-
Medical journals
- Career
Expression of the active caspase-3 in children and adolescents with classical Hodgkin lymphoma
Authors: Michaela Čepelová 1; Kateřina Kamaradová 2; Edita Kabíčková 1; Jan Starý 1; Eliška Čumlivská 3; Roman Kodet 2; Petr Gajdoš 1
Authors‘ workplace: Department of Pediatric Haematology and Oncology, nd Medical Faculty, Charles University and University Hospital in Motol Prague, Czech Republic. 1; Department of Pathology and Molecular Medicine, 2nd Medical Faculty, Charles University and University Hospital in Motol Prague, Czech Republic. 2; Department of Radiology, 2nd Medical Faculty, Charles University and University Hospital in Motol, Prague, Czech Republic. 3
Published in: Čes.-slov. Patol., 50, 2014, No. 1, p. 40-44
Category: Original Article
Overview
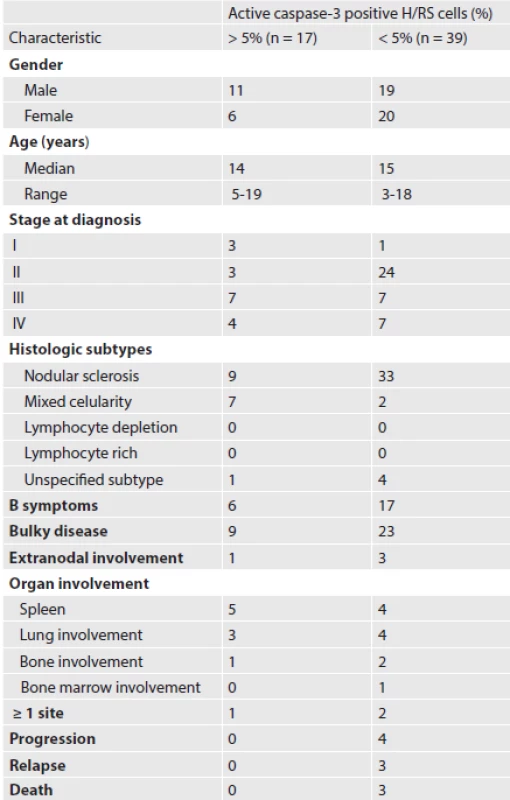
The aim of the study was to determine whether the expression of active caspase-3 in neoplastic Hodgkin and Reed-Sternberg (H/RS) cells correlates with the treatment response and provides prognostic information on treatment outcome. In this retrospective study, we included 56 patients with classical Hodgkin lymphoma treated at the Department of Paediatric Haematology and Oncology between January 2000 and June 2005. Active caspase-3 was detected by immunohistochemistry in primary biopsy specimens. Seventeen patients (29.3%) were evaluated as caspase-3 positive and remained alive in the first complete remission. This stood in contrast to patients with less than 5% caspase-3 positive cells, five of whom experienced relapse and three patients died. Adequate treatment response was achieved in 11 patients (19.6%). Comparison of event-free survival with regard to the percentage of caspase-3 positive tumour cells showed a tendency for a better clinical outcome in patients with 5% or more active caspase-3 positive cells.
Keywords:
classical Hodgkin lymphoma – apoptosis – active caspase-3 – therapy response – clinical outcomeClassical Hodgkin lymphoma (cHL) is a systemic lymphoproliferative disorder derived typically from pre-apoptotic germinal centre B-lymphocytes but rarely from transformed T-cells (1-4). Combined modality treatment (multidrug chemotherapy regimens, radiotherapy) has improved the outcome of cHL patients, with a 5-year overall survival of 90 % (5). Therefore, the aim of current treatment protocols is to individually tailor the therapy to specific risk factors (clinical and biological prognostic factors including caspase-3 (6,7)) with a reduction of late treatment-related complications and maintenance of high cure rates (8,9).
Chemotherapeutic approaches are based on proper activation of an apoptotic pathway and the consequential activation of effector caspase-3. In some cases of Hodgkin lymphoma, chemotherapy resistance can be caused by ineffective or functionless execution or progression of the apoptotic cascade in neoplastic cells.
Apoptosis, as a pathway of programmed cell death, is a result of a complex activation of various molecules including caspases (cysteine-containing aspartic acid-specific proteases). Caspases are present in the cytoplasm of both normal and neoplastic cells. Their activation includes the cleavage of two different inactive subunits and formation of a functional tetramer. Caspases are divided into two groups: initial caspases (caspase 2, 8, 9 and 10) and effector caspases (caspase-3, 6 and 7). Activation of initial caspases is provided either by T-lymphocytes reacting with a cell membrane death receptor (Fas/CD95) or by cytotoxic stress via releasing intracellular cytochrome c. Active initial caspases react with effector caspase proenzymes. Effector caspase-3 is present in cytoplasm as a proenzyme with a molecular weight of 32 kD. Caspase-3 activation comprises an initial cleavage at its p20 cleavage site and an autocatalytic cleavage to form a functional p17 subunit. In vitro studies have demonstrated the presence of the large subunit in cytoplasm and its redistribution to the nucleus (10,11). The function of caspase-3 is executed through forming a heteromer from separately nonfunctional subunits. Activated caspase-3 is responsible for releasing CAD (caspase-activated DNase) from its inhibitor and activating other effector caspases (caspase-6 and -7), as well as a consequential cascade of apoptotic nuclear changes and cell death (12,13).
Apoptosis is regulated by several antiapoptotic protein families, such as bcl-2, bax and IAPs (inhibitors of apoptosis), at different steps of an apoptotic pathway. Increased or aberrant bcl-2 expression was considered a possible cause of apoptotic cascade blockade upstream of caspase-3.
Based on the presence of active caspase-3 in neoplastic cells, we proposed that patients could be stratified into two groups: one group with a functional apoptotic cascade and the other with a probable blockage of apoptosis. Bcl-2 expression and its potential correlation with caspase-3 expression were also investigated.
MATERIALS AND METHODS
Patients Characteristics
Between January 2000 and July 2005, 78 children with newly diagnosed, biopsy proven cHL were treated at our department. Among them, only 56 children were eligible for this study because the paraffin tissue blocks of 20 patients were not available for further immunohistochemical analysis (mainly consultation cases), and two patients were excluded from the analysis due to their violation of the treatment protocol. Five children with nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) were not enrolled because of the previously described absence of procaspase-3 expression in NLPHL (14).
Essential characteristics were extracted from the patients’ medical records: age at diagnosis, staging, gender, presence of B symptoms, chemotherapy regimen, radiotherapy dosage, treatment response and cause and time of death. The median follow-up was 44.5 months (range, from 6 to 71 months).
Staging and Treatment
All patients underwent full staging investigations before the start of therapy (chest X-ray, ultrasonography, computed tomography of chest, abdomen and pelvis and positron emission tomography scans, as well as bone scans and bone marrow aspiration in advanced-stage cHL). Organ functions were assessed in all patients including cardiac ejection fraction, spirometry, a routine serum chemistry panel and blood count. The staging was performed according to the Ann Arbor Staging Classification.
Low-risk patients (stage IA, IIA and IIIA) received 2 or 4 cycles of DBVE chemotherapy (on day 1 and 15: doxorubicin 25 mg/m2, bleomycin 10 mg/m2 and vincristine 1.5 mg/m2; on days 1-5: etoposide 100 mg/m2) with involved-field (IF) radiotherapy. Patients in the high-risk group received 3 or 5 cycles of DBVE-PC chemotherapy (doxorubicin 30 mg/m2 on day 1 and 2; bleomycin 10 mg/m2 on day 1 and 8; vincristine 1.4 mg/m2 on day 1 and 8; etoposide 75 mg/m2 on days 1-5; prednisone 40 mg/m2 on day 1-10; cyclophosphamide 800 mg/m2 on day 1) and subsequent IF radiotherapy. Chemotherapy cycles were repeated every 3 weeks. Radiotherapy was delivered to all nodal and extranodal sites of initial disease, with the exception of bone marrow and lungs, at a total dose of 21 Gy with 1.8 Gy per fraction, one fraction per day.
Treatment response was evaluated by computed tomography, ultrasonography, magnetic resonance and positron emission tomography (PET). FDG-PET scan was performed in 44 patients (78.6 %). Complete remission (CR) was defined as complete disappearance of disease symptoms a more than 75% reduction of initial tumour volume. CR achieved after two cycles of DBVE chemotherapy or three cycles of DBVE-PC was considered adequate treatment response.
Survival curves were constructed according to the Kaplan-Meier method. Log-rank test was used to analyse differences between the curves. Multivariate analysis was performed using the Cox regression analysis.
Immunohistochemistry
Pretreatment biopsy specimens of the patients were selected from the Tumour Registry of the Department of Pathology and Molecular Medicine. Formalin-fixed paraffin-embedded samples of all cases were reevaluated, and the diagnosis of cHL was confirmed both by morphology and by immunohistochemistry using a standard panel of antibodies (CD30, CD15, CD3, CD20, fascin and bcl-2).
Caspase-3 and Bcl-2 Immunohistochemistry
All cases were examined by immunohistochemistry using a primary monoclonal antibody against the active caspase-3 (primary antibody to caspase-3 (active); Alexis Biochemicals, Lausen, Switzerland). This antibody recognises cleaved caspase-3 in the cytoplasm and nucleus. Antigen epitopes in tissue samples were revealed by boiling the slides in citrate buffer or in a microwave oven, and they were visualized by the streptavidin-biotin complex method using diaminobenzidine as a chromogen. The expression of active caspase-3 was semiquantitatively evaluated. We evaluated its nuclear and cytoplasmic positivity in neoplastic RS and Hodgkin cells using a positive control in apoptotic bodies and lymphocytes (15). At least 75-100 neoplastic cells were counted. The cut-off of positivity was set as 5% of positive neoplastic cells in accordance with previous publication by Dukers et al. (7).
Any cytoplasmic positivity of bcl-2 was evaluated as positive (16).
RESULTS
Demographics
Patient demographics are listed in Table 1. The male to female ratio was 1.1 : 1.
According to the Ann Arbor Classification, patients were mostly stage II (48.2 %). Common sites of involvement were mediastinal, supraclavicular and cervical lymph nodes. B symptoms (fever > 38 0C, drenching night sweats and greater than 10% weight loss within the previous 6 months) were present at diagnosis in 23 patients (41.1 %). Bulky disease (mediastinal mass occupying more than one third of the transthoracic diameter on a chest radiograph or a peripheral lymph node greater than 6 cm in maximal axial diameter) was observed in 32 patients (57.1 %). Thirteen patients were assigned to the low-risk treatment group and 43 to the high-risk treatment group.
Treatment results
Treatment response was not evaluated in three patients because they achieved CR by surgery. In the low-risk group, five patients (50.0 %) achieved CR after two cycles of chemotherapy. In the group of patients with an advanced-stage disease, six patients (13.9 %) achieved CR after three cycles of DBVE-PC.
At 44.5-month median follow-up (range, 6 to 71 months), five relapses and four progressions were recorded in four girls and three boys.
Immunohistochemistry results
Histological subtypes were classified according to the WHO modification of the Rye classification. Nodular sclerosis was the predominant histological subtype (75.0 %). Mixed cellularity was less frequent (16.1 %). In five cases, the histological subtype was not specified due to limited availability of the tissue submitted for diagnosis.
Evaluation of active caspase-3 expression
The percentage of caspase-3 positive neoplastic cells ranged between 0 % and 25 % with the mean value of 3 % (Figure 1A, B and C). The cut-off of positivity was determined as 5 % of positive neoplastic cells according to experiences published in adult patients by Dukes et al (7).
Figure 1. Evaluation of active caspase 3 immunoexpression in the pretreatment biopsy. A, B: Two positive cases – active caspase 3 cytoplasmic and nuclear staning (original magnification 200x). C: Negative control (original magnification 400x). 
None of the 17 patients with 5 % or more active caspase-3 positive cells died, whereas three of 39 patients with less than 5 % active caspase-3 positive RS cells died.
An adequate treatment response was achieved in three patients with early-stage disease and one patient with advanced-stage disease accompanied by 5 % or more active caspase-3 positive cells. All patients with stable or progressive disease had less than 5 % of caspase-3 positive cells in the pretreatment specimens.
Among 56 patients in our study, 51 are currently alive in the first CR, one in the second CR and one in the third CR. One patient died from complications related to autologous stem cell transplantation after the second relapse of Hodgkin lymphoma, and two patients died of refractory disease. The overall and event-free survival (EFS) rates in patients with 5% or more caspase-3 positive cells were both 100 %; in patients with less than 5 % caspase-3 positive cells the overall survival reached 95.7 %, and EFS was 83.2 % (Figure 2).
Figure 2. EFS according to the number of caspase-3-positive RS cells in 56 cHL patients (17 patients with 5% or more active caspase-3-positive cells, 39 patients with less than 5% active caspase-3-positive cells, p=0.08, SE±0.359, 95% confidence interval 5.262-6.668 limited to 6.921, mean 5.965). 
Comparison of EFS according to the number of caspase-3 positive RS cells showed a tendency to a better clinical outcome in patients with 5 % or more active caspase-3 positive RS cells, and this tendency was apparent even in stage IV patients. However, these results were not statistically significant (p = 0.08 and p = 0.3, respectively).
In multivariate analysis, none of the analysed parameters (age at diagnosis, gender, clinical stage and B symptoms) was found to be significant, which confirms that prognostic factors gradually lose their predictive power when treatment is successfully adapted to the disease burden (17).
Expression of an antiapoptotic regulator, bcl-2
We detected positivity of an antiapoptotic regulator bcl-2 in 62.5 % of our cases. Active caspase-3 versus bcl-2 expression status in the investigated group of patients is listed in Table 2. We did not observe any significant correlation between bcl-2 expression and active caspase-3 expression.
2. Expression of bcl-2 antiapoptotic regulator 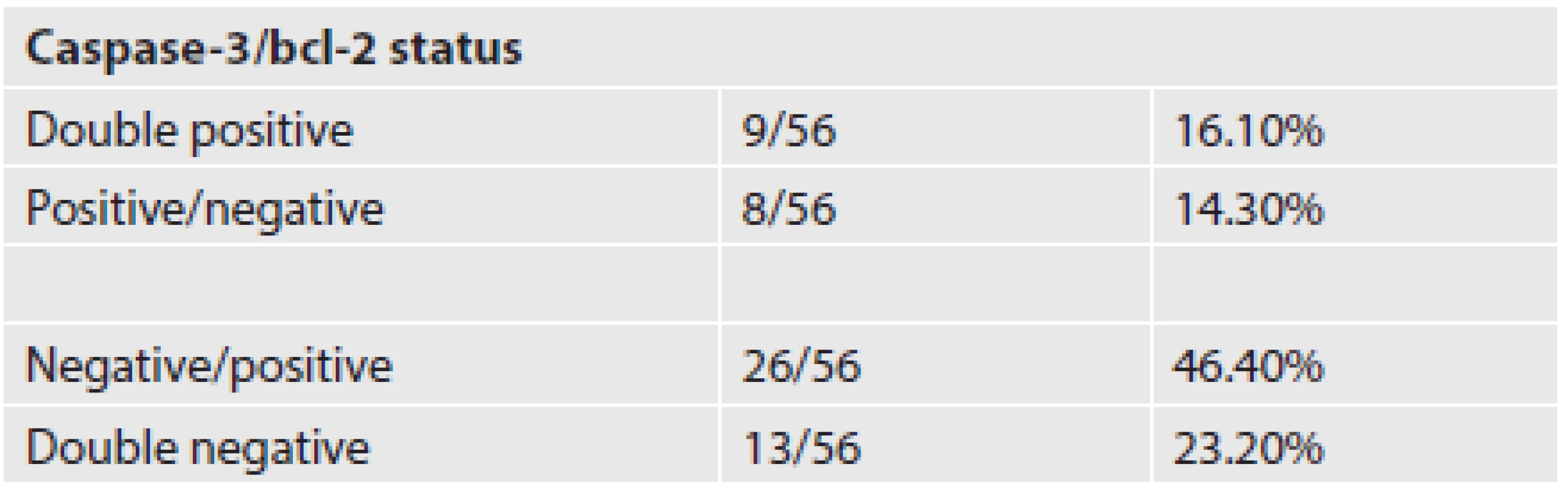
DISCUSSION
Proapoptotic or antiapoptotic status of a cell depends on the activation or inhibition of different signalling pathways at different steps of cell death execution. In case of neoplastic cells, the antiapoptotic status is predominant. Although the molecular pathogenesis of cHL remains unclear, it has been shown that neoplastic H/RS cells demonstrate constitutive activation of nuclear factor kappa-B (NF-κB), resulting in an increased proliferation and survival of the cells (18,19). Furthermore, several other proteins linked to the NF-κB pathway are involved in apoptosis resistance, such as cellular FADD-like interleukin-1-inhibitory protein (c-FLIP) (20). c-FLIP is an inhibitor of caspase-8 activity and mediates cell resistance to death receptor (CD95/Fas)-induced apoptosis that is known to be insufficient in H/RS cells (21,22), but it does not affect caspase-3 activity. Contrariwise, caspases can be inhibited by the IAP-family molecules (inhibitors of apoptotic proteins) such as XIAP (23) or cIAP2. The latter seems to be overexpressed in H/RS cells, and it interferes with otherwise active caspase-3 (24).
We have shown that the expression of active caspase-3 in more than 5 % of H/RS neoplastic cells of Hodgkin lymphoma in pretreatment biopsies may predict a more favourable clinical outcome in patients with an advanced-stage disease. We did not find a correlation between an expression of caspase-3 in the neoplastic cells and the achievement of an adequate treatment response. All 17 patients with 5 % or more caspase-3 positive cells are alive in the first complete remission, but only 4 patients (23.5 %) achieved an adequate treatment response. In the group of 39 patients with less than 5 % caspase-3 positive cells, 2 patients died of progressive disease, and 5 relapses were diagnosed. None of the patients with adequate treatment response relapsed, irrespective of their numbers of caspase-3 positive neoplastic cells. Comparison of EFS according to the number of caspase-3 positive RS cells showed a tendency towards a better clinical outcome in patients with 5 % or more active caspase-3 positive RS cells.
Our study in children and adolescents unfortunately hasn´t reproduced previously published results in adult patients with significant differences in OS and EFS according to percentages of active caspase-3 - positive H/RS cells (7). Statistical significance was not achieved probably due to the limited number of patients in our study and the short follow-up (median 44.5 months). Therefore, larger paediatric studies need be conducted.
Several previous studies revealed bcl-2 as a possible prognostic marker of poor outcome in cHL adult patients (25,26). Our previous study on expression of the antiapoptotic protein, bcl-2, suggested an inverse correlation between its expression and active caspase-3 expression. However, we did not observe any significant correlation between the expression levels of these two factors in the present study, probably due to a more complex regulation of the apoptotic pathway in these cases. This is in accordance with findings of a study by Bai et al. (27). In their study, both pro - and antiapoptotic proteins of the bcl-2 family were variably expressed, very often in a high percentage of the cells, suggesting that it is mainly the antiapoptotic proteins that counteract the expression of proapoptotic proteins and thereby contribute to cell survival. Furthermore, there was no inverse correlation of bcl-2 and active caspase-3 expression.
A relation between a high number of active caspase-3 positive H/RS neoplastic cells and a better prognosis of cHL patients supports the theory that a proper action of chemotherapy and radiotherapy depends on a proper activation of the apoptotic cascade, especially the effector caspases. The possibility to detect or even control the ineffective or inappropriate activation of apoptosis could facilitate the development of new methods in cancer management, although the complexity of the process disallows to the reliability solely on one factor.
ACKNOWLEDGEMENTS
This research was supported by grants from the Teaching Hospital in Motol (No. 9747), from the Ministry of Health (No. VZ MZ 00064203) and from the Ministry of Education (No. MSM 0021620813). The authors thank Dr. W. Hamish Wallace for his critical reading of the manuscript.
Correspondence address:
Cepelova Michaela, MD
Department of Pediatric Haematology and Oncology
2nd Medical Faculty, Charles University
and University Hospital Motol
V Úvalu 84, Prague 5, 150 06, Czech Republic
tel.: + 420 224 436 431, fax: + 420 224 436 420
e-mail: michaela.cepelova@fnmotol.cz
Sources
1. Jaffe ES, Harris NL, Stein H, Vardiman JW (Eds.). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of haemopoietic and lymphoid tissues. IARC Press, Lyon, 2001.
2. Küppers R, Hansmann ML. The Hodgkin and Reed/Sternberg cell (Cells in focus). Int J Biochem Cell Biol 2003; 37 : 511-517.
3. Re D, Küppers R, Diehl V. Molecular Pathogenesis of Hodgkin’s Lymphoma. J Clin Oncol 2005; 23 : 6379-6386.
4. Schmitz R, Stanelle J, Hansmann M-L, Küppers R. Pathogenesis of Classical and Lymphocyte-Predominant Hodgkin Lymphoma. Annu Rev Pathol 2009; 4 : 151-157.
5. Schellong G, Pötter R, Brämswig J, et al. High cure rates and reduced long-term toxicity in pediatric Hodgkin´s disease: The German-Austrian Multicenter Trial DAL-HD 90. J Clin Oncol 1999; 17 : 3736-3744.
6. Vassilakopoulos TP, Pangalis GA. Biological prognostic factors in Hodgkin’s lymphoma. Haema 2004; 7(2): 147-164.
7. Dukers DF, Meijer CJ, Ten Berge RL, et al. High numbers of active caspase-3 positive Reed-Sternberg cells in pretreatment biopsy specimen of patients with Hodgkin disease predict favourable clinical outcome. Blood 2002; 100(1): 36-42.
8. Meadows AT, Baum E, Fossati-Bellani F, et al. Second malignant neoplasms in children: an update from the Late effects Study Group. J Clin Oncol 1985; 3 : 532-538.
9. Van Leeuwen FE, Klokman WJ, Veer MB, et al. Long-term risk of second malignancy after Hodgkin´s disease in a collaborative British cohort: the relationship to age at treatment. J Clin Oncol 2000; 18 : 498-509.
10. Chhanabhai M, Krajewski S, Krajewska M, et al. Immunohistochemical Analysis of Interleukin-1β-Converting Enzyme/Ced3 Family Protease, CPP32/Yama/Caspase-3, in Hodgkin Disease. Blood 1997; 90(6): 2451-2455.
11. An S, Park MJ, Park IC, Hong SI, Knox K. Procaspase-3 and its active large subunit localized in both cytoplasm and nucleus are activated following application of apoptotic stimulus in Ramos-Burkitt lymphoma B cells. Int J Mol Med 2003; 12 : 311-317.
12. Jänicke MU, Sprengart ML, Wati MR et al. Caspase-3 Is Required for DNA Fragmentation and Morphological Changes Associated with Apoptosis. J Biol Chem 1998; 273(16): 9357-9360.
13. Trisciuoglio L, Bianchi ME. Several Nuclear Events during Apoptosis Depend on Caspase-3 Activation but Do Not Constitute a Common Pathway. Plos One 2009 : 4(7): e6234.
14. Izban KF, Wrone-Smith T, Hsi ED, et al. Characterization of the Interleukin-1β-Converting Enzyme/Ced-3-Family Protease, CPP32/ Caspase-3 in Hodgkin Disease; Lack of Caspase-3 Expression in Nodular Lymphocyte Predominance Hodgkin’s Lymphoma. Am J Pathol 1999; 154 (5): 1439-1447.
15. Donoghue S, Baden HS, Lauder I, Sobolewski S, Pringle JH. Immunohistochemical Localization of Caspase-3 Correlates with Clinical Outcome in B-cell Diffuse Large-cell Lymphoma. Cancer Res 1999; 59 : 5386-5391.
16. Tsujimoto Y, Shimizu S. Bcl-2 family: Life-or-death switch. FEBS lett. 2000; 466 : 6-10.
17. Hasenclever D. The disappearance of prognostic factors in Hodgkin´s disease. Annals of Oncology 2002; 13(Suppl1): 75-78.
18. Izban KF, Ergin M, Huang Q, et al. Characterization of NF-κB in Hodgkin’s Disease: Inhibition of Constitutively Expressed NF-κB Results in Spontaneous Caspase-Independent Apoptosis in Hodgkin and Reed-Sternberg Cells. Mod Pathol 2001; 14(4): 297-310.
19. Küppers R. The biology of Hodgkin’s lymphoma. Nat Rev Can 2009; 9 : 15-27.
20. Mathas S, Lietz A, Anagnostopoulos I, et al. c-FLIP mediates resistance of Hodgkin/Reed-Sternberg cells to death receptor-induced apoptosis. J Exp Med 2004; 19; 199(8): 1041-1052.
21. Re D, Hofmann A, Wolf J, Diehl V, Staratchek-Jox A. Cultivated H-RS cells are resistant to CD95L-mediated apoptosis despite expression of wild-type CD95. Exp Hematol 2000; 28(1): 31-35.
22. Los M, Herr I, Friesen C, et al. Cross-resistance of CD95 - and drug-induced apoptosis as a consequence of deficient activation of caspases (ICE/Ced-3 proteases). Blood 1997; 90 : 3119-3129.
23. Kashkar H, Haefs C, Shin H, et al. XIAP-mediated caspase inhibition in Hodgkin’s lymphoma-derived B cells. J Exp Med 2003; 198(2): 341-347.
24. Dürkop H, Hirsch B, Hahn C, Stein H. cIAP2 is highly expressed in Hodgkin/Reed-Sternberg cells and inhibits apoptosis by interfering with constitutively active caspase-3. J Mol Med 2006; 84 : 132-141.
25. Rassidakis GZ, Medeiros LJ, Vassilakopoulos TP, et al. BCL-2 expression in Hodgkin and Reed-Sternberg cells of classical Hodgin disease predicts a poorer prognosis in patients treated with ABVD or equivalent regimens. Blood 2002; 100(12): 3935-3941.
26. Sup SJ, Alemañy CA, Pohlman B, et al. Expression of bcl-2 in classical Hodgkin’s lymphoma: an independent predictor of poor outcome. J Clin Oncol 2005; 23 : 3773-3779.
27. Bai M, Papoudou-Bai A, Horianopoulos N, et al. Expression of bcl-2 family proteins and active caspase-3 in classical Hodgkin’s lymphoma. Hum Path 2007; 38 : 103-113.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2014 Issue 1-
All articles in this issue
- Lynch syndrome in the hands of pathologists
- Detection of chromosome changes by CGH, array-CGH and SNP array techniques in tumours
- Cell cultures
- Granular cell variant of atypical fibroxanthoma. A case report
- Expression of the active caspase-3 in children and adolescents with classical Hodgkin lymphoma
- Uterine tumors resembling ovarian sex cord tumors (UTROSCT). Report of a case with lymph node metastasis
- Gynecomastia with pseudoangiomatous hyperplasia and multinucleated giant cells in a patient without neurofibromatosis
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Lynch syndrome in the hands of pathologists
- Cell cultures
- Detection of chromosome changes by CGH, array-CGH and SNP array techniques in tumours
- Uterine tumors resembling ovarian sex cord tumors (UTROSCT). Report of a case with lymph node metastasis
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career