-
Medical journals
- Career
Prolonged Treatment of Chronic Renal Insufficiency, Acquired Cystic Kidney Disease, Simultaneous Precancerous Lesions and Multiple Tumors of Left Kidney
Authors: A. Böör; I. Jurkovič; Z. Havierová; P. Kočan
Authors‘ workplace: Department of Pathology, Faculty of Medicine, P. J. Šafárik University in Košice, Slovak Republic
Published in: Čes.-slov. Patol., 46, 2010, No. 4, p. 105-110
Category: Original Article
Overview
Purpose of the investigation:
Description of precancerous lesions and kidney tumors developing in a patient with chronic uremia treated by long-term hemodialysis.Most important methods.
Light microscopy, polarization and immunohistochemistry with CK1/CK3, CK5/6, CK7, CK8, CK20, EMA, Renal cell, CD10, Ki-67, PCNA, p53 and E-cadherin antibodies were used.Main findings:
After 11 years of hemodialysis treatment of end-stage diabetic nephropathy and chronic tubulointerstitial nephritis an urgent left-sided nephrectomy was performed because of pain and massive intrarenal bleeding. Biopsy revealed acquired cystic kidney disease associated with multiple precancerous lesions, several small papillary adenomas and a multifocal renal cell carcinoma with conventional and papillary structures with admixture of small foci of highly cellular sarcomatoid features. Severe vascular nephrosclerosis and uremic oxalosis were additional findings. The upper pole of the kidney was massively hemorrhagic.Principal conclusions:
This case illustrates the association of chronic renal insufficiency, uremic oxalosis, long-term hemodialysis, acquired cystic kidney disease and development of variable precursor intratubular and intracystic lesions progressing to several papillary adenomas and multifocal renal cell carcinomas with variegated microscopic structures in one kidney.Kew words:
prolonged treatment of chronic renal insufficiency – acquired cystic kidney disease – precancerous lesions – kidney tumorsIntroduction
With the introduction and prolonged use of hemodialysis as a treatment modality for end-stage kidney patients Dunnill et al. (6) and many consecutive authors have later reported appearance of a new disease form called „acquired cystic kidney disease“ (ACKD), frequently complicated by renal cell neoplasms. The incidence of these tumors was higher than in the general population. A complex review of this topic has recetly been published by Bisceglia et al. (2).
Besides typical forms known in the category of spontaneous adult malignant renal tumors accumulated data have shown that these tumors may occasionally have distinctive histological features not easily referable to the categories described in the current WHO classification of kidney tumors (8). Also it has been noted that many of these tumors reveal tendency for multicentricity, bilaterality, earlier occurence, frequent presence of oxalate crystals within the tumors, and possibly an overall better prognosis (15). Therefore, attention was concentrated on various studies, including immunohistochemical, fluorescence in situ hybridization (FISH) and chromosome analysis along with reporting pertinent clinical data (4).
Rioux-Leclercq and Epstein (29) expressed the hope that their study will stimulate presentation of additional information on various aspects of this topic from other countries. The aim of our report supports this proposal. It is of interest that most of the up to now published data on this theme were published by Japanese authors.
Material and Methods
The formalin-fixed whole-kidney biopsy specimen measured 12 x 6,5 x 5 centimeters. The surface of the organ was uneven due to the presence of numerous expanding cysts varying widely in diameter and some reaching up to 15 milimeters. On the cut section, parenchymal atrophy, numerous cysts, fibrosis and loss of structure and multiple white solid neoplastic tissue replacing and infiltrating original parenchyma were seen leading to the complete loss of original structure and disappearance of the corticomedullary junction. Cysts were mostly filled with clear serous fluid and their content was occasionally hemorhagic. The neoplastic infiltration was irregularly nodular with the biggest solid tumor measuring 11 milimeters in diameter. The upper pole area was intensively hemorrhagic. The consistency of the kidney was firmly elastic.
Multiple blocks were excised, fixed, embedded in parrafin and stained with HE, van Gieson stain, PAS stain and Weigert stain for elastin. Several slides were immunohistochemically stained with a panel of the following monoclonal antibodies: AE1/AE3, CK 5/6, CK7, CK8, CK 20, RC (renal cell antibody), CD10, Ki-67, PCNA, p53 and E-cadherin.
Results
Clinical features
A 72-old-year patient underwent regular hemodialysis through an 11-year-long time interval because of terminal stage of chronic renal insufficiency due to diabetic nephropathy associated with chronic tubulointerstitial nephritis. The urgent nephrectomy was performed because of lumbar pain and massive intrarenal bleeding. Due to symptoms of progression of the primary disease, cachexia, anemia, anorhexia, splenomegaly, immobility and terminal respiratory infection his general condition was extremely poor and he has died 4 months after nephrectomy. Unfortunately, the autopsy was not performed.
Macroscopical findings
Macroscopical details seen in the nephrectomy specimen were described in the chapter „Material and Methods“. The cystic and neoplastic changes explained why almost normal lenght and width of the organ was preserved. Althogether, the thickness of the kidney appeared slightly enlarged than normal mostly due to presence of cystosis and multifocal neoplastic growth.
Microscopical findings
Microscopically, a complex picture of end stage kidney disease consisting of diabetic nephropathy, chronic tubulointerstitial nephritis, pyelitis cystica, severe vascular nephrosclerosis, secondary uremic oxalosis, focal hemosiderosis, acquired cystic kidney disease and pyelitis cystica was found. These changes were associated with variably dysplastic intratubular and intracystic changes, several papillary adenomas and multifocal renal cell carcinomas with conventional (clear-cell) and papillary structure with rare small areas of sarcomatoid character. Multiple tumor emboli were observed in small intrarenal veins and lymphatics.
Two categories of cyst epithelial lining were observed. In the one part the lining epithelium appeared atrophic, while the epithelium of other cysts was proliferating and revealing focally different grades of dysplasia (Figs. 1–4). Similar multiple proliferative and dysplastic epithelial changes were focally observed within some tubules (Figs. 5, 6). Some of these cysts and tubules contained calcium oxalate crystals. Unfrequently, areas with presence of psammoma bodies were found (Fig. 5). The dysplastic epithelium variably expressed Ki-67 in approximately 10 per cent of cells, p53 in 20 per cent of cells and conspicuous expression of PCNA was found in about 40 per cent of cells. While no expression of these antibodies was found in normal or atrophic tubules, 20 per cent of tubular dysplastic nephrocytes were positive with p53 and up to 30 per cent of cells expressed PCNA. Ki-67 staining left the cells unstained. Tab. 1 and 2 presents a complex review of our immunohistochemical analysis.
Fig. 1. Transition from a low cylindrical to cribriform epithelium lining the wall of a small renal cyst. HE, 420x 
Fig. 2. Some of the larger cysts were lined by papillary epithelium. HE, 420x 
Fig. 3. Lining of many small cysts was complex and frequently individually dysplastic. HE, 420x 
Fig. 4. Detail from a similar renal cyst lined by a higher cribriform epithelium. HE, 420x 
Fig. 5. A group of renal tubules lined by variable epithelium containing a few psammoma bodies. HE, 420krát 
Fig. 6. Spectrum of tubular epithelia from atrophic to hyperplastic forms. HE, 420krát 
1. Immunohistochemical expression of a series of antibodies in tubular changes 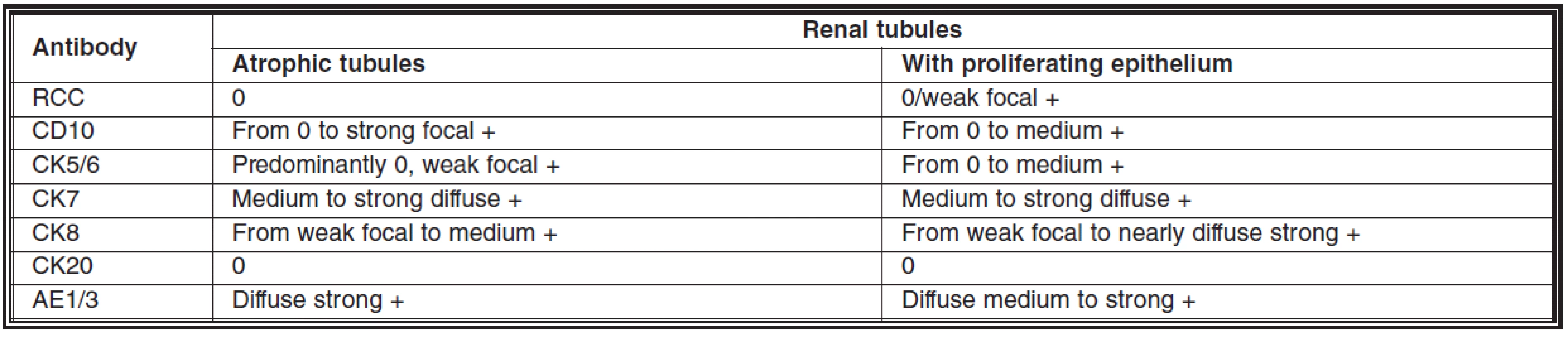
2. Immunohistochemical expression of a series of antibodies in cystic changes 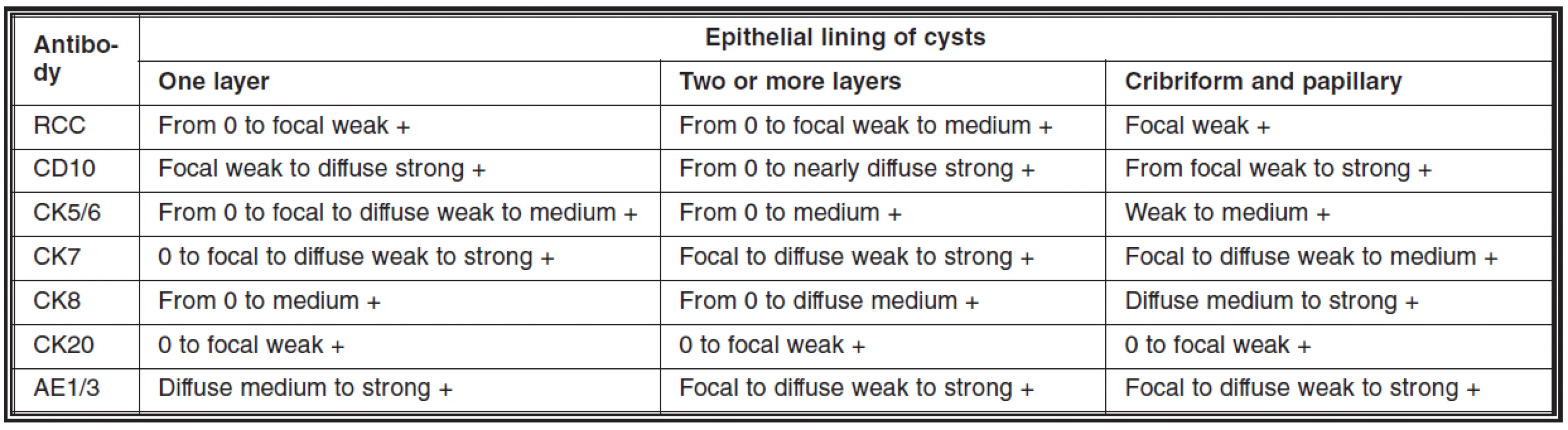
Multiple small renal cell carcinomas were found in many parts of the kidney: papillary (the largest tumor, 11 milimeters in diameter) and conventional (clear-cell) with occasional small areas of sarcomatoid differentiation. Areas of oncocytic differentiation were also rarely encountered. In the largest tumors scattered areas of necrosis were found.
Immunohistochemical expression of a group of antibodies in these tumors is summarized in the tab. 3.
3. Immunohistochemical expressions of a series of antibodies in kidney tumors 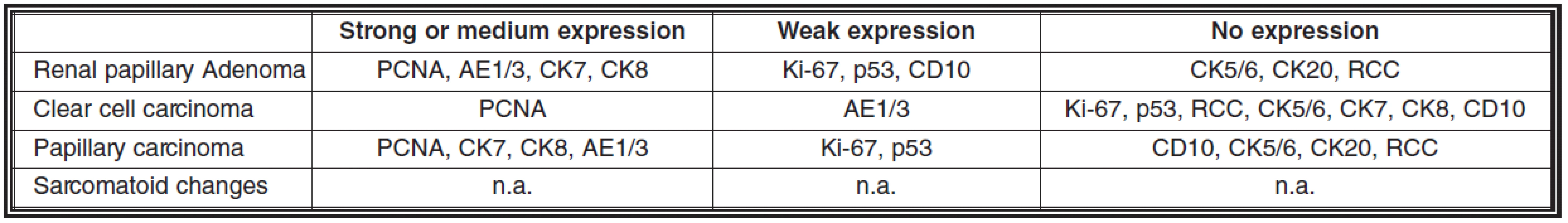
n.a. = not analyzed because of minute areas of these changes not easily identifiable in slides RCC = renal cell carcinoma Discussion
Renal cysts or cystoses may have non-neoplastic (congenital, developmental or acquired) or neoplastic origin. Cysts of the first category are distributed diffusely and bilaterally (35). Neoplastic cysts represent a characteristic feature of the renal tumor, and in the rest of the parenchyma their incidence is unusual. The number of such cystic kidney tumors is relatively large and includes cystic nephroma, cystic variant of the congenital mesoblastic nephroma, mixed epithelial and stromal kidney tumor, cystic lymphangioma of the kidney, cystic renal cell carcinoma and cystic sarcoma of the kidney (2).
Pathogenesis of ACKD was not yet definitively clarified (15). Among the possible pathogenic factors the following data are discussed in the literature: secondary immunodeficiency due to chronic uremia, depressed antioxidant activity and increased synthesis of free radicals, influences of mutations, influence of growth factors, including hepatocyte growth factor and its receptor C-met involved in renal cyst formation and subsequent tumor transformation (16) and activated oncogens, including a protooncogen c-Jun, playing a role in cell cycle progression and neoplastic transformation (26), immunosuppressive therapy of the basic disease or after transplantation, accumulation of carcinogens during the course of chronic renal inssuficiency, possible supportive influence of oxalates on tumorigenesis (due to obstruction, atrophy and rupture of nephrons and iniciating peritubular inflammation). The latter factor was extensively studied by Sule et al. (33). Konda et al. (17) conclude, that hypoxia-inducible protein 2, hypoxia-inducible factor 1alpha and phosphorylated nuclear factor-kappaB may be involved in a continuous process of the evolution of phenotypic expression from a simple cyst to epithelial hyperplasia and eventually to tumor. According to Peces et al. (27) the duration of renal disease rather than the dialysis procedure itself appears to be the main determinant of development of ACKD. The work of Huang et al. (11) represents an extremely rare example of a rat model of cystic transformation and development of a renal tumor.
The biochemical environment in end-stage kidney patients represents according to Truong et al. (35) unrepeated situation of an „acquired predisposition“ to series of steps leading to the development of cysts, development of hyperplasia of their epithelial lining and finally, gradual transformation of epithelial dysplastic lesions to life-threatening renal cell carcinoma. In ACKD the types of the cysts include simple and atypical cysts (3).
Morphological changes of the end-stage renal disease observed in necropsy or biopsy studies during the pre-hemodialysis era were sufficiently described in the literature. With some time delay, since the introduction of long-term dialysis treatment of the end-stage renal disease or nephrosclerosis of either origin a combination of pathogenic factors may support development of acquired cystosis and in protracted cases also premalignant, as well as finally malignant neoplastic changes (6). In these circumstances especially the ultimate microscopical picture is extremely variable and complex.
The ACKD develops within the first three years of therapy in 10-20% of patients, after the first five years of hemodialysis in 40-60% of patients, and after ten or more years of therapy in more than 90% of cases (13). It also is well known that ACKD can develop in end-stage kidney disease without dialysis therapy as well as during the chronic rejection phase after renal transplantation (31, 32).
An intersting report of a patient who was on hemodialysis for 37 uninterrupted years was published by Rifkin (28). His article also presents a list of 8 patients living on uninterrupted hemodialysis for at least 33 years.
Lining of the cysts is variable. On the one side of the spectrum there is mostly flattened or low cuboid epithelium, which sometimes contains larger, more spherical cells with faintly eosinophilic cytoplasm. The degree of epithelial atrophy depends on the dimesions of cysts. On the other side of the spectrum the epithelium reveals different structural forms, terminology, and architecture, e.g. atypical hyperplasia, stratified epithelial projections, intracystic epithelial hyperplasia, atypical hyperplastic cyst, monostratified epithelial intracavitary projections, atypical papillary hyperplasia, papillary tufts and cribriform epithelial proliferations. According to Sule et al. (33) such epithelial changes represent precancerous lesion which are apt to neoplastic transformation.
ACKD proceeds more frequently (86%) asymptomatically. The data published by Truong et al. (35) describing complications include spontaneous rupture and intracystic bleeding as well as bleeding into perirenal or retroperitoneal soft tissues (68%), renal colic (11,5%), persistent hematuria (the frequency not shown), elevated or progressively lowered hematocrit (4,9% and 1,6%), unexplained and prolonged fever (9.8%), rare hypoglycemia and hypocalcemia, and finally precancerous changes and bilateral (10%) and in 50% a multicentric renal cell carcinoma, rarely associated with spontaneous renal rupture and massive perirenal hematoma (22). Significant kidney volume increase due to ACKD and neoplasia was evaluated by Ishikawa et al. (13) in a long-term study reviewing an 15 year long interval. Furthermore, it was found to be still more intensive after 20 years. Non-papillary renal cell carcinomas received shorter period of dialysis than papillary tumors.
Surgical biopsy typing of kidney tumors developing in dialysis-related ACKD includes papillary or tubular adenomas („microadenomas“), oncocytomas (mahagony-brown tumors) and renal cell carcinomas (9, 25). According to Ticoo et al (34) these tumors may reveal most frequently papillary structures, or in a lesser frequency, they correspond to conventional clear cell type carcinoma, occasionally with a cystic subtype, or to rare chromophobe cell carcinoma (7) and sarcomatoid collecting duct carcinoma (1, 20). The largest series o0f observations has been published by Denton et al. (5). It was also noted, that in cases of multiplicity and bilaterality of renal cell carcinoma different parallel types of tumors may be encountered, mostly as a simultaneous presence of a clear-cell and papillary type as well as sometimes described as intermediate between clear cell and papillary types. In the case described by Mazzucchelli et al. (23) the principal disease leading to a series of discussed complications was diabetic glomerulosclerosis, as in our case. Besides renal oncocytosis, multiple papillary adenomas, papillary carcinoma also a B cell lymphoma was found. In the study of 14 patients by Hora et al. (10) the list of tumors included also one myxoid liposarcoma.
In approximately 60% of observed cases new and unusual histological patterns of malignant kidney tumors arise de novo under specific pathogenic conditions, namely renal cell carcinoma composed of large cells with ill-defined cell membranes, abundant eosinophilic granular cytoplasm, large nuclei and prominent nucleoli arranged in solid, acinar, or tubulocystic architecture in ACKD and clear-cell papillary renal cell carcinoma in end-stage-renal-disease (ESRD), with different immune profiles and proximal tubular differentiation (4, 30, 33). A rare combination of multiple renal cell carcinoma and pheochromocytoma in ACKD and long-term hemodialysis was described by Nagashima et al. (25).
As additional characteristics of these latter tumors such features as bilaterality (14, 36), multifocality (27), satellite tumor lesion in renal-cell carcinoma (15), oncocytosis (24), elevated kidney volumes (13), intracystic form of neoplastic growth and micro - or macrocystic structures must be considered. Fortunately, many of these renal cell carcinomas are of small dimensions at the time of diagnosis.
Along with analysis of descriptive features in atypical epithelial proliferations as well as in ACKD-related renal cell carcinoma studies aimed at karyotypic features and the demonstration of immunohistochemical findings have been presented by a small group of authors (3, 4).
In our observation the cytogenetic study was not performed and therefore will not be discussed in details. The rare published data on this topic have demonstrated chromosomal aberrations in the form of gain of various chromosomes depending on the histologic substrate in atypical epithelial proliferation and in the tumors. There appears to be sequential accumulation of cytogenetic abnormalities in the spectrum of epithelial proliferations leading to the emergence of carcinoma, with gain of chromosome 7 as an early event, followed by gain of chromosome 17. It was stressed, this scenario is reminiscent of the adenoma-carcinoma sequence (3, 4, 18, 19).
The presence of several morphologically recognizable types of atypical epithelial proliferations provides an unparalleled opportunity also to evaluate immunohistochemical profiles of atypical epithelial proliferations and in neoplastic cells in our observation, developing due to chronic renal insufficiency and ACKD. Cosu-Rocca et al. (4) have presented immunohistochemical data obtained by analysis of three renal cell carcinomas, but no information has been presented regarding of such findings in precancerous lesions. The tumor cells in their observation expressed CD10, AE1/AE3, α‑methyl-acyl-CoA racemase, CAM5.2 and vimentin and were EMA, CK7, HMWK, 34ßE12 negative. In their surgical biopsy material analysis Aita et al. (1) have used immunohistochemistry and lectins. Carcinomatous tumor cells were positive for EMA, CK and reactive to soybeen and peanut agglutinin, whereas the sarcomatous cells were positive for vimentin as well as EMA. This spectrum confirmed that the tumor originated from the medullary collecting ducts. Using PCNA immunohistochemical labelling Ikeda et al (12) have concluded, that ACKD-acquired renal cell carcinomas are less proliferative and at the same time less aggressive neoplasms than typical renal cell carcinomas.
According to Kojima et al. (15), Scandling et al. (31) and Schwarz et al. (32) the elevated incidence of renal cell carcinoma supports the need of periodical annual sonographic, computerized axial tomography (CAT) screening and magnetic resonance imaging (MRI) in dialysis patients. The former authors have found longer duration of dialysis and smaller tumors with better prognosis in their series. For these patients prophylactic bilateral nephrectomy with the perspective of kidney trasplantation was recommended. The category of benign tumors complicating the ACKD and supported by long-term hemodialysis may be best managed with nephron sparing surgery conservatively (21).
Address for correspondence:
Professor Andrej Böőr, MD, PhD
Institute of Pathology, Faculty of Medicine, P. J. Šafárik University
Rastislavova Street 43
041 90 Košice
Slovak Republic
EU, 0421 55 615 2592
E-mail: andrej.boor@upjs.sk
Sources
1. Aita, K., Tanimoto, A., Fujimoto, Y. et al.: Sarcomatoid collecting duct carcinoma arising in the hemodialysis-associated acquired cystic kidney: an autopsy report. Pathol. Int. 53, 2003, p. 463–7.
2. Bisceglia, M., Galliani, C.A., Senger, C. et al.: Renal cystic diseases: a review. Adv. Anat. Pathol. 13, 2006, p. 26–56.
3. Cheuk, W.,Lo, E.S.F., Chan, A.K.C. et al.: Atypical epithelial proliferations in acquired renal cystic disease harbor cytogenetic aberrations. Hum. Pathol. 33, 2002, p. 761–5.
4. Cossu-Rocca, P., Eble, J.N., Zhang, S. et al.: Acquired cystic disease-associated renal tumors: an immunohistochemical and fluorescence in situ hybridization study. Mod. Path. 19, 2006, p. 780–7.
5. Denton, M.D., Magee, C.C., Ovuworie, C. et al.: Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: A pathologic analysis. Kidney Int. 61, 2002, p. 2201–2209.
6. Dunnill, M.S., Millard, P.R., Oliver, D.: Acquired cystic diseae of the kidneys: A hazard of long-term intermittent maintainance hemodialysis. J. Clin. Patol. 30, 1977, p. 868–877.
7. Fujimoto, K., Anai, S., Okajima, E. et al.: Chromophobe cell renal carcinoma with acquired cystic disease of the kidney in a long-term hemodialysis patient. Int. J. Urol. 10, 2003, p. 99–102.
8. Hammerschmied, C.G., Walter, B., Hartmann, A.: Nierenzellkarzinom 2008. Pathologe 29, 2008, p. 354–63.
9. Hashimoto, T., Togo, Y., Yasuda, K. et al.: Oncocytoma associated with acquired cystic disease of kidney (ACKD): a case report. Hinyokika Kiyo 51, 2005, p. 747–9.
10. Hora, M., Hes, O., Reischig, T. et al.: Tumors in end-stage kidney. Transplant. Proc. 40, 2008, p. 3354–8.
11. Huang, X., Wang, X., Zhu, J. et al.: Chemical-induced polycyst with renal tumor and expression of 8-OHdG in kidney tissue. Thonghua Wai Ke Za Zhi 38, 2000, p. 226–8.
12. Ikeda, R., Tanaka, T., Moriwama, M.T. et al.: Proliferative activity of renal cell carcinoma associated with acquired cystic disease of the kidney: a comparison with typical renal cell carcinoma. Hum. Pathol. 33, 2002, p. 230–5.
13. Ishikawa, I., Saito, A., Chikazawa, Y. et al.: Cystic renal cell cell carcinoma, suspected because of lack of regression of renal cysts after renal transplantatioon in a dialysis patient witrh acquired renal cystic disease. Cli. Exp. Nephrol. 7, 2003, p. 81–4.
14. Kato, T., Takahasi, Y., Nakane, K. et al.: Bilateral renal cell carcinoma associated with polycystic kidney disaese: case report and literature review. Hinyokika Kiyo 53, 2007, p. 117–119.
15. Kojima, Y., Takahara, S., Mivake, O. et al.: Renal cell carcinoma in dialysis patients: a single center experience. Int. J. Urol. 13, 2006, p. 1045–8.
16. Konda, R., Sato, H., Hatafuku, F. et al., T.: Expression of hepatocyte growth factor and its receptor C-met in acquired renal cystic disease associated with renal cell carcinoma. J. Urol. 171, 2004, p. 2166–70.
17. Konda, R., Sugimura, J., Sohma, F. et al.: Over expression of hypoxia-inducible protein 2, hypoxia-inducible factor-1alpha and nuclear factor kappa B is putatively involved in acquired renal cyst formation and subsequent tumor transformation in patients with end stage renal failure. J. Urol. 180, 2008, p. 481–5.
18. Kovacs, G.: Molecular cytogenetics or renal cell tumors. Adv. Cancer. Res. 62, 1993, p. 89–124.
19. Kovacs, G.: High frequency of papillary renal-cell tumours in end-stage kidneys – is there a molecular genetic explanation?. Nephrol. Dial. Transplant. 10, 1995, p. 595–6.
20. Kuroda, N., Tamura, M., Taguchi, T. et al: Sarcomatoid acquired cystic disease-associated renal cell carcinoma. Histol. Histopathol. 23, 2008, p. 1327–31.
21. Lu, T.C., Lim, P.S., Hsu, W.M. et al.: Renal oncocytoma in acquired renal cystic disease. J. Formos. Med. Assoc. 100, 2001, p. 488–91.
22. Matsui, F., Kobori, Y., Amano, T. et al.: Renal cell carcinoma in acquired cystic disease of the kidney manifested by spontaneous renal supture. Hinyokika Kiyo 49, 2003, p. 239–41.
23. Mazzucchelli, R., Cheng, L., Lopez-Beltran, A. et al.: Renal oncocytosis and multiple papillary adenomas with oncocytoma as dominant nodule coexisting with papillary carcinoma in a patient with diabetic glomerulosclerosis, acquired renal cystic disease and B cell lymphoma. APMIS 116, 2008, p. 934–8.
24. Menéndez, C.L., Pobes, A., Corte Torres, M.G. et al.: A case of acquired renal cystic disease ACKD) with oncocytosis, a dominant nodule (oncocytoma), mutliple adenomas and a microscopic papillary renal cell carcinoma associated with crescentic glomerulonephritis. Virchows. Arch. 450, 2007, p. 365–7.
25. Nagashima, A., Ikemoto, I., Furuta, N. et al.: A case of pheochromocytoma associated with incidental multiple renal cell carcinoma originating from acquired cystic disease of the kidney in hemodialysis. Hinyokika Kiyo 52, 2006, p. 557–60.
26. Ova, M., Mikami, S., Mizuno, E. et al.: C-jun activation in acquired kidney disease and renal cell carcinoma. J.Urol. 174, 2005, p. 726.
27. Peces, R., Martinez-Ara, J., Miguel, J.L.: Renal cell carcinoma co-existent with other renal disease: clinico-pathological features in pre-dialysis patients and those receiving dialysis or renal transplantation. Nephrol. Dial. Transplant. 19, 2004, p. 2789–96.
28. Rifkin, S.I.: Thirty-seven uninterrupted years of hemodialysis: a case report. Medscape J. Med. 10, 2008, p. 231–5.
29. Rioux-Leclercq, N.C., Epstein, J.I.: Renal cell carcinoma with intratumoral calcium oxalate crystal depositions in patients with acquired cystic disease of the kidney. Arch. Pathol. Lab. Med. 127, 2003, p. 89–92.
30. Rivera, M., Tickoo, S.K., Saqi, A. et al.: Cytologic findings of acquired cystic disease-associate renal cell carcinoma: a report of two cases. Diagn. Cytopathol. 36, 2008, p. 344–7.
31. Scandling, J.D.: Acquired cystic kidney disease and renal cell cancer after transplantation: time to rethink screening?. Clin. J. Am. Soc. Nephrol. 2, 2007, p. 750–6.
32. Schwarz, A., Vatandaslar, S., Merkel, S. et al.: Renal cell carcinoma in transplant recipients with acquired cystic kidney disease. Clin. J. Am. Soc. Nephrol. 2, 2007, p. 621–2.
33. Sule, N., Yakupoglu, U., Shen, S.S. et al.: Calcium oxalate deposition in renal cell carcinoma associated with acquired cystic kidney disease: a comprehensive study. Am. J. Surg. Pathol. 29, 2005, p. 443–451.
34. Ticoo, S.K., de Peralta-Venturina, M.N., Salama, M. et al.: Spectrum of epithelial tumors in end stage renal disease (ESRD): emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Lab. Invest. 83, 2003, .... 173A.
35. Truong, L.D., Choi, Y.J., Shen, S.S. et al.: Renal cystic neoplasms and real neoplasms associated with cystic renal diseases: pathogenetic and molecular links. Adv. Anat. Pathol. 10, 2003, p. 135–159.
36. Vaseemuddin, M., Kraus, M.A.: Quiz page. Acquired kidney disease (ACKD) with associated bilateral renal cell carcinoma. Am. J. Kidney Dis. 46, 2005, p. 47–9.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2010 Issue 4-
All articles in this issue
- IgG4-related Systemic Sclerosing Disease: a Review
- Muir-Torre Syndrome – a Phenotypic Variant of Lynch Syndrome
- Lymphatic System: Morphology and Pathology Update
- Prolonged Treatment of Chronic Renal Insufficiency, Acquired Cystic Kidney Disease, Simultaneous Precancerous Lesions and Multiple Tumors of Left Kidney
- The Role of Molecular Biology in Detection and Monitoring of Prostate Cancer
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Muir-Torre Syndrome – a Phenotypic Variant of Lynch Syndrome
- Lymphatic System: Morphology and Pathology Update
- IgG4-related Systemic Sclerosing Disease: a Review
- Prolonged Treatment of Chronic Renal Insufficiency, Acquired Cystic Kidney Disease, Simultaneous Precancerous Lesions and Multiple Tumors of Left Kidney
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

