-
Medical journals
- Career
Current use of beta adrenergic receptors blocking medication in paediatric heart failure
Authors: Z. Slavík
Authors‘ workplace: Department of Paediatrics, Royal Brompton and Harefield NHS Foundation Trust, London, United Kingdom
Published in: Čes-slov Pediat 2012; 67 (3): 192-196.
Category: Review
Overview
Congenital heart disease and cardiomyopathy represent the most common cause of chronic congestive heart failure in children. To the contrary of published evidence from adult series, very little convincing evidence for clear clinical benefit of beta adrenergic receptors blocking medication exists in these patients in paediatric age group. Small series of patients, wide age range of studied subjects, variable doses and types of medications used, single nucleotide polymorphism in human population, and heterogeneity of congenital heart defects in individual series of patients with known difference in beta receptor density between right and left ventricle in disease states are but a few reasons for the lack of convincing positive clinical impact of beta receptor blockade found in treated children with chronic heart failure. Preliminary data suggest promising effect of beta receptor blocking medication in myocardial diastolic dysfunction in selected patients following surgical treatment of critical congenital heart defects. Much wider use of beta receptor blockers is to be expected in paediatric intensive care in future (e.g. sepsis, burns, portal hypertension, post-traumatic stress disorder).
Key words:
beta-blockers, heart failure, children
Paediatric congestive heart failure is associated with acquired and congenital heart disease, sepsis, administration of cardiotoxic medication, and severe dysrhythmias to name but a few most common causes. The incidence of acquired heart disease including myocarditis and various forms of cardiomyopathy ranges between 0.3–1.2/100 000 children [1, 2, 3]. It has to be said though that not all of these cases lead to heart failure requiring medical treatment.
Given the relatively low incidence of rheumatic heart disease in paediatric age group in the developed countries, congenital heart disease is currently the most common cause of chronic heart failure in children [4]. It is somewhat surprising that about 25% of children undergoing cardiac surgical treatment for congenital heart defect have developed low cardiac output state as a form of acute congestive heart failure in the early postoperative period in 1970’s and in the recent era [5, 6, 7].
EVIDENCE FOR THE USE OF MEDICATION IN CHRONIC PAEDIATRIC HEART FAILURE
Treatment of chronic heart failure in children includes diuretics despite no evidence from randomised controlled study and doses extrapolated from adult studies [8]. Anecdotal evidence for their beneficial effect makes any future randomised, placebo controlled study in children with heart failure unlikely. The use of angiotensin converting enzyme inhibitors (captopril and/or enalapril) in paediatric heart failure patients was documented in 5 published series including total of 257 patients (0.3–16 years of age) between 1998 and 2010 [9, 10, 11, 12, 13]. The results showed either no effect or improvement in echocardiografic findings of left ventricular size, mass or wall stress. Translation of these results into clinical effect is uncertain.
BETA ADRENERGIC RECEPTORS AND CARDIOVASCULAR SYSTEM
Before we look closely at the role of beta adrenergic receptor blocking medication in paediatric heart failure, short overview of the receptors’ distribution and function in cardiovascular tissues is warranted. Beta 1 and beta 2 receptors are evenly distributed in the myocytes of the left and right ventricles in healthy heart as opposed to differences in their distribution of morphologically abnormal heart [14]. Their stimulation leads to increase in heart rate and myocardial contractility. Stimulation of beta 2 receptors present in vascular smooth muscle cells leads to arterial and venous vasodilatation. Beta 1 receptors are linked with intracellular G stimulating protein and beta 2 receptors with both the stimulating and inhibiting G proteins. Beta receptor stimulation in a myocyte is followed by increase in cytosolic cyclic adenosine monophosphate and calcium concentrations with ensuing increase in myocardial contraction. The ratio of beta 1 and beta 2 receptors in healthy myocardium is about 3 : 1 in favour of beta 1 receptors. This ratio changes to 2 : 1 still in favour of beta 1 receptors under chronic overstimulation.
Moreover, this overstimulation due to high levels of endogenous or exogenous catecholamines in chronic heart failure leads to down-regulation (loss of receptors) accompanied by desensitization (loss of signalling) followed by increase in cytosolic calcium levels and adverse cardiac remodelling (myocyte hypertrophy, apoptosis, fibrosis, extracellular matrix proliferation). Beta 3 adrenergic receptor identified in myocytes in 1996 is coupled with G inhibiting protein and appears upregulated in failing myocardium. This upregulation leads to increase in intracellular cyclic guanosine monophosphate and drop in cyclic adenosine monophosphate and intracellular calcium. This antagonistic effect to beta 1 receptor stimulation and resistance to desensitization following chronic adrenergic stimulation in failing heart makes beta 3 receptor the focus of intense research. Its stimulation was shown to play promising role in slowing progression of heart failure in experimental setting [15].
CLINICAL USE OF BETA BLOCKERS IN CHILDREN WITH CHRONIC HEART FAILURE
The following beta adrenergic receptor blocking medication has been used in the treatment of paediatric heart failure – propranolol, atenolol, esmolol, metoprolol, bisoprolol, carvedilol (Table 1). Carvedilol was considered a promising agent with its beta 1, beta 2, and alfa 1 receptors blocking properties leading to direct myocardial effect but also to peripheral arterial vasodilatation and anti-oxidant and anti-apoptotic effect. It is therefore particularly disappointing that it had no positive impact on paediatric heart failure in the largest prospective, placebo controlled trial conducted to date [16] (Table 2).
1. List of beta-blockers used clinically. 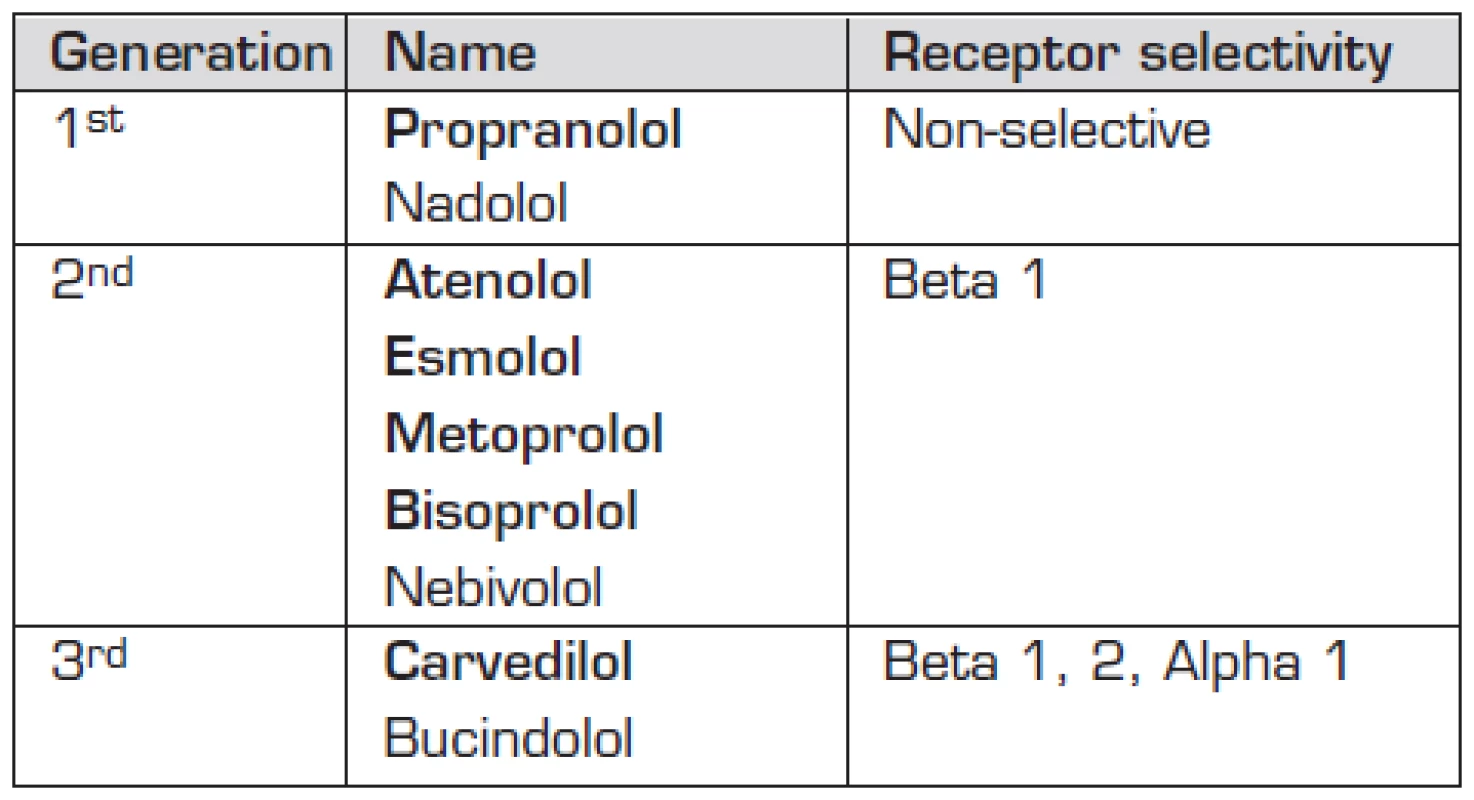
Names in bold depict beta-blockers used in paediatric patients 2. Use of beta-blockers in paediatric heart failure. 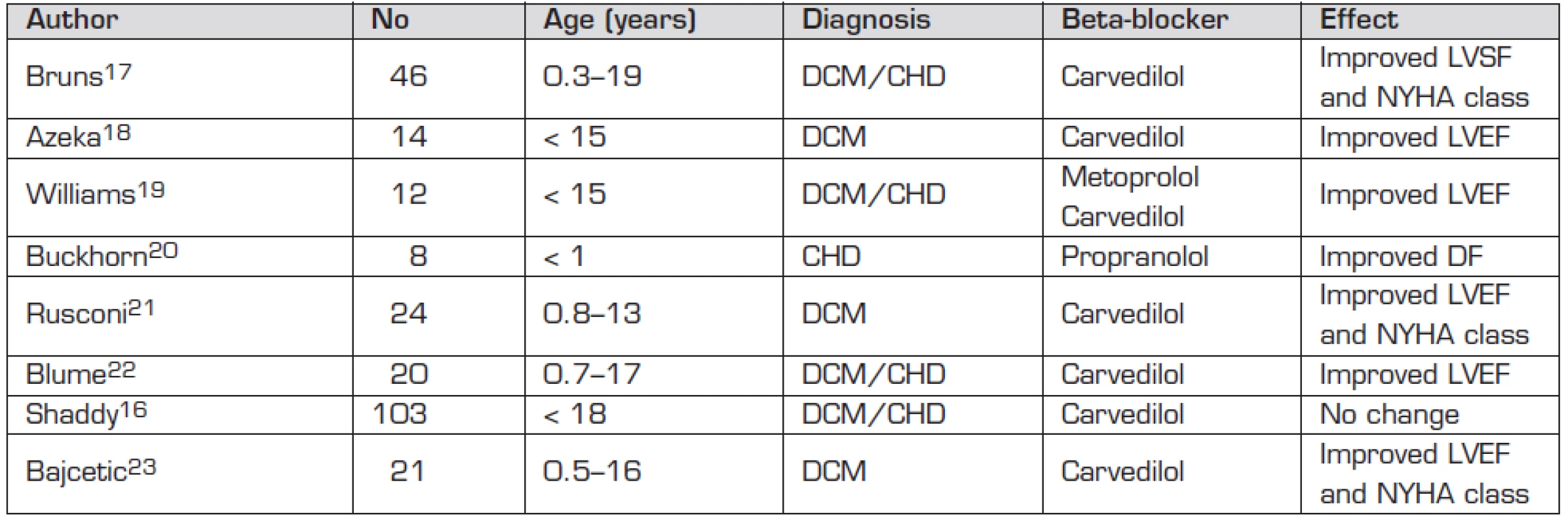
Abbreviations: CHD – congenital heart disease; DCM – dilated cardiomyopathy; DF – diastolic function; LVEF – left ventricular ejection fraction; LVSF – left ventricular shortening fraction; NYHA – New York Heart Association There are several speculative reasons why the so far published series did not show any convincing beneficial impact on failing myocardium in paediatric age group [16–23]. Small series of patients, wide age range of studied subjects, and variable doses of medications used (same Carvedilol plasma levels found in infants treated by 3 mg/kg/day and in adults treated by 0.7 mg/kg/day [24]) are the most obvious shortcomings. Single nucleotide polymorphism was found to lead to functional changes in beta 1 and beta 2 receptors and in G protein kinase responsible for disappointing outcome of a large prospective study in adult patients [25, 26]. This polymorphism was linked with patients’ racial origin. Heterogeneity in the studied patients with congenital heart disease as to their systemic ventricular morphology is likely to play a role given the known difference in beta receptor density between diseased left and right ventricles [21, 27].
USE OF BETA BLOCKERS IN ACUTE MYOCARDIAL FAILURE
There is intriguing anecdotal evidence of beneficial effect of beta blocking medication administered in acute heart failure. Administration of esmolol to a single adult patient with acute left ventricular failure while recording pressure-volume curves showed decreased end-systolic and arterial elastance as markers of decreased stroke volume and dP/dt, and afterload, respectively. More importantly the area of pressure volume loop was reduced as a marker of reduced myocardial oxygen consumption [28]. Left ventricular diastolic myocardial dysfunction follows surgical repair of critical neonatal congenital heart defects with obstructed left ventricular outflow despite successful initial treatment and very good systolic function in some patients. Low cardiac output states present in many such patients lead to high serum levels of catecholamines with concomitant tachycardia and increased postoperative morbidity.
We added esmolol infusion in this setting to milrinone and digoxin treatment in an infant requiring artificial ventilation 1 month after Ross-Konno operation for severe aortic stenosis with left ventricular end-diastolic blood pressure of 24 mmHg, well preserved systolic myocardial function, and persistent sinus tachycardia. Reduction of mean daily heart rate from 160 bpm to 130 bpm without changes to systemic blood pressure or central venous pressure allowed for weaning from ventilator support 3 days later [29]. Heart rate lowering medication has been used successfully in adult population with myocardial dysfunction of various etiology for some time now [30]. Theoretical benefit of heart rate reduction in clinical scenario of diastolic myocardial dysfunction is based on longer diastolic phase of cardiac cycle and potentially longer ventricular filling time. However, positive effect of beta-receptor blocking medication on myocardial dysfunction is likely to be multi-factorial and highly individual in each patient treated [28].
There is also evidence for benefit from beta adrenergic receptor blocking medication related to prevention of postoperative junctional ectopic tachycardia in patients with tetralogy of Fallot pre-treated with propranolol [31] and to reduction of myocardial ischaemia/reperfusion injury through reduced myocardial apoptosis by late administration of esmolol and milrinone in experimental setting [32]. Moreover, experimental findings show improved myocardial relaxation and increased ventricular end-diastolic volume under esmolol treatment in sepsis [33].
CONCLUSIONS
No strong proof of clinical beta blocker treatment benefit in chronic paediatric myocardial failure exists at present. There is limited experience with beta blocker use in acute paediatric myocardial failure with promising new avenues to explore. Much wider use of beta blockers is to be expected in paediatric intensive care in future (e.g. in sepsis, burns, portal hypertension, post-traumatic stress disorder).
Došlo: 19. 2. 2012
Přijato: 22. 4. 2012
Doc. MUDr. Zdeněk Slavík, DM (UK), FRCPCH
Department of Paediatrics
Royal Brompton and Harefield NHS Foundation Trust
Sydney Street
London SW3 6NP
United Kingdom
e-mail: Zdenek.Slavik@rbht.nhs.uk
Sources
1. Nugent AW, Daubeney PE, Chondros P, et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med 2003; 348 : 1639–1646.
2. Wilkinson JD, Landy DC, Colan SD, et al. The pediatric cardiomyopathy registry and heart failure: key results from the first 15 years. Heart Fail Clin 2010; 6 : 401–413.
3. Kay JD, Colan SD, Graham TP Jr. Congestive heart failure in pediatric patients. Am Heart J 2001; 142 : 923–928.
4. Šamánek M, Slavík Z, Zbořilová B, et al. Prevalence, treatment,and outcome of heart disease in live-born children: A prospective analysis of 91,823 live born children. Pediatr Cardiol 1989; 10 : 205–211.
5. Parr GVS, Blackstone EH, Kirklin JW. Cardiac performance and mortality early after intracardiac surgery in infants and young children. Circulation 1975; 51 : 867–874.
6. Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 1995; 92 : 2226–2235.
7. Hoffman TM, Wernovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 2003; 107 : 996–1002.
8. van der Vorst MM, Kist JE, van der Heiiden AJ, et al. Diuretics in pediatrics: current knowledge and future prospects. Paediatr Drugs 2006; 8 : 245–264.
9. Hsu DT, Zak V, Mahony L, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation 2010; 122 : 333–340.
10. Gisler F, Knirsch W, Harpes P, et al. Effectiveness of angiotensin-converting enzyme inhibitors in pediatric patients with mid to severe aortic valve regurgitation. Pediatr Cardiol 2008; 29 : 906–909.
11. Mori Y, Nakazawa M, Tomimatsu H, et al. Long-term effect of angiotensin-converting enzyme inhibitor in volume overloaded heart during growth: a controlled pilot study. Am Coll Cardiol 2000; 36 : 270–275.
12. Calabro R, Pisacane C, Pacileo G, et al. Hemodynamic effect of a single oral dose of enalapril among children with asymptomatic chronic mitral regurgitation. Am Heart J 1999; 138 : 955–961.
13. Alehan D, Ozkutlu S. Beneficial effect of 1-year captopril therapy in children with chronic aortic regurgitation who have no symptoms. Am Heart J 1998; 135 : 598–603.
14. Patel AR, Shaddy RE. Role of beta-blocker therapy in pediatric heart failure. Ped Health 2010; 4 : 45–58.
15. Ufer C, Germack R. Cross-regulation between beta 1 - and beta 3-adrenoceptors following chronic beta-adrenergic stimulation in neonatal rat cardiomyocytes. Br J Pharmacol 2009; 158 : 300–313.
16. Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure. JAMA 2007; 298 : 1171–1179.
17. Bruns LA, Chrisant MK, Lamour JM, et al. Carvedilol as therapy in pediatric heart failure: an initial multicenter experience. J Pediatr 2001, 138 : 505–511.
18. Azeka E, Franchini Ramirez JA, Valler C, Alcides Bocchi E. Delisting of infants and children from the heart transplantation waiting list after carvedilol treatment. J Am Coll Cardiol 2002; 40 : 2034–2038.
19. Williams RV, Tani LY, Shaddy RE. Intermediate effects of treatment with metoprolol or carvedilol in children with left ventricular systolic dysfunction. J Heart Lung Transplant 2002; 21 : 906–909.
20. Buckhorn R, Hulpke-Wette M, Ruschewski W, et al. Effects of therapeutic beta blockade on myocardial function and cardiac remodelling in congenital cardiac disease. Cardiol Young 2003; 13 : 36–43.
21. Rusconi P, Gomez-Arin O, Rossique-Gonzales M, et al. Carvedilol in children with cardiomyopathy: 3-year experience at a single institution. J Heart Lung Transplant 2004; 23 : 832–838.
22. Blume ED, Canter CE, Spicer R, et al. Prospective, single-arm protocol of carvedilol in children with ventricular dysfunction. Pediatr Cardiol 2006; 27 : 336–342.
23. Bajcetic M, Kokic Nikolic A, Djukic M, et al. Effects of carvedilol on left ventricular function and oxidative stress in infants and children with idiopathic dilated cardiomyopathy: a 12-month, two-center, open-label study. Clin Ther 2008; 30 : 702–714.
24. Albers S, Meibohm B, Mir TS, et al. Population pharmacokinetics and dose simulation of carvedilol in paediatric patients with congestive heart failure. Br J Clin Pharmacol 2008; 65 : 511–522.
25. BEST trial investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001; 344 : 1659–1667.
26. Dorn GW, Liggett SB. Mechanism of pharmacogenomic effect of genetic variation within the cardiac adrenergic network in heart failure. Mol Pharm 2009; 76 : 466–480.
27. Kaufman BD, Desai M, Reddy S, et al. Genomic profiling of left and right ventricular hypertrophy in congenital heart disease. J Cardiac Fail 2008; 14 : 760–767.
28. Zaugg M, Schaub MC, Pasch T, et al. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88 : 101–123.
29. Furck AK, Desai A, Rodrigues W, et al. Beta-receptor blocking medication as a rescue treatment for severe postoperative myocardial diastolic dysfunction in infants. Cardiol Young 2011; 21: S158.
30. Cucherat M. Quantitative relationship between resting heart rate reduction and magnitude of clinical benefit in post-myocardial infarction: a meta-regression of randomised clinical trials. Eur Heart J 2007; 28 : 3012–3019.
31. Mahmoud AB, Tantawy AE, Kouatli AA, et al. Propranolol: a new indication for an old drug in preventing postoperative junctional ectopic tachycardia after surgical repair of tetralogy of Fallot. Interact Cardiovasc Thorac Surg 2008; 7 : 184–187.
32. Huang MH, Wu Y, Nguyen V, Rastogi S, et al. Heart protection by combination therapy with esmolol and milrinone at late-ischemia and early reperfusion. Cardiovasc Drugs Ther 2011; 25 : 223–232.
33. Gore DC, Wolfe RR. Hemodynamic and metabolic effects of selective beta1 adrenergic blockade during sepsis. Surgery 2006; 139 : 686–694.
Labels
Neonatology Paediatrics General practitioner for children and adolescents
Article was published inCzech-Slovak Pediatrics

2012 Issue 3-
All articles in this issue
- Incidence of dyslipidemia in a obese children population
- Pyruvate kinase deficiency in children
- Unusual manifestations of parvovirus B19 infection in children
- Determination of prealbumin and selenium in serum for monitoring the nutrition status of phenylketonuric and hyperphenylalaninemic patients
- Variability in clinical manifestation of noroviral infection in a newborn: from fulminant necrotizing enterocolitis to asymptomatic course
- Benign infantile seizures associated with noroviral gastroenteritis
- Reading about speech and language therapy – 1st Part
- Current use of beta adrenergic receptors blocking medication in paediatric heart failure
- Czech-Slovak Pediatrics
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Unusual manifestations of parvovirus B19 infection in children
- Pyruvate kinase deficiency in children
- Variability in clinical manifestation of noroviral infection in a newborn: from fulminant necrotizing enterocolitis to asymptomatic course
- Benign infantile seizures associated with noroviral gastroenteritis
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

