-
Medical journals
- Career
Comparison of enhanced recovery protocol with conventional care in patients undergoing urogynecological surgery
Authors: G. Yilmaz 1; E. Can 2; D. O. Omaygenc 3; N. Tuten 2; F. Olmez 2; H. Kiyak 2; P. Y. Bahat 2; A. Akca 2; Z. Salihoglu 4
Authors‘ workplace: Department of Anesthesiology, Kanuni Sultan Suleyman Training and Research Hospital, Istanbul, Turkey 1; Department of Obstetrics and Gynecology, Kanuni Sultan Suleyman Training and Research Hospital, Istanbul, Turkey 2; Department of Anesthesiology, Istanbul Haseki Training and Research Hospital, Istanbul, Turkey 3; Department of Anesthesiology, Faculty of Medicine, Istanbul University – Cerrahpasa, Istanbul, Turkey 4
Published in: Ceska Gynekol 2022; 87(4): 232-238
Category: Original Article
doi: https://doi.org/10.48095/cccg2022232Overview
Objective: The impact of enhanced recovery after surgery (ERAS) protocol on postoperative outcomes after urogynecological surgery is yet to be a matter of investigation. This study sought to evaluate this issue by comparing the patients who had conventional or ERAS--guided perioperative care for several clinical end-points including ambulation, length of hospital stay (LOS), readmissions, and postoperative complications. Materials and methods: A total of 121 patients undergoing pelvic organ prolapse surgery were allocated to two study arms, ERAS protocol (Group E) or conventional care (Group C). Variables reflecting the restoration of appetite and bowel movements, bleeding events, other complications, LOS and readmissions were compared between the groups. Results: The patients in Group C significantly received a more intensive intravenous fluid treatment compared to Group E (2,760 ± 656 vs. 1,045 ± 218 mL, P < 0.001). Time required for first flatus, first defecation, eating solid food, and ambulation (P < 0.001) were also longer in the former group of patients. Moreover, LOS was significantly reduced when the ERAS protocol was applied (2.5 ± 1.1 vs. 2.0 ± 0.6 days, P < 0.001). On the other hand, the two groups were similar with respect to the frequency of the postoperative complications, including surgical site infections, cardiovascular complications, non-specific abdominal pain, sub-ileus, blood loss and readmission rate. Conclusion: In our sample population, ERAS protocol led to early initiation of oral intake, early recovery of bowel function, early mobilization, and early discharge of patients without compromise in safety concerns after urogynecological surgery.
Keywords:
Gynecologic surgery – postoperative complications – pelvic organ prolapse – Enhanced Recovery After Surgery – postoperative care
Introduction
The cardinal strategy in perioperative management had been used to be passive, featuring a “wait and see” protocol, until enhanced recovery after surgery (ERAS) techniques were firstly introduced in colorectal surgery. While physicians reacted only in response to postoperative adverse event occurrences in the former, perioperative interventions were carried out more proactively in the ERAS algorithm which led to a huge paradigm shift [1].
ERAS, also named the “fast-track” protocol, mainly aims to improve patient satisfaction along with procedure-related advantages such as reduction of complications and duration of hospital stay. ERAS protocol has dedicated preoperative, intraoperative, and postoperative measures targeting improved postoperative outcomes. At the preoperative stage, stabilizing the chronic disease symptoms, maintaining the optimal energy expenditure and volume status, and getting prepared for minimally invasive techniques to the utmost are the basic components of the ERAS protocol [2]. In the meantime, providing adequate analgesia, avoiding hypothermia, goal-directed fluid administration and early nutrition, ambulation and removal of surgical instruments are the key features of intraoperative and postoperative ERAS care; respectively [3].
The positive influence of the ERAS protocols on hindering postoperative complications, diminishing the length of stay (LOS) and improving postoperative analgesia and rapid mobilization is well-established for the subjects undergoing urologic, gastrointestinal, and pancreatic surgery [4–7]. However, the evidence concerning the role of ERAS protocol on postoperative outcomes in patients undergoing urogynecological surgery is considerably sparse.
Here, we sought to investigate this issue by comparing the patients who had conventional or ERAS-guided perioperative care for several clinical endpoints including ambulation, length of hospital stay, readmissions, and postoperative complications after urogynecological surgery.
Materials and methods
Patient selection
One hundred and twenty-one subjects, out of 128 eligible subjects undergoing urogynecological procedures between April 2019 and December 2019 in our institution, were enrolled in this prospective, randomized, and controlled study. The pathologic states and the surgical procedures performed for treatment were demonstrated in Fig. 1. The study was approved by the local ethical committee (Protocol. 2019-4-92, Date of approval April 2019), hence was per-formed in accordance with the Helsinki Declaration. Informed consent was obtained from all subjects. Exclusion criteria were as follows: emergent surgery, preoperative sepsis, admission to intensive care unit following surgery, reoperation for hernia recurrence, and advanced liver or kidney disease. Seven patients were excluded during the recruitment period by this means (four patients for intensive care unit follow-up and three patients in need of reoperation). The remaining participants were allocated to one of the treatment arms: ERAS care (Group E) or conventional care (Group C) by computer-generated randomization considering a match-up for age and body mass index (BMI) between the groups. The study groups of the subjects were recorded on a card and placed into sealed envelopes by a researcher blinded to the procedural features for being opened during admission for surgery. The flow diagram of the study was displayed in Fig. 2.
Fig. 1. Distribution of the diseases creating the indication for surgery (A), and the procedures performed for treatment (B) were displayed in pie-charts.
A/P CR – anterior/posterior colporrhaphy, IVS – intravaginal sling, LAVH – laparoscopy-assisted vaginal hysterectomy, SSF – sacrospinal fixation, TOT – transobturator tape, VH – vaginal histerectomy
Obr. 1. Rozdělení onemocnění určujících indikaci k operaci (A) a výkonů provedených pro léčbu (B) byly znázorněny v koláčových grafech.
A/P CR – přední/zadní kolporhapie, IVS – intravaginální závěs, LAVH – laparoskopicky asistovaná vaginální hysterektomie, SSF – sakrospinální fixace, TOT – antiinkontinenční pásky, VH – vaginální hysterektomie
Fig. 2. Flow diagram of the study protocol – ERAS.
Obr. 2. Vývojový diagram protokolu studie – ERAS.
Features of the treatment arms
Conventional care – the sealed envelopes were opened at the time of admission. The patients assigned to Group C were scheduled for surgery the day after admission. A urinary catheter was placed and cefoperazone (1,000 mg, i.v.) was administered to all participants as a prophylactic antimicrobial agent who was subsequently received general anesthesia. Intravenous fluid infusion (100 mL/hour) was invariably initiated at admission and sustained until first oral intake after the surgery. Postoperative analgesia was achieved with paracetamol (1,000 mg, p. o.), and diclofenac sodium (50 mg, i.v.) was added if necessary.
ERAS protocol – the ERAS group subjects were scheduled for surgery on the day of admission. Our institutional ERAS protocol (in accordance with the recent guidelines) was followed for the patients in Group E (Tab. 1) [8]. The subjects were encouraged to cease smoking and alcohol consumption four weeks prior to surgery. Intravenous iron was supplemented in the subjects with anemia; hematology counseling was reserved for advanced cases. Extended fasting, bowel cleansing, and premedication were avoided in this group. Clear fluids were permitted up to 2 hours prior to anesthesia induction and solids rich in carbohydrates were withdrawn 6 hours before the procedure. Routine antibiotic prophylaxis with cefoperazone (1,000 mg, i.v.) was given 60 minutes before the skin incision, and thromboembolism prophylaxis was performed in selected high-risk patients. Intraoperative normothermia was achieved by warmed intravenous fluids. Short-acting anesthetic agents were used for epidural anesthesia/analgesia [8]. Conservation of surgical instruments (drains, catheters, nasogastric tubes, etc.) and volume and electrolyte overload were strictly avoided in this group. Intravenous paracetamol was administered for postoperative analgesia before the completion of the surgical procedure. Ondansetron was the agent of choice for the management of postoperative nausea. Diclofenac sodium (50 mg, i.v.) was administered for rescue analgesia. Oral intake and ambulation were initiated as early as possible [9].
1. Recommendations regarding ERAS care.
Tab. 1. Doporučení týkající se péče ERAS.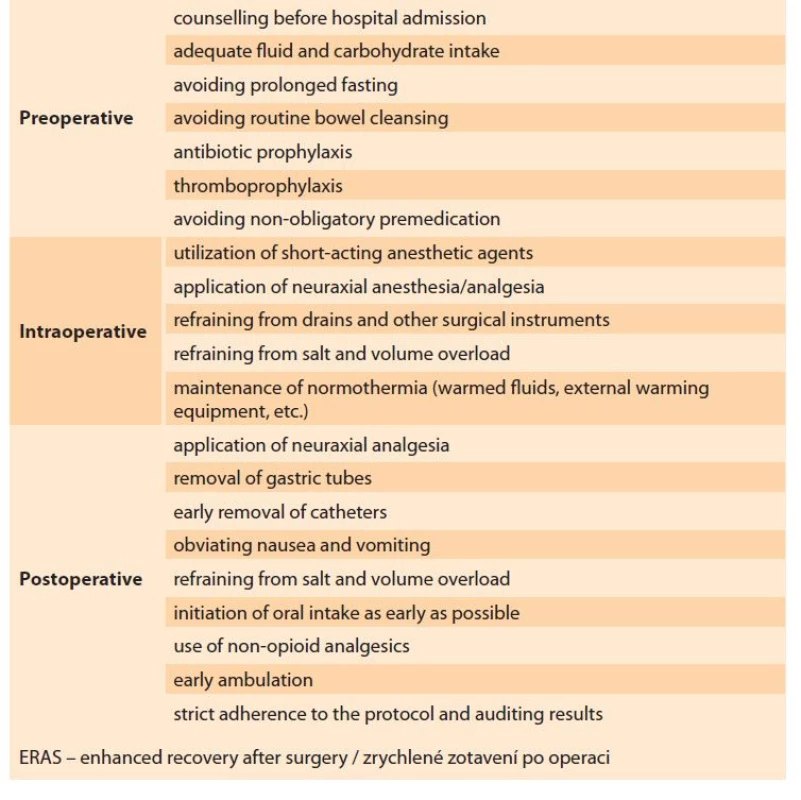
Outcome measures
The primary measures compared between treatment arms were surgical or anesthetic complication occurrence, perioperative bleeding, LOS, and readmission rates. The secondary measures were related to postoperative compliance including time to first flatus, defecation, ambulation, and solid food intake.
Statistical analysis
The entire set of data was analyzed by using SPSS for Windows, version 17 (SPSS, Chicago, IL, USA). Power calculations based on our pilot study with 20 subjects revealed that (effect size: 0.70, alpha error: 0.5 power: 0.95) 45 subjects should be enrolled at minimum for each group to reach statistical significance [10]. The normal distribution of the variables was studied with the Kolmogorov-Smirnov test. Continuous variables were presented as the mean ± standard deviation and categorical variables as a percentage. The Student t-test was used for parametric comparisons. The Chi-square test was used for distinguishing the groups when it came to categorical variables. Two-sided P ≤ 0.05 was interpreted as statistically significant.
Results
Group E was comprised of 71 patients (mean age 57.5 ± 12 years) and Group C consisted of 50 patients (mean age 54.6 ± 10 years). The two groups were similar with respect to the age (P = 0.168), BMI (P = 0.355), ASA score (P = 0.667), the preoperative diagnosis, and the surgical procedure performed (Tab. 2).
2. Comparison of the perioperative characteristics of study groups.
Tab. 2. Srovnání perioperačních charakteristik sledovaných skupin.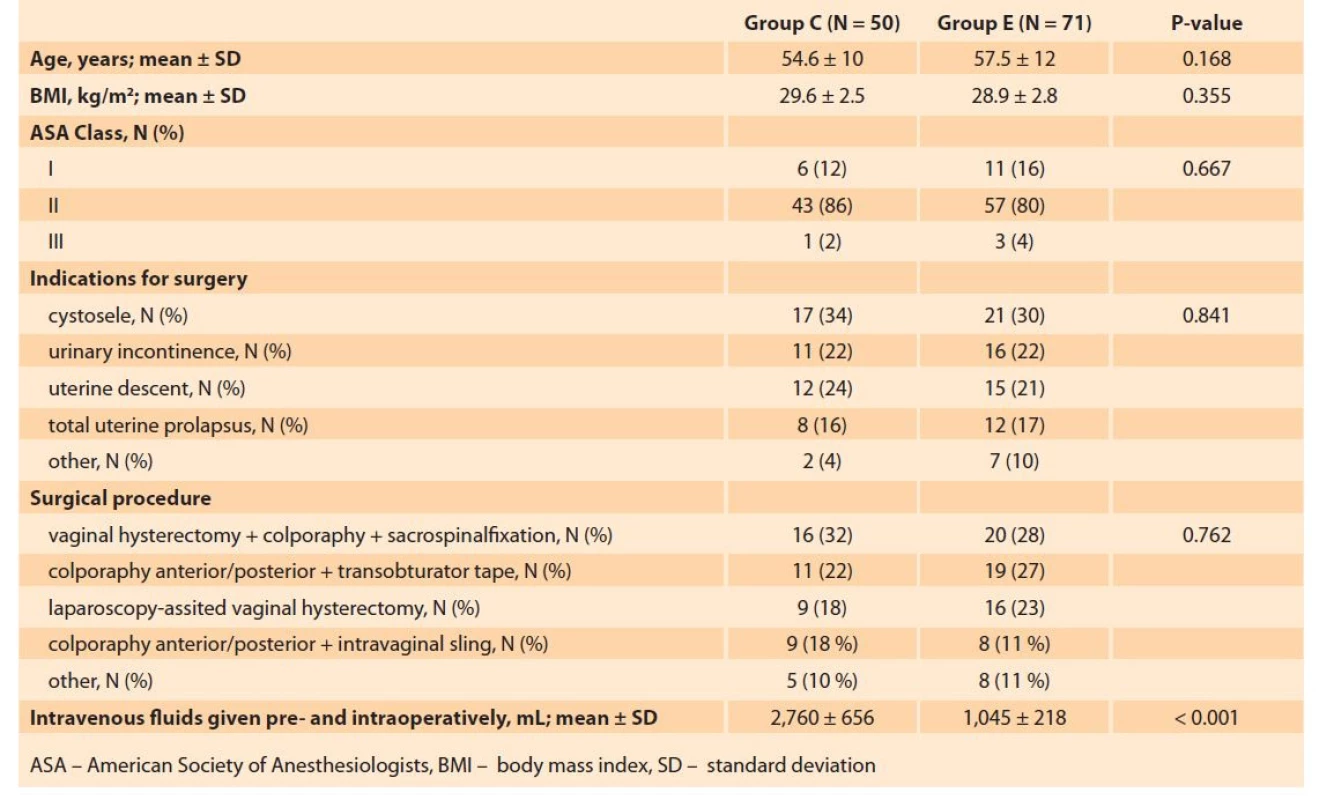
The total volume of fluids infused at the preoperative (1,760 ± 656 vs. 1,045 ± 218 mL, P < 0.001) and postoperative stages (1,840 ± 320 vs. 1,160 ± 230 mL, P < 0.001) was significantly higher in Group C. Time to first flatus (11.5 ± 2.9 vs. 14.4 ± 4.2 hours, P < 0.001), time to first defecation (22.4 ± 6.2 vs. 29.8 ± 6.7 hours, P < 0.001), time to first solid food intake (18.3 ± 4.2 vs. 23.8 ± 6.1 hours, P < 0.001), and time to ambulation (26.1 ± 7.1 vs. 38.4 ± 11.3 hours, P < 0.001) were shorter in the ERAS group (Tab. 3). LOS was also found to be significantly lower in the ERAS protocol as compared to conventional care (2.0 ± 0.6 vs. 2.5 ± 1.1 days, P < 0.001).
3. Comparison of the postoperative measures related to functional recovery.
Tab. 3. Srovnání postoperačních opatření spojených s funkční obnovou.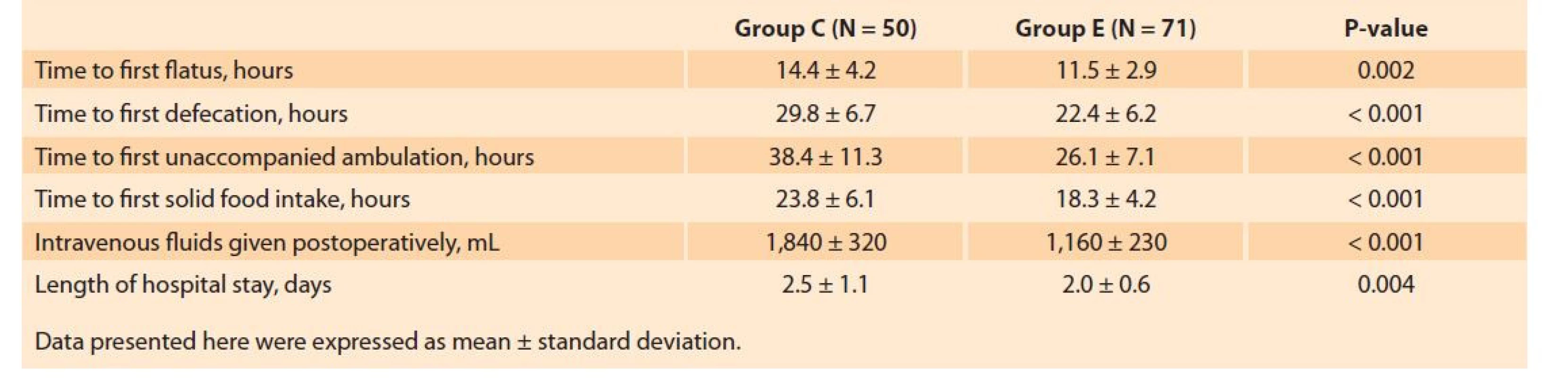
The treatment arms were similar concerning the postoperative complications including surgical site infections (14.8 vs. 12.3%, P = 0.758), cardiovascular complications (4.0 vs. 2.8%, P = 0.754), non-specific abdominal pain (12.1 vs. 9.9%, P = 0.770), and sub-ileus (3 vs. 1.4%, P = 0.569). Blood loss (189 ± 56 vs. 197 ± 63 mL, P = 0.411), overall complication rate (26.0 vs. 16.9%, P = 0.258) and readmission rate (2.0 vs. 4.2%, P = 0.639) were also comparable between the groups (Tab. 4).
4. Comparison of the complication and readmission rates between the study groups.
Tab. 4. Srovnání komplikací a míry opětovné hospitalizace mezi sledovanými skupinami.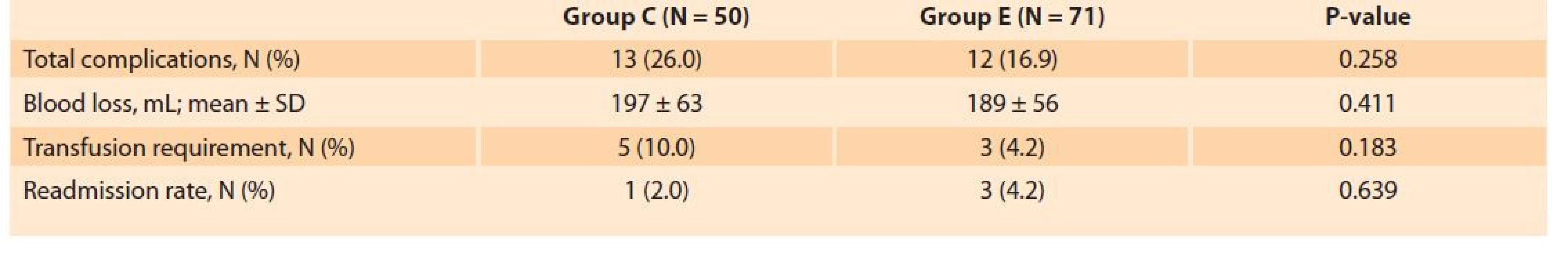
Discussion
The introduction of multimodal treatment programs, including ERAS protocol, has become one of the most promising advancements in the perioperative care of the patients undergoing surgery. Rapid recovery and shortened hospitalization achieved with the implementation of the ERAS protocol not only leads to increased patient satisfaction but also improves certain postoperative outcomes [11]. Beyond the rapid recovery, ERAS protocol also has been shown to reduce complication rates up to 50% [12,13]. The recent guidelines published by the international ERAS Study group standardized the basics of ERAS care for the subjects scheduled for surgery [8,9].
Surgical metabolic stress and the potential negative impact of interventions performed for rapid restoration of homeostasis might be alleviated with ERAS algorithms with the intention of enhanced and expedited recovery. The surgical distress results in a catabolic state and increased insulin resistance, which further provokes a global metabolic functional alteration paving the way for postoperative complications [14]. ERAS care has been shown to improve insulin sensitivity and reduce surgical stress through the abstention of prolonged starvation periods [15].
Maintaining the fluid balance in surgical patients is another cornerstone of ERAS care. The conventional care of surgical patients includes the administration of isotonic saline solutions for the prevention of intra - and postoperative hypotension. However, the results of the recent studies revealed that inelaborate fluid administration might even be deteriorating due to the risk of fluid retention. Hence, goal-directed fluid management strategies are recommended in this population [16]. Restriction of fluid management was shown to reduce the complication rate as compared to the non-selective strategies. Rapid infusion of intravenous solutions was reported to increase perioperative adverse events during colonic resection surgery, particularly if the total amount had exceeded 3,000 mL [13]. Moreover, previous evidence indicates that recovery of the bowel function may be delayed in the subjects receiving non-selective fluid administration [17]. Avoidance of sedative agents prior to surgery and limiting the use of opioids in addition to the utilization of short-acting anesthetics as a part of the ERAS care reduces postoperative nausea and thus allows oral intake within hours following the surgery [18]. ERAS protocol potentiates the benefit of the aforementioned care measure. Thus, the resultant synergistic effect yields to an improved outcome attributed to ERAS protocols.
The efficacy of ERAS care in reducing LOS, complication rate and healthcare costs during gastrointestinal, urologic, and pancreatic surgery has been well-established [19–22]. The changes in outcome variables after implementation of the ERAS protocol have also been studied in gynecologic surgery. Wijk et al reported in their observational study that ERAS care had significantly shortened LOS following abdominal hysterectomy [23]. Similar findings were also mentioned for vaginal surgery of uterovaginal prolapse [24]. ERAS care has also been demonstrated to reduce the amount of intraoperative and total fluid administration in women undergoing surgery for gynecologic cancer [25].
The restoration of bowel function and early ambulation, which are critical for early discharge, had also been significantly improved when ERAS protocols were used. A double-blind, randomized, controlled trial of Hansen et al revealed that ERAS protocol facilitates early defecation after abdominal hysterectomy [26]. Two studies also reported that ERAS care was associated with early ambulation and early discharge in patients scheduled for abdominal hysterectomy and uterovaginal prolapse surgery [24,26]. Moreover, the improvements achieved with ERAS care in gynecologic surgery were acquired without an increased rate of postoperative complications [3].
The evidence regarding the benefits of ERAS care in major gynecologic surgery is widely acclaimed; however, the data demonstrating the beneficial effect of ERAS protocol in urogynecological surgery is limited [27]. Our findings indicating the association of ERAS care with earlier ambulation, oral intake, defection and discharge as compared to the conventional care in this population might be noteworthy in this sense. Nevertheless, complication and readmission rates were similar between the groups in our study. In other words, ERAS care was associated with rapid recovery, but similar postoperative complication rate with conventional care in the subjects scheduled for urogynecological surgery. The findings of this study are consistent with the previous data exhibiting significant improvement in recovery and hospital stay with ERAS care. With this regard, we suggest that the implementation of ERAS protocol in the subjects scheduled for urogynecological surgery may facilitate recovery and probably reduce healthcare costs.
Implementation of ERAS care requires a multimodal and multidisciplinary approach. Therefore, there was a major limitation in this study about unblinded nursing staff and physicians providing health care.
Conclusion
The findings of this study indicated that ERAS care was associated with early recovery represented with oral intake, return of bowel function, mobilization, and discharge without an increase in complication rate in patients undergoing urogynecological surgery. In this manner, we claim that developing an individual ERAS protocol on the institutional basis in accordance with the relevant guidelines may improve the recovery of the patients scheduled for these procedures.
ORCID numbers
G. Yilmaz 0000-0003-2984-156X
E. Can 0000-0001-7851-4026
D. O. Omaygenc 0000-0003-1037-8915
N. Tuten 0000-0001-8609-4770
F. Olmez 0000-0003-4281-1226
H. Kiyak 0000-0001-7580-9179
P. Y. Bahat 0000-0003-2558-1924
A. Akca 0000-0002-8644-7908
Z. Salihoglu 0000-0002-6905-2664
Submitted/Doručeno: 13. 3. 2022
Accepted/Přijato: 24. 5. 2022
Derya Ozden Omaygenc, MD
Department of Anesthesiology
Istanbul Haseki Training and Research Hospital
Ugur Mumcu Belediye Str. No. 7
34265 Sultangazi/Istanbul
Turkey
Sources
1. Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003; 362 (9399): 1921–1928. doi: 10.1016/ S0140-6736 (03) 14966-5.
2. Yilmaz G, Akca A, Aydin N. Enhanced recovery after surgery (ERAS) versus conventional postoperative care in patients undergoing abdominal hysterectomies. Ginekol Pol 2018; 89 (7): 351–356. doi: 10.5603/GP.a2018.0060.
3. Scheib SA, Thomassee M, Kenner JL. Enhanced recovery after surgery in gynecology: a review of the literature. J Minim Invasive Gynecol 2019; 26 (2): 327–343. doi: 10.1016/j.jmig.2018.12.010.
4. Mortensen K, Nilsson M, Slim K et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) society recommendations. Br J Surg 2014; 101 (10): 1209–1229. doi: 10.1002/bjs.9582.
5. Cerantola Y, Valerio M, Persson B et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clin Nutr 2013; 32 (6): 879–887. doi: 10.1016/ j.clnu.2013.09.014.
6. Lassen K, Coolsen MM, Slim K et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clin Nutr 2012; 31 (6): 817–830. doi: 10.1016/j.clnu.2012.08.011.
7. Greco M, Capretti G, Beretta L et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 2014; 38 (6): 1531–1541. doi: 10.1007/s00268-013-2416-8.
8. Nelson G, Altman AD, Nick A et al. Guidelines for pre - and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) society recommendations – part I. Gynecol Oncol 2016; 140 (2): 313–322. doi: 10.1016/j.ygyno.2015.11.015.
9. Nelson G, Altman AD, Nick A et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations – part II. Gynecol Oncol 2016; 140 (2): 323–332. doi: 10.1016/j.ygyno.2015.12.019.
10. Faul F, Erdfelder E, Lang AG et al. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39 (2): 175–191. doi: 10.3758/bf03193146.
11. Zychowicz A, Pisarska M, Laskawska A et al. Patients’ opinions on enhanced recovery after surgery perioperative care principles: a questionnaire study. Wideochir Inne Tech Maloinwazyjne 2019; 14 (1): 27–37. doi: 10.5114/wiitm. 2018.77261.
12. Gatt M, Khan S, MacFie J. In response to: Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr 29 (2010) 434–440. Clin Nutr 2010; 29 (5): 689–690; author reply 691–682. doi: 10.1016/j.clnu.2010.06.005.
13. Gustafsson UO, Hausel J, Thorell A et al. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 2011; 146 (5): 571–577. doi: 10.1001/archsurg.2010.309.
14. Ljungqvist O. Jonathan E. Rhoads lecture 2011: Insulin resistance and enhanced recovery after surgery. JPEN J Parenter Enteral Nutr 2012; 36 (4): 389–398. doi: 10.1177/0148 607112445580.
15. Ljungqvist O. Modulating postoperative insulin resistance by preoperative carbohydrate loading. Best Pract Res Clin Anaesthesiol 2009; 23 (4): 401–409. doi: 10.1016/j.bpa.2009.08.004.
16. Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc 2010; 69 (4): 488–498. doi: 10.1017/S0029665110001734.
17. Brandstrup B, Tønnesen H, Beier-Holgersen R et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003; 238 (5): 641–648. doi: 10.1097/01.sla.0000 094387.50865.23.
18. Ljungqvist O. ERAS – enhanced recovery after surgery: moving evidence-based perioperative care to practice. JPEN J Parenter Enteral Nutr 2014; 38 (5): 559–566. doi: 10.1177/ 0148607114523451.
19. Adamina M, Kehlet H, Tomlinson GA et al. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery 2011; 149 (6): 830–840. doi: 10.1016/j.surg.2010.11.003.
20. Lin T, Li K, Liu H et al. Enhanced recovery after surgery for radical cystectomy with ileal urinary diversion: a multi-institutional, randomized, controlled trial from the Chinese bladder cancer consortium. World J Urol 2018; 36 (1): 41–50. doi: 10.1007/s00345-017-2108-3.
21. Ding J, Sun B, Song P et al. The application of enhanced recovery after surgery (ERAS) /fast-track surgery in gastrectomy for gastric cancer: a systematic review and meta-analysis. Oncotarget 2017; 8 (43): 75699–75711. doi: 10.18632/oncotarget.18581.
22. Perinel J, Adham M. ERAS and pancreatic surgery: a review. Updates Surg 2016; 68 (3): 253–255. doi: 10.1007/s13304-016-04 06-8.
23. Wijk L, Franzen K, Ljungqvist O et al. Implementing a structured Enhanced Recovery After Surgery (ERAS) protocol reduces length of stay after abdominal hysterectomy. Acta Obstet Gynecol Scand 2014; 93 (8): 749–756. doi: 10.1111/aogs.12423.
24. Ottesen M, Sørensen M, Rasmussen Y et al. Fast track vaginal surgery. Acta Obstet Gynecol Scand 2002; 81 (2): 138–146. doi: 10.1034/j.1600-0412.2002.810209.x.
25. Mendivil AA, Busch JR, Richards DC et al. The impact of an enhanced recovery after surgery program on patients treated for gynecologic cancer in the community hospital setting. Int J Gynecol Cancer 2018; 28 (3): 581–585. doi: 10.1097/IGC.0000000000001198.
26. Hansen CT, Sørensen M, Møller C et al. Effect of laxatives on gastrointestinal functional recovery in fast-track hysterectomy: a double-blind, placebo-controlled randomized study. Am J Obstet Gynecol 2007; 196 (4): 311.e1–311.e7. doi: 10.1016/j.ajog.2006.10.902.
27. Başgül A, Hancı A, Atalan G. Our experiences of regional anaesthesia in urologic surgery. Med Bull Sisli Etfal Hosp 2006; 40 (3): 35–38.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inCzech Gynaecology

2022 Issue 4-
All articles in this issue
- Barriers to the cervical cancer screening attendance among Czech women
- Cesarean scar pregnancy – a retrospective analysis of cases in the years 2012–2021
- Enhanced myometrial vascularity
- A patient with primary adenocarcinoma of the appendix metastasizing to the ovary
- SARS-CoV-2 placentitis as a cause of intrauterine death of a fetus in a patient with covid-19 infection and ongoing HELLP syndrome
- Cephalothoracoomphalopagus – a rare type of conjoined twins from the pathologist’s perspective
- Unilateral macrocystic dysplasia and contralateral agenesis in a monoamniotic twin
- Uterus sparing surgery in adenomyosis and its impact on reproductive outcomes
- The role of power morcellation in minimally invasive gynecologic surgery
- Uterine perforation during intrauterine procedures and its management
- Comparison of enhanced recovery protocol with conventional care in patients undergoing urogynecological surgery
- Amniotic fluid embolism – review and multicentric case analysis
- Czech Gynaecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Uterine perforation during intrauterine procedures and its management
- Uterus sparing surgery in adenomyosis and its impact on reproductive outcomes
- Amniotic fluid embolism – review and multicentric case analysis
- Cesarean scar pregnancy – a retrospective analysis of cases in the years 2012–2021
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

