richardson RL, Zincke H, Burch PA. Predictors of survival from urachal cancer: a Mayo Clinic study of 49 cases. Cancer 2007; 110(11): 2434-2440.
36. Sheldon CA, Clayman RV, Gonzalez R, Williams RD, Fraley EE. Malignant urachal lesions. J Urol 1984; 131(1): 1-8.
37. Herr HW, Bochner BH, Sharp D, Dalbagni G, Reuter VE. Urachal carcinoma: contemporary surgical outcomes. J Urol 2007; 178(1): 74-78.
38. Paner GP, Annaiah C, Gulmann C, et al. Immunohistochemical evaluation of novel and traditional markers associated with urothelial differentiation in a spectrum of variants of urothelial carcinoma of the urinary bladder. Hum Pathol 2014; 45(7): 1473-1482.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2014 Issue 4-
All articles in this issue
- International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia 2012
- Current status of urinary cytology in the evaluation of bladder neoplasms
- Morphological and electrophysiological changes of the heart atria in necropsy patients with atrial fibrillation – a pilot study
- Bart´s syndrome associated with epidermolysis bullosa junctionalis and with pyloric atresia. An autopsy case report
- A concise update on prostate pathology
- Update on urinary bladder pathology
- An isolated metastasis to the heart from a malignant phyllodes tumor with osteosarcomatous differentiation
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Current status of urinary cytology in the evaluation of bladder neoplasms
- Bart´s syndrome associated with epidermolysis bullosa junctionalis and with pyloric atresia. An autopsy case report
- International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia 2012
- A concise update on prostate pathology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

