-
Medical journals
- Career
A concise update on prostate pathology
Authors: Weisheng Xu 1; Ming Zhou 1,2
Authors‘ workplace: Departments of Pathology, New York University Langone Medical Center, New York, NY, USA 1; Departments of Pathology, New York University Langone Medical Center, New York, NY, USA 2
Published in: Čes.-slov. Patol., 50, 2014, No. 4, p. 120-128
Category: Review Articles - Uropathology
Overview
Prostate carcinoma is the most common non-cutaneous malignancy in men in developed countries and the incidence has been steadily rising in the developing countries. Active research in recent years has led to tremendous progress in our understanding of the biology and genetics, and marked improvement in diagnosis and treatment of prostate cancer. Gleason grading has remained as the cornerstone for management of patients with prostate cancer. However, the grading system has continuously evolving since its inception in response to changes in the clinical practice of diagnosis and treatment of prostate cancer. The modification of Gleason grading system implemented by the International Society of Urological Pathology in 2005 has profoundly changed the way prostate cancer is graded and consequently how patients are managed. Several prostate cancer histological types with distinct clinical and pathological features have been rediscovered or redefined. Finally, elucidations of the molecular and genetic mechanism helps not only better understand the pathogenesis of prostate cancer, but also identify biomarkers for improved diagnosis, risk stratification and clinical management. This article briefly reviews the most recent advances in the Gleason grading system, new histological types and molecular genetics of prostate cancer.
Keywords:
prostate - Gleason grade - intraductal carcinoma - neuroendocrine differentiation - molecular genetics
Prostate carcinoma (PCa) is the most common non-cutaneous malignancy in men in developed countries and the incidence has been steadily rising in the developing countries as well (1). Active research has led to tremendous progress in our understanding of the biology and genetics, and marked improvement in diagnosis and treatment of PCa in the last decade. This article briefly reviews the most recent advances in the Gleason grading system, several PCa histological types that have been recently redefined and molecular genetics of PCa that are most relevant to surgical pathologists’ practice.
CONTEMPORARY GLEASON GRADING SYSTEM
Gleason grading system, developed by Dr. Donald Gleason in 1967 (2,3), remains as the cornerstone for the management of prostate cancer. The system is relatively simple and reasonably reproducible to apply (4,5). It is one of the key parameters for planning treatment, and remains as the most important prognostic factor in predicting pathological findings in radical prostatectomy (RP), biochemical failure, local and distant metastasis after therapy and PCa specific mortality.
The Gleason grading system has undergone continuous modification and changes in response to changes in the clinical practice of diagnosis and treatment of prostate cancer since its inception (6). The most significant changes were introduced in 2005 at the auspices of the International Society of Urological Pathology (ISUP) (7) and further modification also ensued (Fig. 1) (4). The resulting contemporary grading system is referred to as “2005 ISUP modified Gleason grading system”. However, it is important to stress that the changes put forth by ISUP simply codified what have already been used in practice by many pathologists. It is important for surgical pathologists to be acquainted with and apply the modified grading criteria in their practice.
1. Gleason grading system. The original system (A) and 2005 International Society Urological Pathology (ISUP) modified system (B) differ significantly in the definition of grades 3 and 4. Poorly formed glands and majority of cribriform glands are graded as grade 4 and only well formed discrete glands are graded as grade 3 in 2005 ISUP modified system. In the further modification, all cribriform glands are considered grade 4 (C). 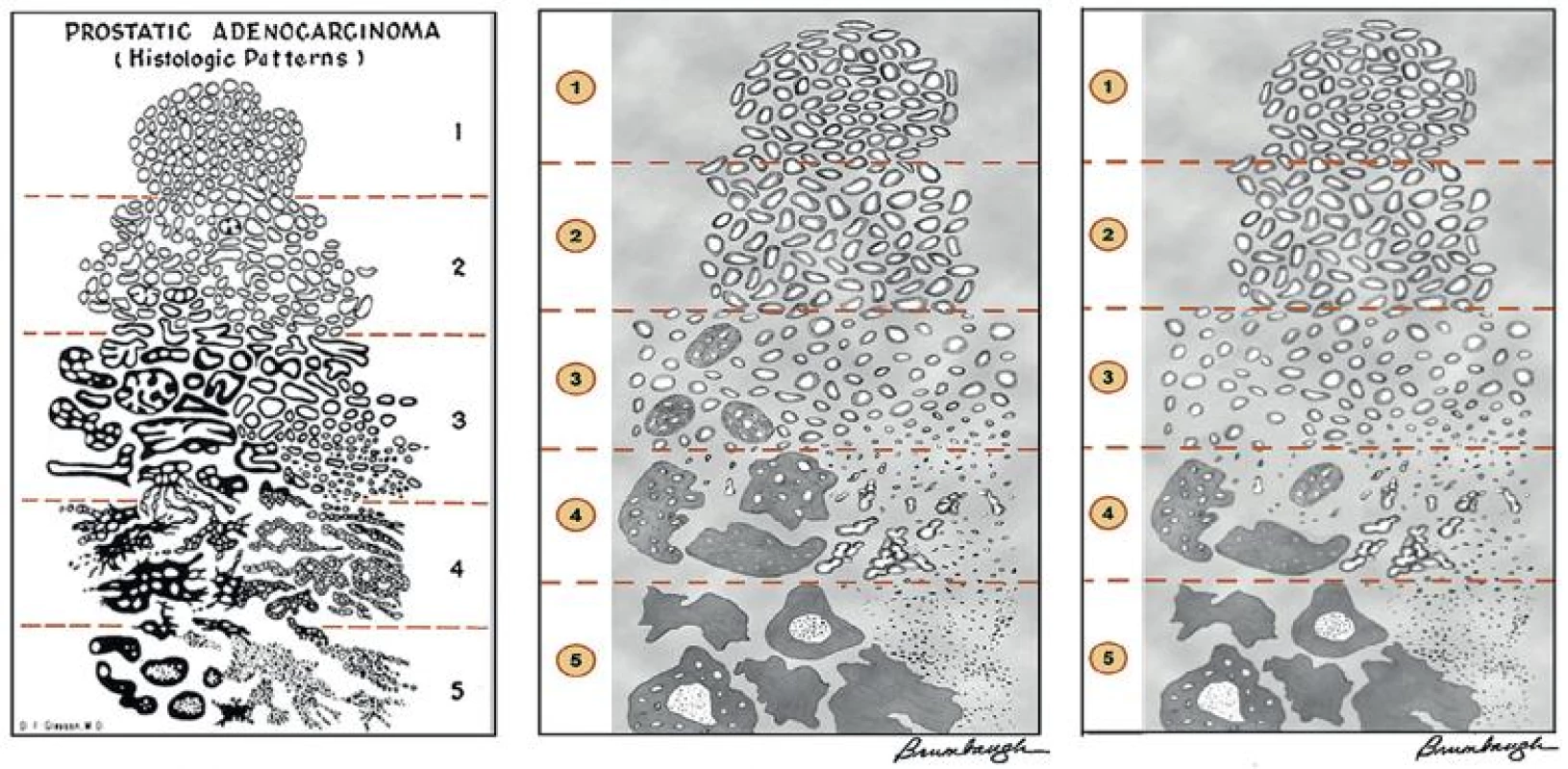
Important changes in 2005 modified Gleason grading system
Some of the changes are definitional, including precise definition of each Gleason grade and grading criteria for PCa morphological variants. Others are operational, i.e., how to report Gleason grade in special circumstances, including reporting of secondary pattern of lower or higher grade when present to a limited extent, tertiary pattern in both biopsy and prostatectomy specimen, etc.
1. Definitional changes
The most important change is perhaps the strict definition of each grade. A Gleason score of 1+1=2 should not be rendered, with only rare exception, regardless of the specimen type. Gleason scores 2-4 should rarely be rendered in needle biopsies, if ever. They should rarely be used in transurethral resection (TURP) and radical prostatectomy (RP) specimens. Therefore, Gleason grade starts at 3 and Gleason score starts at 6 in prostate biopsy specimens and most of TURP and RP specimens.
Grade 3 is strictly defined as discrete, well-formed cancer glands. Ill-defined glands with poorly formed glandular lumens are considered grade 4, together with other grade 4 patterns such as fused, cribriform and hypernephroid glands. However, grade 4 poorly formed glands should be differentiated from small glands resulting from tangential sectioning. The latter typically encompasses only a few poorly formed glands that are adjacent to or intermingle with other well-formed small glands (Fig. 2). A few poorly formed glands adjacent to other small grade 3 glands are not grade 4. Most cribriform patterns are diagnosed as grade 4 (Fig. 3). A recent study found that all cribriform cancer glands should be diagnosed as grade 4 (8).
2. Grade 4 poorly formed glands (A) should be differentiated from small glands resulting from tangential sectioning (B, arrows). The latter typically encompasses only a few poorly formed glands that are adjacent to or intermingle with other well-formed small glands. 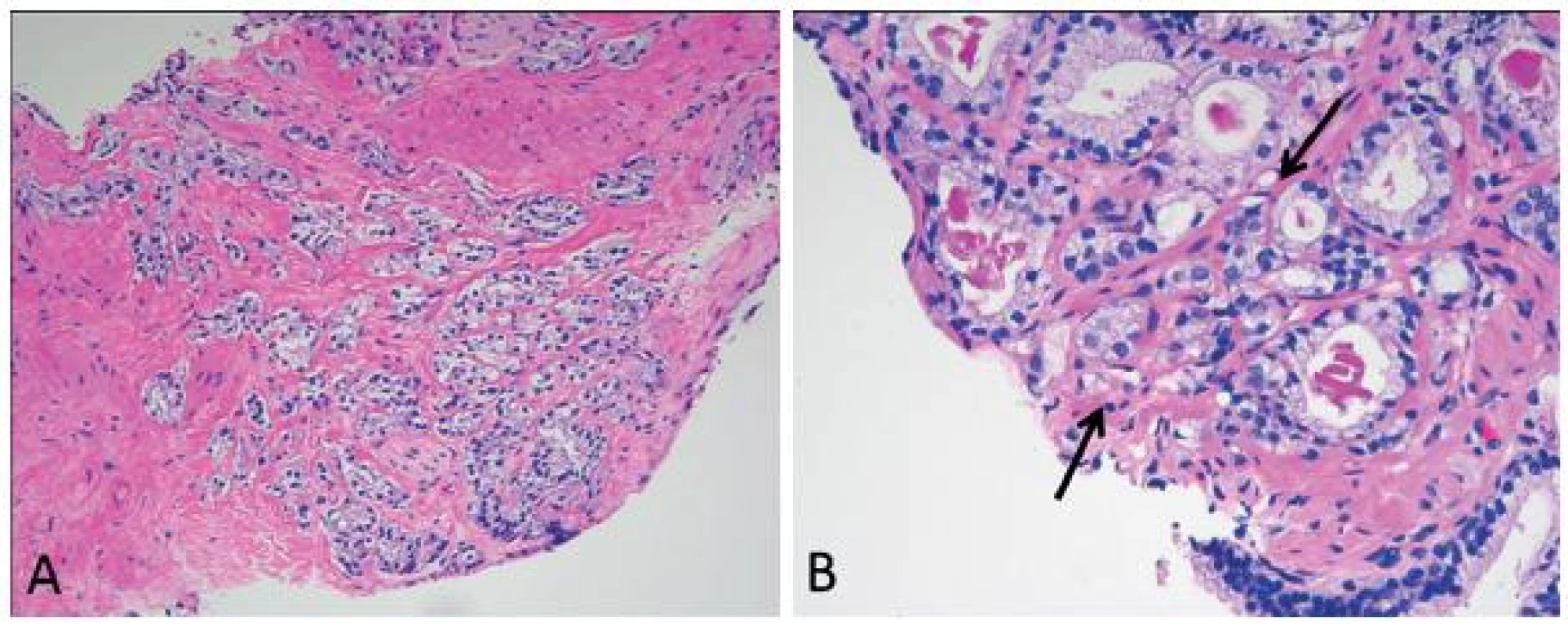
3. All cribriform cancer glands are graded as grade 4, including those small ones with smooth counter. 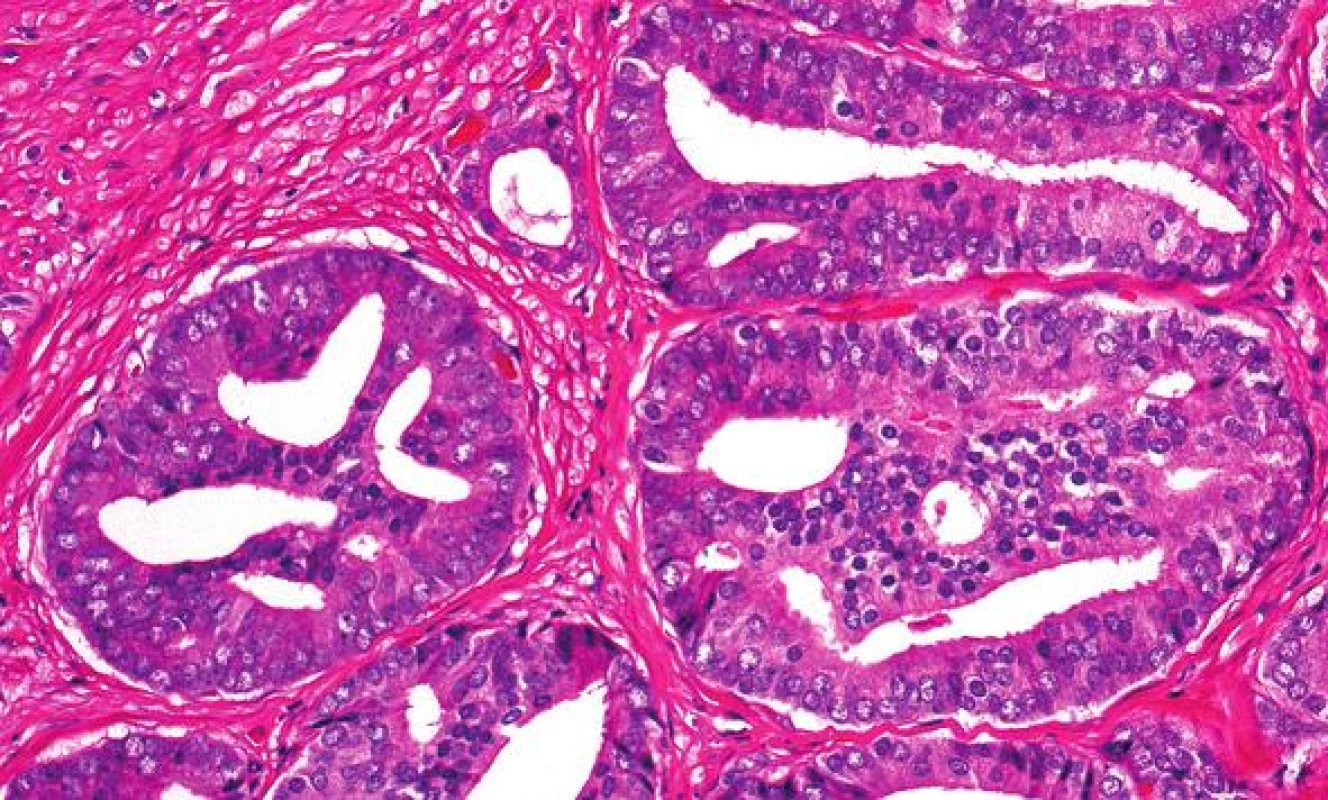
Grading of PCa with histologic patterns such as glomerulation and mucinous fibroplasia and the histologic variants (Table 1) is based on the underlying glandular architecture and the peculiar namesake histologic pattern or variation should be ignored.
1. Grading of histologic variants and patterns of prostate cancer. 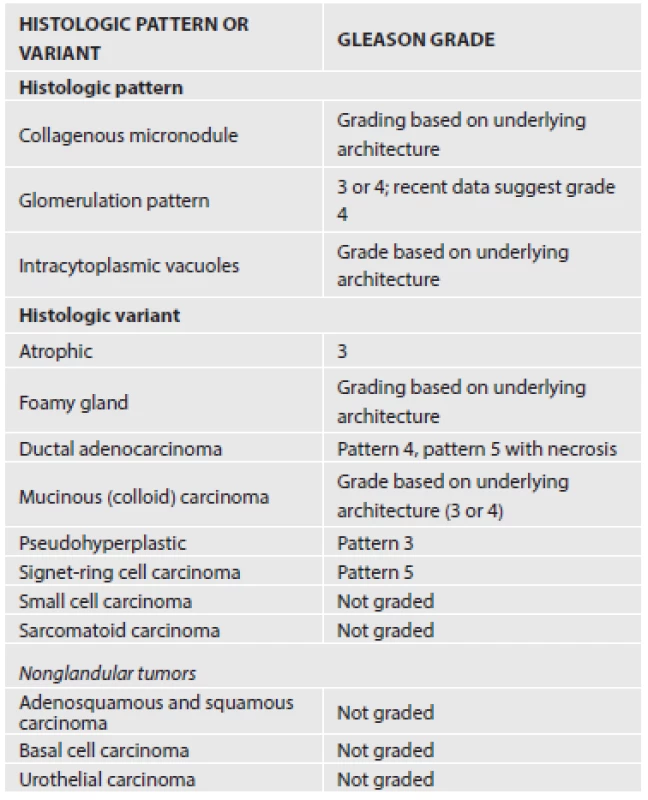
2. Operational changes
There are several specific issues regarding the grading of prostate needle biopsies. In needle biopsy, high score tumor (grade 4 and 5) of any quantity should be included in the final Gleason score as first or second pattern. Tertiary pattern is not reported for biopsy. Secondary patterns of lower-grade cancer, when present to a limited extent (<5 %) in the setting of a high-grade cancer, should be ignored and not reported. For example, a biopsy containing 98 % of Gleason grade 4 and 2 % Gleason grade 3 cancer is graded as 4+4=8, not 4+3=7.
For biopsies with different cores showing different scores, each core should be assigned an individual score if they are submitted in separate containers or their anatomic site is specified by urologists (by different inking) even when they are submitted in the same container. An overall or global Gleason score is optional. When multiple cores are placed in a container without site specification and two or more cores contain PCa, some pathologists grade each core separately, while others would only provide an overall Gleason score. If, however, the cores are fragmented, an overall score should be given.
In radical prostatectomy specimens, a tertiary pattern higher than the primary and secondary grades should be included in the final Gleason score as the secondary grade when it is > 5 % of the tumor. It can be reported as tertiary pattern if it is < 5 % of the tumor. PCa frequently presents as multifocal disease with heterogeneity in Gleason score (GS) and genetic alterations (9). The concept of dominant, or index, tumor nodules is adopted for the convenience of reporting GS of the entire case and procurement of tissue for research. Most often, the dominant nodule is the largest tumor, and has highest stage and grade. However, in significant number of cases the largest tumor volume, highest GS and staging parameters (such as extraprostatic extension) do not always concur in the same tumor nodule (10). In these cases, pathologists should de-emphasize the concept of dominant tumor nodules. Instead, they should place the emphasis on the multifocal nature of the disease and document the pathological features of all independent tumor foci that have largest tumor size, highest GS and staging parameters.
Implications of modified Gleason grading system
1. Gleason score 6 prostate cancer has become a homogeneous group with uniformly excellent prognosis
Strict definition of Gleason grade 3 cancers and inclusion of any high grade (grade 4 and 5) tertiary pattern in the final Gleason score in prostate biopsy have led to reassigning many GS 6 cancers to GS 7. One immediate effect of such changes is that GS 6 cancers have become more homogeneous in their clinical behavior and have excellent prognosis when diagnosed in both radical and biopsy specimens. Eggener et al. studied the 15-year cancer specific mortality following radical prostatectomy from 1987 to 2005. Of 9557 patients with organ-confined, GS 6 PCa, only 3 (0.03%) died of cancer (11). Since patients in this cohort were enrolled before the modification of Gleason grading system was implemented, authors inferred that “… we may have observed even fewer cancer specific deaths in men with pathological Gleason 6 or less cancer had surgical specimens been subjected to a contemporary pathological review”. Similarly, Hernandez et al. found that patients with pathologically organ-confined, Gleason score ≤ 6 PCa, biochemical recurrence and local recurrence following radical prostatectomy were extremely rare and no patients experienced distant metastasis nor prostate cancer specific death (12). GS 6 PCa diagnosed on biopsy also has excellent prognosis despite of sampling issue and potential upgrade to > 7 at radical prostatectomy. Pierorazio et al. studied 5205 patients with GS 6 PCa diagnosed in biopsy (13). Almost 1/3 (31.7 %) cases were upgraded to > GS 7 on radical prostatectomy. However, the 5-year biochemical recurrence free survival was 94.7 % (vs 82.7 % for GS 7 on biopsy). The excellent prognosis of GS 6 PCa sparked a debate whether GS 6 PCa should be labeled as “cancer” in radical prostatectomy (14). Our opinion is that the cancer label should be retained for GS 6 cancer, as these cancers are morphologically and genetically similar to higher grade PCa and can invade extraprostatic tissue. Furthermore, GS 6 PCa in prostate biopsy is upgraded in radical prostatectomy in significant percentage of cases (15).
2. Is the modified Gleason grading system better than the original system?
To claim the modified Gleason system is better than the original one, it has to show that it can improve the inter-observer reproducibility among pathologists who use it, and improve the biopsy and radical prostatectomy Gleason score concordance. Ultimately, it has to demonstrate a better correlation with clinical outcomes.
Studies have so far shown that the inter-observer reproducibility increased from 60 % with the original system to 80 % with the modified system (16-18). The improvement has been in particular impressive for GS 7 PCa. The inter-observer reproducibility has increased from 27 % in a study conducted in 1997 (19) to 68 % in a study conducted in 2008 (17).
The modified Gleason grading system has also improved the concordance between the biopsy and radical prostatectomy (RP) GS. Before the 2005 modifications, Gleason scores were concordant between biopsy and RP specimens in 28 - 68 % of cases. The discordance was mainly due to biopsy under-grading and accounted for 24 - 60 % of the discordant cases. Biopsy over-grading was less of a problem and accounted for 5 - 32 % of the discordant cases. In general, there was a better concordance in high grade PCa. After the modified Gleason grading system was implemented, there was a 12 - 15 % increase in overall exact concordance between biopsy and RP (17,20). However, biopsy under-grading is still responsible for the majority of the discordance.
The most important question is how the modified Gleason grading system affects the outcome prediction. Biopsy GS is incorporated in several preoperative nomograms to predict pathological findings in RP, such as Partin tables (21) and Kattan nomogram (22), and risk of progression after RP, such as Stephenson model (23) and Han table (24). However, the impact of modified Gleason grading on outcome prediction requires large cumulative data to clarify. So far there are only very limited studies. Several studies have shown that the correlation between biopsy GS and the risk of biochemical recurrence or PCa specific survival was significantly better using the modified grading (25-28). A study by Delahunt et al., however, reported that the original system outperformed the modified one in predicting PSA nadir following external-beam radiation therapy and hormone therapy (29). More studies are needed before a definitive conclusion can be reached.
3. Impact of modified Gleason grading system on patient management
Biopsy GS plays a pivotal role in treatment decision making. For example, the US National Cancer Center Network Practical Guidelines (http://www.nccn.org/) stratify PCa patients into 6 recurrence risk groups based on several clinicopathological parameters, including biopsy GS and extent, clinical stage, serum PSA and PSA density. Patients within different risk groups are offered different therapeutic modalities. Therefore, it is expected an upward shift in GS resulting from the modified grading system will impact how patients are managed.
Increasing number of patients are choosing active surveillance (AS), in which patients are monitored closely and definitive treatment such as surgery, radiation and hormonal ablation is withheld until there is sign of progression. The AS criteria vary from institution to institution (30), but require GS < 6 in most criteria. With modified Gleason grading system, fewer cases are graded as GS 6 and more cases as GS 7. Therefore, fewer patients would qualify for AS, which will potentially worsen the problem of overtreatment for PCa. However, patients on AS will be safer with less likelihood to progress to definitive treatment as GS 6 PCa constitutes a homogeneous group with excellent prognosis when graded with modified Gleason grading system. Furthermore, recent studies have shown that GS 3+4=7 PCa diagnosed on biopsy is associated with more favorable prognosis and these findings raised the possibility for AS to be a management option for intermediate risk PCa. Bul et al. followed patients with low risk (cT1/2, PSA < 10ng/mL, PSAD < 0.2 ng/mL/mL, GS ≤ 6, positive cores ≤ 2) and intermediate risk PCa (PSA 10 – 20ng/mL, GS = 7) and found that the 10-year metastasis free survival and disease specific survival are similar between low risk and intermediate risk patients, suggesting that AS is a safe approach for intermediate risk PCa (31). Therefore reduced enrollment of patients into AS due to upward grade shift caused by modified Gleason grading system is effectively counter balanced by less progression to definitive treatment for patients already on AS, and more patients with intermediate risk being managed with AS.
4. Limitations of modified Gleason grading system
There have been such significant changes to the Gleason grading system that the modified system is essentially a different system from the original one. It is therefore difficult to compare the outcome data in contemporary series with the historical ones. Another issue is the artificial improvement of prognosis due to grade migration, so called the Will Rogers phenomenon (32). The modified Gleason system has practically eliminated GS 2 – 4. Furthermore, some PCas that were graded as grade 3 in the original system are now graded as grade 4 due to strict definition of grade 3. As the result, some PCa in the lower grade group (GS 6) with better prognosis is moved into a higher grade group (≥ GS 7) and therefore improves the prognosis of the higher grade group.
5. Further modification of Gleason grading system
A very important limitation of the Gleason grading system, both the original and modified systems, is that the numerical scale of Gleason scores does not accurately reflect the biological aggressiveness of the disease. The Gleason scores range from 2 to 10, with 7 further divided to 3+4=7 and 4+3=7. However, the modified Gleason grading system has practically eliminated GS 2-5 in biopsy, and in majority of radical prostatectomy specimens. Therefore the lowest GS in both biopsy and radical prostatectomy is 6. Since 6 is in the middle of the 2-10 numerical scale, patients may therefore reason they have a moderately aggressive cancer despite that GS 6 PCa is the least aggressive tumor. To avoid such confusion, Epstein and his associates proposed a prognostic grouping (13). PCas are stratified into 5 prognostic groups, group I for GS 6, II for GS 3+4=7, III for GS 4+3=7, IV for GS 8 and V for GS 9/10. This approach is supported by other studies (25). It is our opinion that while this prognostic grouping is more closely reflective of the tumor behavior, it cannot replace the Gleason grading system for several reasons. First, this prognostic grouping is still based on Gleason grading, i.e., pathologists have to perform Gleason grading first and then derive the prognostic grouping based on the Gleason scores. Prognostic grouping cannot be rendered de novo. Second, Gleason grading system is so entrenched in pathologists, clinicians and patients, replacing it with a similarly numbered system would cause massive confusion. A reasonable approach would be providing the prognostic grouping along with the Gleason scores.
PROSTATE CANCER HISTOLOGICAL TYPES THAT HAVE BEEN REDEFINED
Recently, several PCa histological types with distinct clinicopathological features have been redefined. Two of them will be briefly reviewed here, including ductal carcinoma and prostate cancer with neuroendocrine differentiation.
Intraductal carcinoma of the prostate
Intraductal carcinoma of the prostate (IDC-P) represents spread of invasive carcinoma into preexisting benign ducts and acini (Fig. 4) and is strongly associated with high-grade (Gleason grades 4/5), large-volume invasive prostate cancers.
4. Intraductal carcinoma of the prostate presents as marked expansile growth of prostate cancer cells (A) within preexisting prostate ducts and acini (A) with at least focally preserved basal cells on P63 immunostain (B). 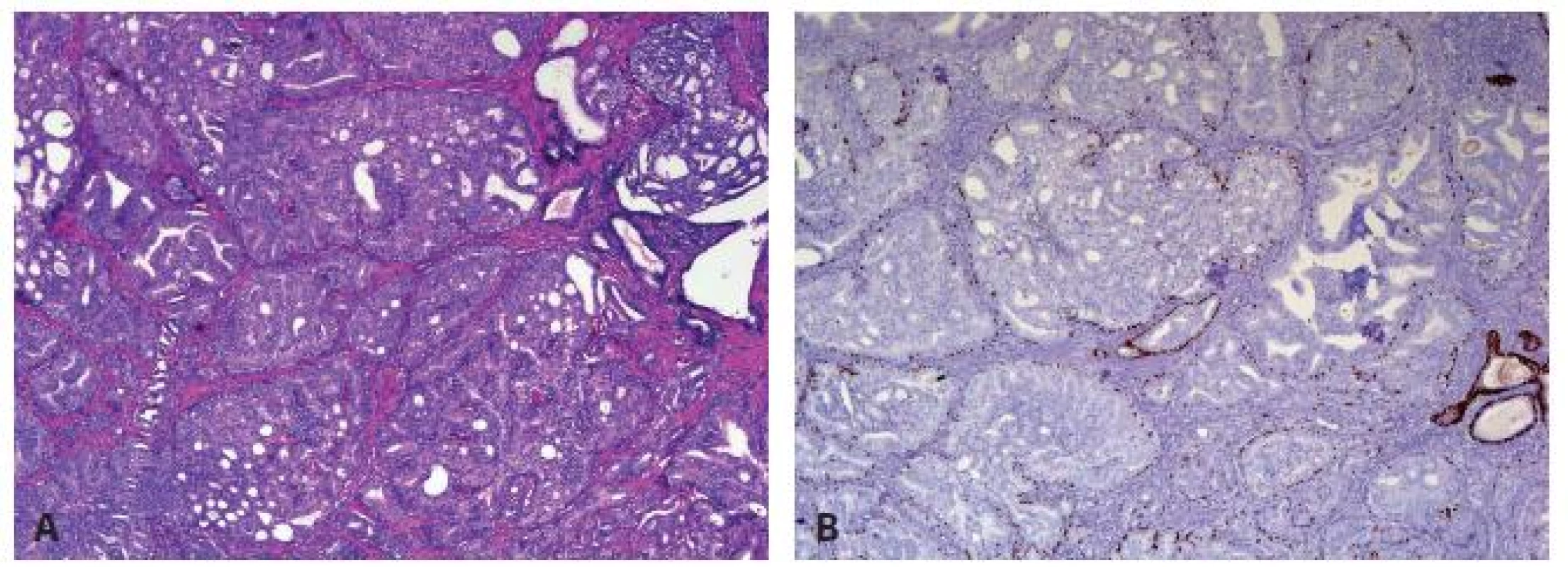
IDC-P glands are larger than normal peripheral zone glands (33,34), and exhibit markedly irregular and branching contours (Fig. 4A) (33-35). Several architectural patterns are observed in IDC-P, including trabecular, loose cribriform (Fig. 5A), dense cribriform (Fig. 5B), and solid (Fig. 5C). Comedonecrosis is diagnostic of IDC-P but is not seen in all cases (Fig. 5D). Neoplastic cells in classic IDC-P are pleomorphic, some 6 times larger than the adjacent nonneoplastic nuclei (Fig. 5E).
Several diagnostic criteria were put forth (33,36), but the one proposed by Guo and Epstein is simple, subjective and reproducible (36). This diagnostic approach is summarized in Figure 5. In addition to the presence of malignant epithelial cells filling large acini and prostatic ducts with preservation of basal cells, the diagnosis of IDC-P required the presence of solid or dense cribriform pattern (Fig. 5B, C). If these features are not present, a diagnosis of IDC-P can be made if there is (1) non-focal comedonecrosis involving ≥ 2 glands (Fig. 5D); or (2) marked nuclear atypia, where the nuclei are at least 6 times larger than adjacent benign nuclei (Fig. 5E).
5. Histological features of intraductal carcinoma of the prostate (IDC-P) include loose cribriform (A), dense cribriform (B), solid growth (C) patterns. Comedonecrosis (D) and marked nuclear pleomorphism with nuclear size >6× of the adjacent non-neoplastic cells (E) are present in some, but not all, the cases. Of these morphological features, dense cribriform and solid patterns, non-focal comedonecrosis, and marked pleomorphic nuclei are diagnostic of IDC-P. 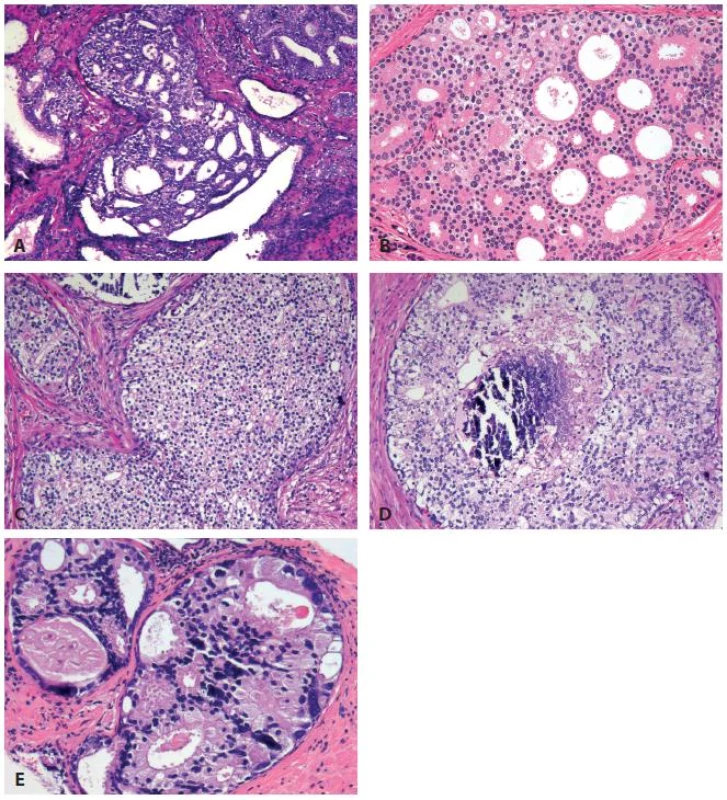
6. Schematic representation of the diagnostic criteria for intraductal carcinoma proposed by Guo and Epstein (36). 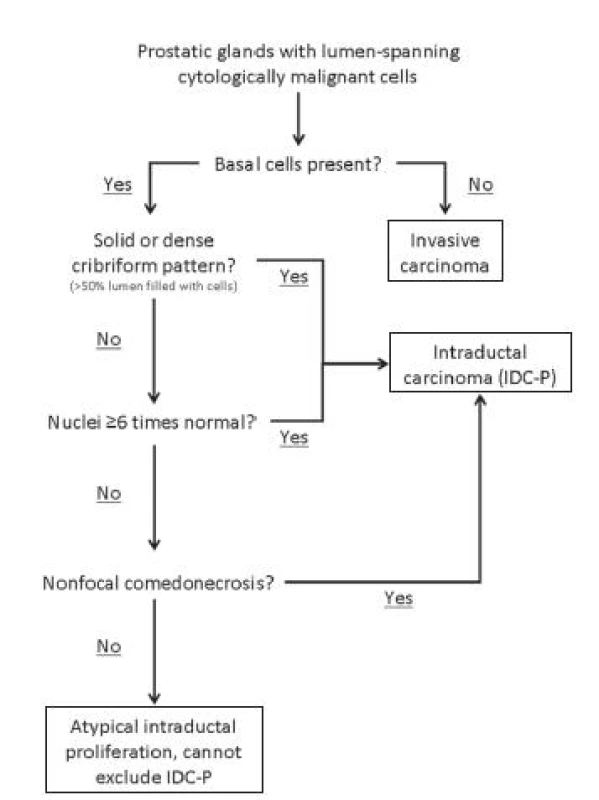
Studies have established that IDC-P represents an aggressive form of PCa and is an adverse pathologic parameter in both radical prostatectomy and needle biopsy specimens. The presence of IDC-P correlated with other adverse pathologic features, including higher Gleason score, larger tumor volume, and greater probability of extraprostatic extension, seminal vesicle invasion, and pelvic lymph node metastasis, in radical prostatectomy. It also correlated with decreased progression-free survival and with postsurgical, biochemical recurrence (34,37-40). IDC-P is uncommon in prostate biopsy; the incidence in a prospective biopsy cohort, i.e., prostate biopsies collected in daily practice, was 2.8 % (41). Isolated IDC-P without concomitant invasive IDC-P was even rarer, seen in 0.26 % cases in the same prospective cohort (41). Increasing body of evidence suggests that IDC-P in prostate biopsies that also contain invasive PCa provides additional prognostic values independent of conventional pathological parameters such as Gleason grade and tumor volume even (41-44); therefore, IDC-P should be reported in biopsy diagnosis.
Epstein and associates reported, in two studies, 66 prostate biopsies in which IDC-P was diagnosed without invasive carcinoma (36,45). They found that the presence of IDC-P, even in the absence of documented invasive carcinoma, was associated with an aggressive clinical course and adverse pathological findings in subsequent radical prostatectomy specimens. Based on their studies of needle biopsy with IDC-P and previous studies in the literature that demonstrated consistent association of IDC-P at radical prostatectomy with multiple adverse prognostic factors, authors recommended definitive therapy in men with IDC-P on needle biopsy, even in the absence of pathologically documented invasive PCa.
Prostate cancer with neuroendocrine differentiation
Neuroendocrine (NE) differentiation can occur de novo with or without concurrent PCa, or as a treatment-emergent transformed phenotype (46). NE phenotype generally confers a more aggressive clinical behavior and less favorable prognosis than conventional PCa. To standardize the diagnosis and facilitate further study, a morphologic classification of NE differentiation in PCa was proposed recently (47) and consists of six categories: 1) usual prostate adenocarcinoma with NE differentiation, 2) adenocarcinoma with Paneth cell-like NE differentiation, 3) carcinoid tumor, 4) small cell carcinoma, 5) large cell NE carcinoma, and 6) mixed NE carcinoma - acinar adenocarcinoma.
Usual PCa with NE differentiation refers to typical acinar or ductal PCa, in which NE differentiation is demonstrated only by immunohistochemical positivity (synaptophysin, chromogranin and CD 56). The clinical significance of NE differentiation in these tumors is uncertain and most of the studies have shown no effect on outcomes (47). Therefore, routine use of immunohistochemistry to detect NE differentiation in an otherwise typical PCa is not warranted.
PCa with Paneth cell-like differentiation is typical PCa containing Paneth cell –like changes with prominent eosinophilic cytoplasmic granules on light microcopy (fig. 7A) and neurosecretory granules by electron microscopy. The Paneth cell-like differentiation may be seen in well-formed cancer glands but can also be present in cords and nests of cancer cells. The clinical significance is not completely understood but studies so far have shown that seemingly poorly differentiated PCa with Paneth cell-like differentiation had favorable prognosis (48). Therefore, it is questionable whether a tumor with Paneth cell-like differentiation and lack of glandular differentiation should be assigned a Gleason score.
7. Prostate carcinoma with neuroendocrine differentiation. (A) Paneth cell-like NE differentiation. (B) Small cell carcinoma. 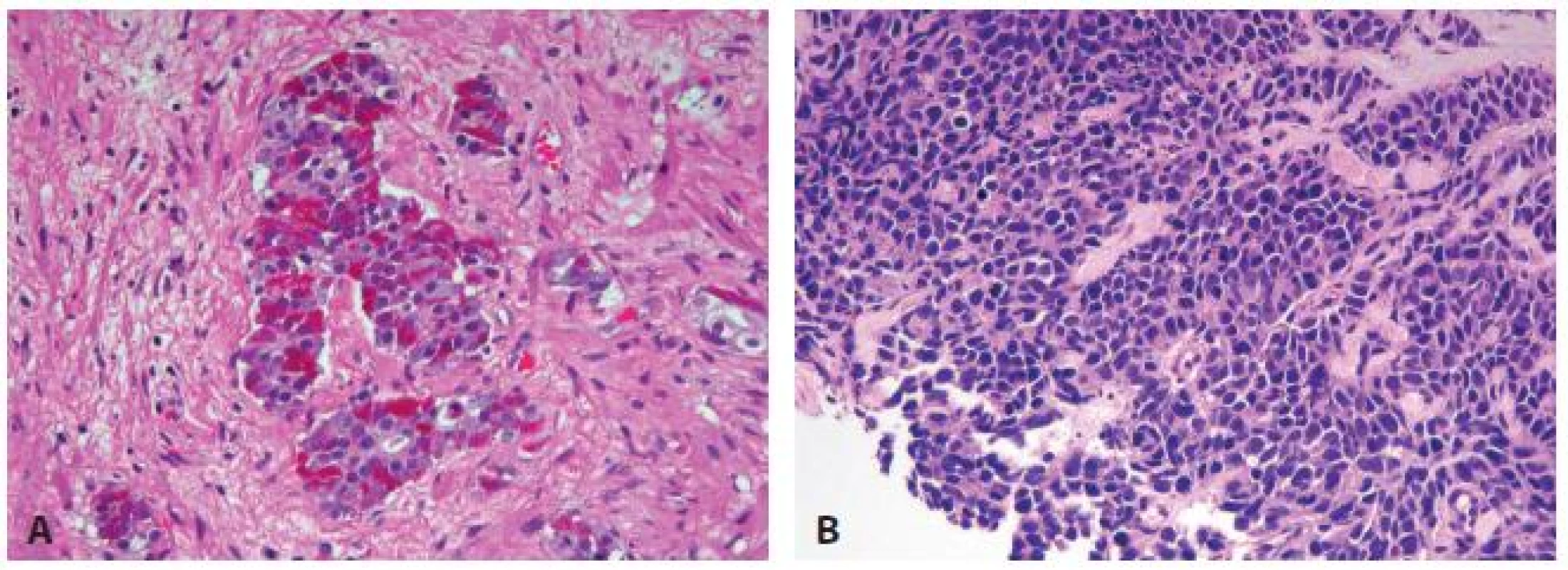
Prostate carcinoid tumor is a well-differentiated NE tumor with classical morphology of carcinoid tumor arising in the prostatic parenchyma. It expresses NE markers but not PSA. It is exceedingly rare and strict diagnostic criteria should be used.
Small cell carcinoma is an aggressive NE tumor recognized by the typical morphology similar to small cell carcinoma of the lung. Immunohistochemically, the small cell component is positive for at least one NE marker in almost 90 % of cases (49, 50), positive for prostate markers such as PSA, often focally, in 17 - 25 % of cases. TTF-1 expression is found in over 50 % of cases (49-52). ERG gene fusion is present in 50 % of prostate small cell carcinoma cases by FISH, similar to acinar PCa (53-57). However, IHC for ERG protein is not reliably positive presumably due to lack of androgen receptor expression in small cell carcinoma (54).
Large cell NE carcinoma is a high grade NE tumor with both morphological NE features (large nests with peripheral palisading, non-small cell nuclear features) and extensive NE marker expression. Majority of cases represent progression from a prior typical PCa following long-standing androgen ablation (58).
Mixed NE carcinoma and acinar PCa comprises distinct components of NE (small cell or large cell) carcinoma and typical acinar PCa with abrupt transition. Most, if not all, cases of mixed small cell carcinoma and PCa represent NE transformation after androgen deprivation therapy, and are hormonal resistant with poor prognosis. While the NE component is not graded, the percentage and grade of the acinar component should be provided.
MOLECULAR GENETICS
The most common genetic alteration in PCa is gene rearrangement between members of the E26 transformation specific (ETS) gene family and androgen-regulated genes, and the fusion between ERG, a ETS gene family member, and transmembrane serine protease 2 (TMPRSS2) is the most frequent one (59), present in about 40 - 50 % of PCa cases. Rearrangement involving other ETS genes accounts for additional 15 - 20 % of cases (60). TMPRSS2/ERG gene fusion can be detected by fluorescence in situ hybridization (FISH). The fusion leads to aberrant expression of ERG, which can be detected by immunohistochemistry (61). Positive ERG immunohistochemistry highly correlates with the ERG gene status. Studies of the prognostic significance of ETS gene alterations produced conflicting results. Some studies showed a poor prognosis for cases with TMPRSS2/ERG fusion (60), while others found no association with Gleason score, tumor stage and prognosis (60,62). TMPRSS2/ERG gene fusion was detected in about 20 % of high-grade prostatic intraepithelial neoplasia (HGPIN) intermingled with or in the vicinity of prostate adenocarcinoma that carries the same fusions, but has not yet been demonstrated in isolated HGPIN, benign prostate tissue, or benign cancer mimics (63). The PCa specific nature of TMPRSS2/ERG fusion makes it a useful marker in the diagnosis of minute focus of cancer (64,65) in challenging cases and confirmation of the prostate origin for metastatic carcinoma of unknown primary (66).
Several other molecular alterations are mutually exclusive with ETS gene rearrangement in PCa, and could define distinct molecular subtypes of PCa. Speckle-type POZ (SPOP) mutations is present in 6 - 15 % of cases of PCa that generally lack ETS rearrangement, PTEN inactivation and p53 mutation (67,68). Cases with SPOP mutation tend to have CHD1 deletion. The initial study failed to show significant correlation between SPOP mutation and rate and time of biochemical recurrence due to small sample size. SPINK1 over-expression is another molecular alteration exclusively identified in ETS rearrangement negative cases, and is present in about 10 % of PCa cases (69). SPINK1 positive cases have worse prognosis.
PTEN inactivation by deletion, less frequently by mutation, is an important event in PCa with poor prognosis. It is identified in about 40 % of PCa (70-72). PTEN inactivation activates AKT pathway in PCa, which can also be activated by other genetic changes, including PI3K and mTOR mutations (73). c-myc amplification and over-expression are detected in about 30 % of cases, and are more frequently present in late stage of PCa and associated with worse prognosis (74,75). Androgen receptor (AR) amplification and mutation play an important role in PCa progression and resistance to androgen deprivation therapy (ADT) (76,77). Other genetic changes significantly detected in PCa include NKX3.1 mutation, FOXA1 elevation, and Rb loss. Most of these genetic changes are more frequently identified in late stage of the disease, and are associated with disease progression and poor prognosis (73).
A potential utility of genetic and molecular markers of PCa is to triage patients for appropriate management. In current practice, the management decision is based primarily on clinical and biopsy pathology findings, including clinical stage, serum PSA level and density, digital rectal examination and biopsy Gleason score and tumor extent. Patients are stratified into different risk groups for which different management regimens are offered. However, such stratification scheme is far from perfection and sometimes results in unnecessary treatment in patients with low risk disease and delayed treatment in patients with high risk disease (78). Molecular and genetic markers, used singularly or in combination, can potentially separate indolent PCa from aggressive ones and help identify patients with indolent disease who can therefore be safely followed and patients with aggressive disease who need definitive treatment (79).
There are several commercially available tests marketed for this purpose. One test is Prolaris® by Myriad Genetics (Salt Lake City, UT, USA). This test measures the gene expression of 31 cell-cycle progression genes and 15 housekeeper genes from biopsy specimens to develop a cell-cycle progression (CCP) score. This CCP score has been shown to stratify men for 10-year PCa death independent of PSA and Gleason score for PCa managed conservatively (80). Recently this test has also been shown to add independent prognostic information to a standard clinical risk score in a contemporary prostatectomy cohort (81). This recent validation study by Cooperberg et al. may help guide decisions regarding adjuvant treatment and in stratifying men for future adjuvant therapy studies.
Another test, Oncotype Dx Prostate®, is marketed by Genomic Health (Redwood City, CA, USA). It tests the expression of genes representing multiple biological pathways and generates a genomic prostate score (GPS). This test, using prostate biopsy tissue, has been prospectively validated as a predictor of PCa aggressiveness in biopsy tissue despite of tumor heterogeneity, multifocality, and limited sampling at time of biopsy (82). The biopsy-based 17-gene GPS improves prediction of the presence or absence of adverse pathology and may help men with PCa make more informed decisions between active surveillance and immediate treatment.
Other similar molecular tests are being developed to augment the clinical and pathological parameters in therapeutic decision making. Even though these tests have shown promising results in early studies and started to be requested by physicians and patients, large prospective validation studies are needed on prostate biopsies before they can be recommended in routine clinical practice for risk stratification and patient management.
CONCLUSION
The ISUP 2005 modification of Gleason grading system has profoundly changed the way PCa is graded and patients are managed. Several PCa histological types with distinct clinical and pathological characteristics have been rediscovered or redefined. Further elucidation of the molecular and genetic mechanism not only helps us better understand the pathogenesis of the disease, but also identify biomarkers for improved diagnosis and clinical management.
✉ Correspondence address:
Ming Zhou, MD, PhD
NYU Langone Medical Center Tisch Hospital
560 First Avenue, TCH-461
New York, NY 10016-6497, USA
phone: 646-501-6976
fax: 212-263-7916
email: ming.zhou@nyumc.org
Sources
1. Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res 2009; 53(2): 171-184.
2. Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974; 111(1): 58-64.
3. Mellinger GT, Gleason D, Bailar J, 3rd. The histology and prognosis of prostatic cancer. J Urol 1967; 97(2): 331-337.
4. Epstein JI. An update of the Gleason grading system. J Urol 2010; 183(2): 433-440.
5. Gleason DF. Histologic grading of prostate cancer: a perspective. Hum Pathol 1992; 23(3): 273-279.
6. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med 2012; 136(4): 426-434.
7. Epstein JI, Allsbrook WC, Jr., Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 2005; 29(9): 1228-1242.
8. Latour M, Amin MB, Billis A, et al. Grading of invasive cribriform carcinoma on prostate needle biopsy: an interobserver study among experts in genitourinary pathology. Am J Surg Pathol 2008; 32(10): 1532-1539.
9. Andreoiu M, Cheng L. Multifocal prostate cancer: biologic, prognostic, and therapeutic implications. Hum Pathol 2010; 41(6): 781-793.
10. Huang CC, Deng FM, Kong MX, Ren Q, Melamed J, Zhou M. Re-evaluating the concept of “dominant/index tumor nodule” in multifocal prostate cancer. Virchows Arch 2014; 464(5): 589-594.
11. Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol 2011; 185(3): 869-875.
12. Hernandez DJ, Nielsen ME, Han M, et al. Natural history of pathologically organ-confined (pT2), Gleason score 6 or less, prostate cancer after radical prostatectomy. Urology 2008; 72(1): 172-176.
13. Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int 2013; 111(5): 753-760.
14. Carter HB, Partin AW, Walsh PC, et al. Gleason score 6 adenocarcinoma: should it be labeled as cancer? J Clin Oncol 2012; 30(35): 4294-4296.
15. Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol 2012; 61(5): 1019-1024.
16. Allsbrook WC, Jr., Mangold KA, Johnson MH, Lane RB, Lane CG, Epstein JI. Interobserver reproducibility of Gleason grading of prostatic carcinoma: general pathologist. Hum Pathol 2001; 32(1): 81-88.
17. Fine SW, Epstein JI. A contemporary study correlating prostate needle biopsy and radical prostatectomy Gleason score. J Urol 2008; 179(4): 1335-1338.
18. Helpap B, Egevad L. The significance of modified Gleason grading of prostatic carcinoma in biopsy and radical prostatectomy specimens. Virchows Arch 2006; 449(6): 622-627.
19. Steinberg DM, Sauvageot J, Piantadosi S, Epstein JI. Correlation of prostate needle biopsy and radical prostatectomy Gleason grade in academic and community settings. Am J Surg Pathol 1997; 21(5): 566-576.
20. Ozok HU, Sagnak L, Tuygun C, et al. Will the modification of the Gleason grading system affect the urology practice? Int J Surg Pathol 2010; 18(4): 248-254.
21. Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology 2001; 58(6): 843-838.
22. Ohori M, Kattan MW, Koh H, et al. Predicting the presence and side of extracapsular extension: a nomogram for staging prostate cancer. J Urol 2004; 171(5): 1844-1849.
23. Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst 2006; 98(10): 715-717.
24. Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol 2003; 169(2): 517-523.
25. Berney DM. The case for modifying the Gleason grading system. BJU Int 2007; 100(4): 725-726.
26. Billis A, Guimaraes MS, Freitas LL, Meirelles L, Magna LA, Ferreira U. The impact of the 2005 international society of urological pathology consensus conference on standard Gleason grading of prostatic carcinoma in needle biopsies. J Urol 2008; 180(2): 548-552.
27. Dong F, Wang C, Farris AB, et al. Impact on the clinical outcome of prostate cancer by the 2005 international society of urological pathology modified Gleason grading system. Am J Surg Pathol 2012; 36(6): 838-843.
28. Uemura H, Hoshino K, Sasaki T, et al. Usefulness of the 2005 International Society of Urologic Pathology Gleason grading system in prostate biopsy and radical prostatectomy specimens. BJU Int 2009; 103(9): 1190-1194.
29. Delahunt B, Lamb DS, Srigley JR, et al. Gleason scoring: a comparison of classical and modified (international society of urological pathology) criteria using nadir PSA as a clinical end point. Pathology 2010; 42(4): 339-343.
30. Iremashvili V, Pelaez L, Manoharan M, Jorda M, Rosenberg DL, Soloway MS. Pathologic prostate cancer characteristics in patients eligible for active surveillance: a head-to-head comparison of contemporary protocols. Eur Urol 2012; 62(3): 462-468.
31. Bul M, van den Bergh RC, Zhu X, et al. Outcomes of initially expectantly managed patients with low or intermediate risk screen-detected localized prostate cancer. BJU Int 2012; 110(11): 1672-1677.
32. Gofrit ON, Zorn KC, Steinberg GD, Zagaja GP, Shalhav AL. The Will Rogers phenomenon in urological oncology. J Urology 2008; 179(1): 28-33.
33. Cohen RJ, Wheeler TM, Bonkhoff H, Rubin MA. A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma. Arch Pathol Lab Med 2007; 131(7): 1103-1109.
34. Shah RB, Magi-Galluzzi C, Han B, Zhou M. Atypical cribriform lesions of the prostate: relationship to prostatic carcinoma and implication for diagnosis in prostate biopsies. Am J Surg Pathol 2010; 34(4): 470-477.
35. McNeal JE, Yemoto CE. Spread of adenocarcinoma within prostatic ducts and acini. Morphologic and clinical correlations. Am J Surg Pathol 1996; 20(7): 802-814.
36. Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: Histologic features and clinical significance. Mod Pathol 2006; 19(12): 1528-1535.
37. Cohen RJ, McNeal JE, Baillie T. Patterns of differentiation and proliferation in intraductal carcinoma of the prostate: significance for cancer progression. Prostate 2000; 43(1): 11-19.
38. Dawkins HJ, Sellner LN, Turbett GR, et al. Distinction between intraductal carcinoma of the prostate (IDC-P), high-grade dysplasia (PIN), and invasive prostatic adenocarcinoma, using molecular markers of cancer progression. Prostate 2000; 44(4): 265-270.
39. Rubin MA, de La Taille A, Bagiella E, Olsson CA, O’Toole KM. Cribriform carcinoma of the prostate and cribriform prostatic intraepithelial neoplasia: incidence and clinical implications. Am J Surg Pathol 1998; 22(7): 840-848.
40. Wilcox G, Soh S, Chakraborty S, Scardino PT, Wheeler TM. Patterns of high-grade prostatic intraepithelial neoplasia associated with clinically aggressive prostate cancer. Hum Pathol 1998; 29(10): 1119-1123.
41. Watts K, Li J, Magi-Galluzzi C, Zhou M. Incidence and clinicopathological characteristics of intraductal carcinoma detected in prostate biopsies: a prospective cohort study. Histopathology 2013; 63(4): 574-579.
42. Cohen RJ, Chan WC, Edgar SG, et al. Prediction of pathological stage and clinical outcome in prostate cancer: an improved pre-operative model incorporating biopsy-determined intraductal carcinoma. Br J Urol 1998; 81(3): 413-418.
43. O’Brien BA, Cohen RJ, Wheeler TM, Moorin RE. A post-radical-prostatectomy nomogram incorporating new pathological variables and interaction terms for improved prognosis. BJU Int 2011; 107(3): 389-395.
44. Van der Kwast T, Al Daoud N, Collette L, et al. Biopsy diagnosis of intraductal carcinoma is prognostic in intermediate and high risk prostate cancer patients treated by radiotherapy. Eur J Cancer 2012; 48(9): 1318-1325.
45. Robinson BD, Epstein JI. Intraductal carcinoma of the prostate without invasive carcinoma on needle biopsy: emphasis on radical prostatectomy findings. J Urol 2010; 184(4): 1328-1333.
46. Aggarwal R, Zhang T, Small EJ, Armstrong AJ. Neuroendocrine prostate cancer: subtypes, biology, and clinical outcomes. J Natl Compr Canc Netw 2014; 12(5): 719-726.
47. Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol 2014; 38(6): 756-767.
48. Tamas EF, Epstein JI. Prognostic significance of paneth cell-like neuroendocrine differentiation in adenocarcinoma of the prostate. Am J Surg Pathol 2006; 30(8): 980-985.
49. Wang W, Epstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol 2008; 32(1): 65-71.
50. Yao JL, Madeb R, Bourne P, et al. Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol 2006; 30(6): 705-712.
51. Agoff SN, Lamps LW, Philip AT, et al. Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol 2000;13(3): 238-242.
52. Ordonez NG. Value of thyroid transcription factor-1, E-cadherin, BG8, WT1, and CD44S immunostaining in distinguishing epithelial pleural mesothelioma from pulmonary and nonpulmonary adenocarcinoma. Am J Surg Pathol 2000; 24(4): 598-606.
53. Guo CC, Dancer JY, Wang Y, et al. TMPRSS2-ERG gene fusion in small cell carcinoma of the prostate. Hum Pathol 2011; 42(1): 11-17.
54. Lotan TL, Gupta NS, Wang W, et al. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod Pathol 2011; 24(6): 820-828.
55. Scheble VJ, Braun M, Wilbertz T, et al. ERG rearrangement in small cell prostatic and lung cancer. Histopathology 2010; 56(7): 937-943.
56. Schelling LA, Williamson SR, Zhang S, et al. Frequent TMPRSS2-ERG rearrangement in prostatic small cell carcinoma detected by fluorescence in situ hybridization: the superiority of fluorescence in situ hybridization over ERG immunohistochemistry. Hum Pathol 2013; 44(10): 2227-2233.
57. Williamson SR, Zhang S, Yao JL, et al. ERG-TMPRSS2 rearrangement is shared by concurrent prostatic adenocarcinoma and prostatic small cell carcinoma and absent in small cell carcinoma of the urinary bladder: evidence supporting monoclonal origin. Mod Pathol 2011; 24(8): 1120-1127.
58. Evans AJ, Humphrey PA, Belani J, van der Kwast TH, Srigley JR. Large cell neuroendocrine carcinoma of prostate: a clinicopathologic summary of 7 cases of a rare manifestation of advanced prostate cancer. Am J Surg Pathol 2006; 30(6): 684-693.
59. Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005; 310(5748): 644-648.
60. Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol 2011; 29(27): 3659-3668.
61. Park K, Tomlins SA, Mudaliar KM, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia 2010; 12(7): 590-598.
62. Xu B, Chevarie-Davis M, Chevalier S, et al. The prognostic role of ERG immunopositivity in prostatic acinar adenocarcinoma: a study including 454 cases and review of the literature. Hum Pathol 2014; 45(3): 488-497.
63. Perner S, Mosquera JM, Demichelis F, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol 2007; 31(6): 882-888.
64. Shah RB, Tadros Y, Brummell B, Zhou M. The diagnostic use of ERG in resolving an “atypical glands suspicious for cancer” diagnosis in prostate biopsies beyond that provided by basal cell and alpha-methylacyl-CoA-racemase markers. Hum Pathol 2013; 44(5): 786-794.
65. Yaskiv O, Zhang X, Simmerman K, et al. The utility of ERG/P63 double immunohistochemical staining in the diagnosis of limited cancer in prostate needle biopsies. Am J Surg Pathol 2011; 35(7): 1062-1068.
66. Yaskiv O, Rubin BP, He H, Falzarano S, Magi-Galluzzi C, Zhou M. ERG protein expression in human tumors detected with a rabbit monoclonal antibody. Am J Clin Pathol 2012; 138(6): 803-810.
67. Barbieri CE, Baca SC, Lawrence MS, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Gen 2012; 44(6): 685-689.
68. Blattner M, Lee DJ, O’Reilly C, et al. SPOP mutations in prostate cancer across demographically diverse patient cohorts. Neoplasia 2014; 16(1): 14-20.
69. Tomlins SA, Rhodes DR, Yu J, et al. The role of SPINK1 in ETS rearrangement-negative prostate cancers. Cancer Cell 2008; 13(6): 519-528.
70. Choucair K, Ejdelman J, Brimo F, Aprikian A, Chevalier S, Lapointe J. PTEN genomic deletion predicts prostate cancer recurrence and is associated with low AR expression and transcriptional activity. BMC Cancer 2012; 12 : 543.
71. Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010; 18(1): 11-22.
72. Yoshimoto M, Cunha IW, Coudry RA, et al. FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br J Cancer 2007; 97(5): 678-685.
73. Schrecengost R, Knudsen KE. Molecular pathogenesis and progression of prostate cancer. Semin Oncol 2013; 40(3): 244-258.
74. Qian J, Hirasawa K, Bostwick DG, et al. Loss of p53 and c-myc overrepresentation in stage T(2-3)N(1-3)M(0) prostate cancer are potential markers for cancer progression. Mod Pathol 2002; 15(1): 35-44.
75. Sato K, Qian J, Slezak JM, et al. Clinical significance of alterations of chromosome 8 in high-grade, advanced, nonmetastatic prostate carcinoma. J Natl Cancer Inst 999; 91(18): 1574-1580.
76. Linja MJ, Visakorpi T. Alterations of androgen receptor in prostate cancer. J Steroid Biochem Mol Biol 2004; 92(4): 255-264.
77. Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Gen 1995; 9(4): 401-406.
78. Dall’Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 2012; 62(6): 976-983.
79. Donovan MJ, Cordon-Cardo C. Predicting high-risk disease using tissue biomarkers. Curr Opin Urol 2013; 23(3): 245-251.
80. Cuzick J, Berney DM, Fisher G, et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer 2012; 106(6): 1095-1099.
81. Cooperberg MR, Simko JP, Cowan JE, et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol 2013; 31(11): 1428-1434.
82. Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to aredict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. In press 2014.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2014 Issue 4-
All articles in this issue
- International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia 2012
- Current status of urinary cytology in the evaluation of bladder neoplasms
- Morphological and electrophysiological changes of the heart atria in necropsy patients with atrial fibrillation – a pilot study
- Bart´s syndrome associated with epidermolysis bullosa junctionalis and with pyloric atresia. An autopsy case report
- A concise update on prostate pathology
- Update on urinary bladder pathology
- An isolated metastasis to the heart from a malignant phyllodes tumor with osteosarcomatous differentiation
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Current status of urinary cytology in the evaluation of bladder neoplasms
- Bart´s syndrome associated with epidermolysis bullosa junctionalis and with pyloric atresia. An autopsy case report
- International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia 2012
- A concise update on prostate pathology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

