Risk factors for endometrial cancer
Authors:
Aus Tariq Ali
Authors‘ workplace:
Department of Chemical Pathology, Tygerberg Hospital and the University of Stellenbosch, Cape Town, South Africa
Published in:
Ceska Gynekol 2013; 78(5): 448-459
Category:
Original Article
Overview
Endometrial cancer is the most common malignancy of women in developed countries, and its incidence is rising among pre- and postmenopausal women. In developed and numerous developing countries endometrial cancer, as well as other types of female cancers are an ever-increasing threat that may be explained, among other reasons, by increased life expectancy and changes in lifestyle factors. Endometrial cancer is more common in postmenopausal women than in premenopausal women. Through a review of the literature it was found that the risk of endometrial cancer is positively correlated with older age, early menarche & late menopause, obesity, family history of endometrial cancer (especially among close relatives), radiation exposure, and infertility particularly in the presence of Polycystic Ovarian Syndrome. Long-term use of unopposed oestrogens for hormone replacement therapy also increases the risk of endometrial cancer. Caucasians have a higher incidence of endometrial cancer than African or Asian women. Obese women were found to be at high risk for developing endometrial cancer, while diabetes, hypertension, and geographical and socioeconomic factors are still inconclusive. Finally, smoking is considered as a protective factor against endometrial cancer due to its anti- estrogenic effect. .
Keywords:
endometrial cancer – older age – race – early menarche – late menopause – obesity – null parity – tamoxifen
INTRODUCTION
Endometrial cancer is the fourth most common diagnosed cancer of the female genital tract in developed countries [1]. It mostly occurs in postmenopausal women affecting women in their sixth and seventh decades of life [2]. Although endometrial cancer occupies a high rank among the incident of cancers in western world’ women and the incidence rate is ever increasing, the death rate from endometrial cancer is low [3]. This is because it has a favourable prognosis as the majority of patients present at an early stage. Endometrial cancer represents over 95% of uterine cancer [4], thus it is the most common type of uterine cancer (figure 1). Endometrial cancer develops along two distinct pathways with defined molecular alterations and distinct histologic and clinical features [5].

The majority (80–90%) of endometrial cancers have endometrioid differentiation and are classified as Type I endometrial cancer. Type I endometrial cancer is usually detected at an early stage. Compared with women with type I, women with type II endometrial cancer tend to be older at diagnosis (figure 2) and their cancers detected at a more advanced stage [5]. Type I endometrial cancer is estrogen dependent and develop through the hyperplasia-carcinoma sequence, whereas type II cancers are not estrogen dependent and develop independently of the endometrial hyperplasia pathway [5]. The other forms of endometrial cancer which fall under subtypes of type II endometrial cancer include; papillary serous adenocarcinoma, and clear cell adenocarcinoma. Because the last two types are less common than endometriod adenocarcinoma, their risk factors are less well identified. They are often detected at advanced stages, and tend to be more aggressive than endometrioid adenocarcinomas. When endometrial cancer has features of more than one subtype it called mixed adenocarcinoma and it represents less than 10% of all endometrial cancers [5].

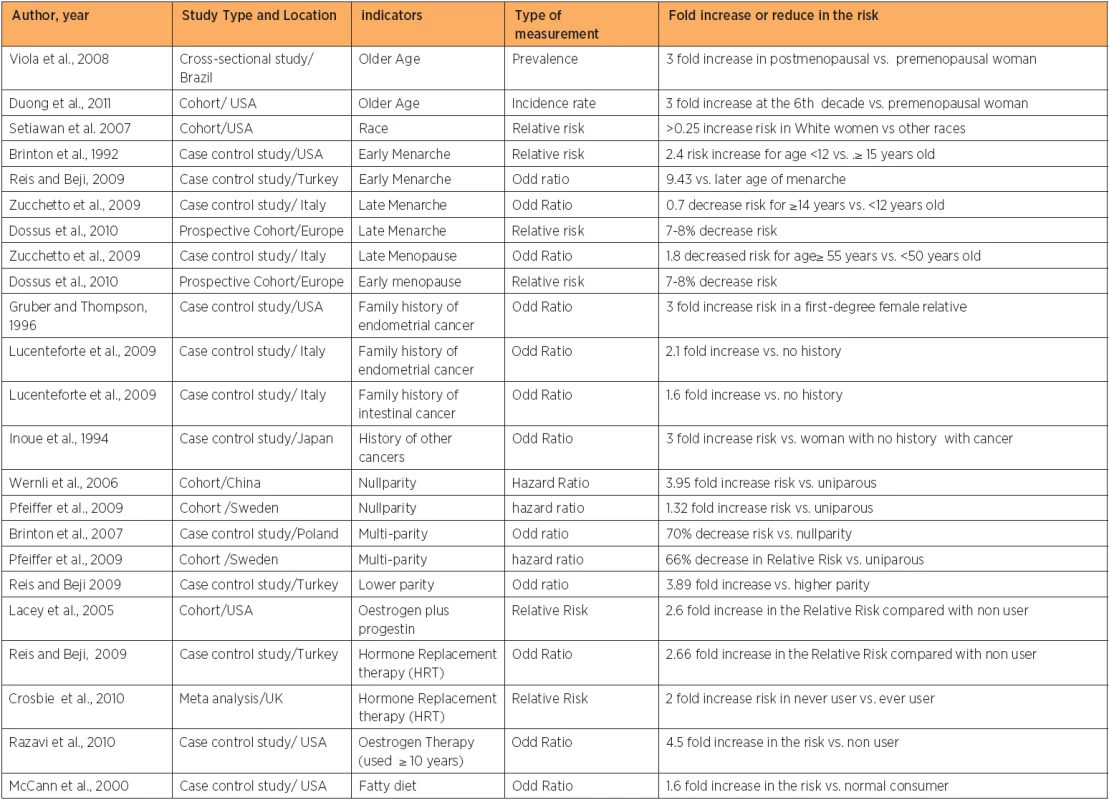
Epidemiological studies have highlighted many risk factors (table 1) associated with an increased endometrial cancer risk. However, the association with these risk factors is based on probabilities, and even woman without any of these risk factors may still get endometrial cancer. The incidence of endometrial cancer is about 10 times higher in developed countries than developing countries [6]. This could be due to an increased life expectancy, increased caloric intake, obesity, adjuvant Tamoxifen use for breast cancer, and a reduction in fertility rates. The risk of the disease has been shown to be increased in Asian and African emigrants to developed countries, possibly due to changes in environmental risk factors [6, 7]. The wide difference in the incidence of endometrial cancer across the world can be also explained by differences in the distribution of known risk factors of the disease. This review will focus on the most important risk factor for endometrial cancer.
OLDER AGE
Endometrial cancer risk is found to be positively correlated with increasing age [5, 8, 9]. As endometrial cancer is more common in post-menopausal women than in premenopausal women, over 90% of the cases are diagnosed after the age of 50 years [10]. Advanced age is also considered as a predictor of poor outcome in patients with endometrial cancer [11]. The incidence peaks can be differed by country, race, and the targeted study population, as different studies showed differences incidence peaks [5, 9, 10, 12, 13, 14]. The incidence of endometrial cancer is higher in developed countries than most developing countries, because of increased life expectancy and changes in lifestyle [5]. On the other hand, the incidence of endometrial cancer is expected to be low in many African countries, mostly due to the decline in life expectancy [15] because of the increased prevalence of HIV, TB, and other diseases.
RACE
Data from Western countries showed differences in the incidence of endometrial cancer and mortality rate among races. White women have a higher risk of developing endometrial cancer than women belonging to other ethnic groups [2]. Endometrial cancer has a higher incidence in White Americans when compared to African Americans, Native Hawaiians, Japanese Americans, and Latinas [16]. However, in comparison with other races the mortality rate in white women is the lowest. Different incidence rates of endometrial cancer among races could be due to differences in life-style, socioeconomic status, and genetic predisposition to developing cancers [17]. Although the incidence of endometrial cancer is 30% less in African American compared with white American, African women usually present with advanced stage tumours resulting in up to 4 times increase in mortality compared with their white counterpart [18]. The high risk of death in black women is attributed to poor access to health care centres, advanced stage at diagnosis, tumour characteristics and delay of treatment [18]. On the contrary, early stage of diagnosis in white women can partially explain their low mortality rate from endometrial cancer compared with the high mortality in black and other race [19]. Early stage of diagnosis could be a reflection of high economic status and high educational level which usually leads to increase awareness and ultimately create the racial gap in life expectancy and survival rate [20].
EARLY MENARCHE & LATE MENOPAUSE
Early age at menarche and late age at menopause are positively associated with some female cancers. Breast-, endometrial- and ovarian cancer were all found to be affected by degree of exposure to oestrogen [21]. Early age at menarche [13, 22, 23] and late age at menopause [24, 25] are associated with increase risk of endometrial cancer and the opposite is true [25, 26]. This is because earlier menarche and the later menopause increased the number of menstrual cycles. Ultimately, the total exposure time to oestrogen will increase [23]. Earlier menarche increases the risk of developing endometrial cancer up to 9 fold compared with woman whose menarche occurred at or above the age of fifteen year [22]. Setiawan and colleagues found a 67 percent elevated risk of endometrial cancer among women whose menopause occurred between the ages of 50 and 54 years, compared with women whose menopause occurred prior to the age of 45 years. The risk increased by 79 percent when menopause occurred after the age of 55 years [16].
FAMILY HISTORY
About 5% of endometrial cancer cases have a family history of the disease among first degree relatives [27]. Family history of endometrial cancer is associated with increased disease risk from two [28] to three fold among premenopausal women [29]. In women less than 50 years old, about 9% of endometrial cancer is due to mutations in mismatch repair genes (MSH1, MSH2, MSH6), which result in Hereditary Non-Polyposis Colorectal Cancer (HNPCC) also known as Lynch II syndrome. These mutations lead to microsatellite instability in 75% of endometrial cancers and 90% of colon cancers [30, 31]. Endometrial cancer is considered to be the second most common cancer in HNPCC and the average age of diagnosis of endometrial cancer has been reported to be between 46 and 62 years old [32]. These mutations raise the lifetime risk of endometrial cancer risk up to 60% by the age of 70 years [33]. A family history of other cancers such as uterine and intestinal cancer is directly associated with an increase in the risk of endometrial cancer [28]. The high incidence of endometrial cancer among young women who have a family history of other cancers, suggests a genetic link [33].
HISTORY OF OTHER FEMALE CANCERS
A woman who has a history of other cancers has a 3 fold increase in the risk of developing endometrial cancer, compared with woman with no history of cancer [34]. Breast-, endometrial- and some ovarian cancers are considered to be oestrogen related cancers, because they share common risk factors such as early age at menarche, late age at menopause, nulliparity and other factors responsible for elevating oestrogen level [35]. Women who had breast or ovarian cancer are at increased risk of developing endometrial cancer [36]. Uterine cancer risk increases 2.7 fold among BRCA1 carriers, which is less than the effect on the risk of breast cancer [37], while no increase in risk is found in BRCA2 mutation carriers [38].
INFERTILITY
The effect of infertility on endometrial cancer is well documented, as it puts the patients at risk of developing endometrial cancer at a younger age [39]. The majority of young patients with endometrial cancer (<40 years) suffers from chronic anovulation [40, 41]. Elevated serum oestrogen levels is one of the main features associated with chronic anovulation, and this creates enough reason to increase the risk of developing endometrial cancer [42]. Thus, women with Polycystic Ovary Syndrome (PCOS) and women with oestrogen-secreting ovarian tumours are more prone to have endometrial cancer especially in their reproductive life [42, 43]. Anovulation or oligoovulation that is associated with PCOS in premenopausal women results in an endometrium that is chronically exposed to unopposed oestradiol. Ultimately this causes proliferation and possibly can lead to neoplastic changes [44]. Almost a third of the cases with endometrial cancer have PCOS [42, 45]. Insulin resistance and PCOS, which are both components of the metabolic syndrome, may play a pivotal role in the pathogenesis of endometrial cancer, likely through disruption of hormonal processes [46]. On the other hand, medication such as clomiphene which is used as an ovulation-stimulating drug in the treatment of infertile woman has recently been reported to be a risk factor for uterine cancer as it increases oestradiol levels [47].
NULLPARITY
Lower parity [22] and/or nullparity [24] were found to increase the risk of developing endometrial cancer up to four fold, while multiparity decreases the risk of endometrial cancer up to 70% [48, 49]. Furthermore, any additional birth among parous women (after the birth of the second child) decreased the risk of developing the disease by 10 % for every new child [48]. This is because parity causes an alteration in the hormonal balance towards increasing progesterone and decreasing oestrogen which suppresses endometrial mitotic activity [48, 50]. The protective effect of parity was found to be stronger in pre-menopausal compared with postmenopausal women [48]. Shedding of the endometrial tissue during delivery could be the reason behind elimination of initiated or precancerous cells [51].
POSTMENOPAUSAL HORMONE THERAPY
Oestrogen Therapy (ET) is the use of oestrogen alone to offset the symptoms of menopause. In the past oestrogen was used alone to treat symptoms of menopause such as hot flushes [52, 53, 54]. Endometrial cancer is associated with a high level of exposure to oestrogen, and the use of oestrogen alone increases the risk of endometrial cancer fivefold as it delays the age of menopause. Since the 1980s, this finding has resulted in a huge reduction in the use of unopposed oestrogen by postmenopausal women who has not undergone a hysterectomy [55]. Thus, also Hormone Replacement Therapy (HRT), also called Oestrogen and Progestin Therapy (EST), which is a diverse combination of oestrogen plus progestin regimens, soon, became available for women with an intact uterus [56]. Results regarding the use of the Oestrogen and Progestin Therapy (EST) and endometrial cancer risk still controversial as some studies have found an increased risk (although it is less than that reported) with the use of ET [6, 22, 57, 58, 59, 60, 61], some found a decreased risk [62], while others found no association [63, 64].
HIGH INTAKE OF FATTY DIET
Endometrial cancer risk is positively correlated with high-fat diet or high energy intake from animal sources [65, 66]. Vegetarian diet, fruits and nutrients such as fibres and vitamins are associated with a reduced risk of endometrial cancer [67, 68], while high energy intake increases the risk up to three fold [69]. This can be explained in two ways. Firstly, a high fat diet can lead to the development of obesity that in turn is considered to be a risk factor for endometrial cancer. Secondly, a fatty diet promotes oestrogen metabolism, which increases the risk of endometrial cancer [70]. Thus, differences among nations in diet contents may be another reason behind differing cancer incidence. African Americans, who usually eat a high-fat diet with less fruits and vegetable, are at high risk compared with white American [71, 72].
OBESITY
Obesity is a well identified risk factor for endometrial cancer (figure 3), both in pre-menopausal and post-menopausal women [73]. The risk of endometrial cancer becomes higher when obesity is associated with infertility or amenorrhoe [74, 75]. This is because obesity increases insulin resistance and oestrogen exposure, which is already high in infertile women and women with anovulation or amenorrhoe [76, 77]. An obese woman has a 2–22 fold increased of the risk of developing endometrial cancer compared to woman with a normal BMI [75, 78, 79, 80]. Endometrial cancer risk increases by 1.2 fold for each 5 kilograms weight gain [78]. Obese premenopausal women have a more than 7 fold increased risk of developing endometrial cancer compared to obese postmenopausal women [80]. Upper body fat is more strongly related with endometrial cancer risk than lower body fat [75, 78]. The positive relationship between obesity and endometrial cancer becomes weaker when obese woman uses Hormone Replacement Therapy (HRT). Compared with current HRT user obese women, who never used Hormone Replacement Therapy, have five fold increases in the risk of developing endometrial cancer [81]. Obesity is associated with a poorer prognosis and increased mortality for both premenopausal and postmenopausal women [82, 83]. Obesity elevates circulating endogenous oestrogens in premenopausal and postmenopausal women, probably due to the conversion of androgen to oestrogen in excess adipose tissue [84]. Thus, and because obesity is also associated with insulin resistance and hyperinsulinemia [85], the positive relationship with endometrial cancer risk becomes even stronger [86].

DIABETES
Previous [23, 87] and recent studies [22, 73, 88, 89] have shown a positive relationship between endometrial cancer risk and diabetes. In comparison with non-diabetic woman, a diabetic woman has a 2 to 3 fold increased risk of developing endometrial cancer [87, 89]. Obesity can exaggerate the effect of diabetes, as most patients with Type II diabetes are obese. Friberg and colleagues found a more than 6 fold increase in the risk of endometrial cancer when diabetes is associated with obesity [89]. The risk rose up to 10 times when obese diabetic women don’t exercise [89]. Women with type1 diabetes have an increased risk of endometrial cancer of up to 3 fold [89]. However, this could be due to effect of confounders, as women with type 1 diabetes have a higher incidence of nulliparity , irregular menstruation and fertility disorders, which are all probable risk factors for endometrial cancer [90].
HYPERTENSION
Hypertensive women have a 3 fold increased risk of developing endometrial cancer compared to healthy women [22, 84]. However, it is unclear whether this positive relationship is due to hypertension or due to the interaction with weight [91] as the relationship between hypertension and endometrial cancer risk usually vanishes after adjusting for body weight [23].
TAMOXIFEN
Tamoxifen is a selective oestrogen receptor modulator often used to treat women with an oestrogen receptor positive breast cancer [92]. Tamoxifen stimulates endometrial proliferation and as the duration of treatment increases the thickness of the endometrium increases [93]. Endometrial cancer risk increases with the duration of tamoxifen use with a relative risks of 2.0 for 2–5 years and 6.9 for at least 5 years when compared to non-users [94]. In addition, the elevated risk of endometrial cancer with long term Tamoxifen use is usually associated with a poor prognosis and poor survival rate [94, 95].
SMOKING
Although smoking is considered a risk factor for endometrial cancer in a limited numbers of studies [13, 77] the majority of studies have considered smoking as a protective factor against endometrial cancer [96, 97, 98, 99, 100]. After analysing 34 studies, Zhou and colleagues have concluded that smoking may decrease the risk of endometrial cancer by up to 30% [101]. Furthermore, the risk of endometrial cancer has declined dramatically with increased numbers of daily cigarettes smoking per day [102] and duration of smoking/years [96, 98]. However, this positive correlation between decreased endometrial risk and smoking was reported only in postmenopausal women [96, 97].
The protective effect of smoking may be attributed to its anti-oestrogenic effect [99, 100]. Smokers have lower endogenous oestrogen levels compared to none-smokers [103, 104]. Smoking also reduced the effect of oestrogen by reducing the age of menopause so that smokers have less menstrual cycles compared non-smokers [105].
RADIOTHERAPY
A percentage of patients with radiotherapy experience face severe complications including late toxicity in normal tissues and significant excess of radiation-induced malignancies [106, 107, 108]. Radiation associated endometrial cancer may develop directly after irradiation the uterus or indirectly after surrounded area like; cervix, rectum or the urinary system [106]. The majority if not all radiation-associated endometrial cancers are second cancers developing in the lining cells of the body and remote from the treatment site [107]. Radiation therapy which used commonly to treat patients from cancers can cause damage to the surrounding tissues’ DNA, which ultimately increases the chance of developing second type of cancer [108].
GEOGRAPHICAL AND SOCIOECONOMIC FACTORS
The incidence of endometrial cancer may fluctuate from country to country or from area to another area in the same country, state, or even within the same city. This could be due to differences in lifestyle and socioeconomic status which usually exist among population, not only in regard with endometrial cancer incidence rate but most kind of cancer [108, 109, 110]. The socioeconomic gradients in cancer risk generally tend to affect women in different ways. It found to be positive (richer women are more affected than their poorer counterpart) for skin melanoma and cancers of the colon, breast, and ovaries, whereas negative association has been observed (i.e., poorer women are more affected than their richer counterpart) for lung, stomach, esophagus, and cervical cancer [111].
Although the relationship between education and endometrial cancer is still controversial, studies found that educated women usually use oestrogen replacement, contraception, and tend have lower parity, compared to un-educated women. This may put educated women under some risk of developing endometrial cancer. On the contrarily, educated women are aware of symptoms of the disease and thus they early diagnosed, get better access to medical care and therefore have less mortality rate, while uneducated women, even though they get the disease, they may died without seeking medical care [112]. From epidemiological point of view, education is found to be inversely associated with the incidence of most cancers, especially those related to avoidable risk factors such as smoking [113, 114, 115, 116].
To conclude, the risk of endometrial cancer increases with increasing age, being Caucasian, early menarche & late menopause, a positive family history of endometrial cancer, a history of other cancers, infertility, long-term use of unopposed oestrogens for hormone replacement therapy, fatty diet, obesity, and long duration of Tamoxifen use. The relationship with other reported risk factors, such as diabetes and hypertension, are still controversial. Smoking was found to be protective against endometrial cancer in most studies. Having one or more of these risk factors does not guarantee the occurrence of the disease but rather increases the risk of developing the disease, and the opposite is also true.
Acknowledgement
I would like to thank Professor Bill Botha, from the Department of Obstetrics and Gynaecology, at Tygerberg Hospital and Stellenbosch Medical School, for his valuable suggestions.
Dr. Aus Tariq Ali, , BSC, MSC-Nutrition, MSC-Medicine, PhD-Chemical Pathology
Department of Chemical Pathology
Tygerberg Hospital 7505
Cape Town, South Africa
e-mail: atali@sun.ac.za
Sources
1. Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2000: Cancer incidence, mortality and prevalence worldwide. IARC Cancer Base No. 5, version 2.0. Lyon: IARC Press, 2004.
2. Buchanan EM, Weinstein LC, Hillson C. Endometrial cancer. Am Fam Physician 2009; 80:1075–1080.
3. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58:71–96.
4. Persson I, Adami HO. Endometrial cancer. In: Adami HO, Hunter DJ, Trichopoulos D, eds. Textbook of Cancer Epidemiology. Oxford, United Kingdom: Oxford University Press; 2002. p. 359–377.
5. Duong LM, Wilson RJ, Ajani UA, Singh SD, Eheman CR. Trends in endometrial cancer incidence rates in the United States, 1999–2006. J Women’s Health 2011; 20,8: 1–7.
6. Lacey JV, Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Endometrial Carcinoma Risks among Menopausal Estrogen plus Progestin and Unopposed Estrogen Users in a Cohort of Postmenopausal Women. Cancer Epidemiol Biomarkers Prev 2005; 14:1724–1731.
7. Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents. vol VII. Lyon: IARC, Scientific Publication No. 155, 2002.
8. Viola AS, Gouveia D, Andrade L, Aldrighi JM, Viola CF, Bahamondes L. Prevalence of endometrial cancer and hyperplasia in non-symptomatic overweight and obese women. Aust Nz J Obstet Gynaecol 2008; 48:207–213.
9. Evans T, Sany O, Pearmain P, Ganesan R, Blann A, Sundar S. Differential trends in the rising incidence of endometrial cancer by type: data from a UK population-based registry from 1994 to 2006. Br J Cancer 2011; 104:1505–1510.
10. Sorosky JI. Endometrial cancer. Obstet Gynecol 2008; 111:436–447.
11. Alektiar KM, Venkatraman E, Abu-Rustum N, Barakat RR. Is endometrial carcinoma intrinsically more aggressive in elderly patiants? Cancer 2003; 98:2368–2377.
12. Manchana T, Khemapech N. Endometrial adenocarcinoma in young Thai women. Asian Pac J Cnacer Prev 2008; 9:283–286.
13. Fujita M, Tase T, Kakugawa Y, et al. Smoking, earlier menarche and low parity as independent risk factors for gynecologic cancers in Japanese : a case-control study. Tohoku J Exp Med 2008; 216:297–307.
14. Soliman PT, Oh JC, Schmeler KM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol 2005; 105:575–580.
15. Who site; http://www.who.int/countries/en/ last accessed 19/12/2012.
16. Setiawan VW, Pike MC, Kolonel LN, Nomura AM, Goodman MT, Henderson BE. Racial/Ethnic differences in endometrial cancer risk: the multiethnic cohort study. Am J Epidemiol 2007; 165:262–270.
17. Allard JE, Maxwell GL. Race disparities between black and white women in the incidence, treatment, and prognosis of endometrial cancer. Cancer Control 2009; 16:53–56.
18. Farley J, Risinger JI, Rose GS, Maxwell GL. Racial disparities in blacks with gynecologic cancers. Cancer 2007; 110:234–243.
19. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin 2006; 56:106–130.
20. Linkov F, Taioli E. Factors influencing endometrial cancer mortality: the Western Pennsylvania Registry. Future Oncol 2008; 4:857–865.
21. Rice LW. Hormone prevention strategies for breast, endometrial and ovarian cancers. Gynecol Oncol 2010; 118:202–207.
22. Reis N, Beji NK. Risk factors for endometrial cancer in Turkish women: results from a hospital-based case control study. Eur J Oncol Nurs 2009; 13:122–127.
23. Brinton LA, Berman ML, Mortel R, et al. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstet Gynecol 1992; 167:1317–1325.
24. Wernli KJ, Ray RM, Gao DL, De Roos AJ, Checkoway H, Thomas DB. Menstrual and reproductive factors in relation to risk of endometrial cancer in Chinese women. Cancer Causes Control 2006; 17:949–955.
25. Zucchetto A, Serraino D, Polesel J, et al. Hormone-related factors and gynecological conditions in relation to endometrial cancer risk. Eur J Cancer Prev 2009; 18:316–321.
26. Dossus L, Allen N, Kaaks R, et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2010; 127:442–451.
27. Parazzini F, La Vecchia C, Moroni S. Family history and the risk of endometrial cancer. Int J of Cancer 1994; 59:460–462.
28. Lucenteforte E, Talamini R, Montella M, et al. Family history of cancer and the risk of endometrial cancer. Eur J Cancer Prev 2009; 18:95–99.
29. Gruber SB, Thompson WD. A population-based study of endometrial cancer and familial risk in younger women. Cancer and Steroid Hormone Study Group. Cancer Epidemiol Biomarkers Prev 1996; 5: 411–417.
30. Lu KH, Schorge JO, Rodabaugh KJ, et al. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol 2007; 25:5158–5164.
31. Lu KH, Dinh M, Kohlmann W, et al. Gynecologic cancer as a „sentinel cancer“ for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol 2005; 105:569–574.
32. Hampel H, Stephens JA, Pukkala E, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology 2005; 129:415–421.
33. Lalloo F and Evans G. Molecular genetics and endometrial cancer. Best Pract Res Clin Obstet Gynaecol 2001; 15: 355–363.
34. Inoue M, Okayama A, Fujita M, Enomoto T, Tanizawa O, Ueshima H. A case-control study on risk factors for uterine endometrial cancer in Japan. Jpn J Cancer Res 1994; 85:346–350.
35. McGrath M, Lee IM, Buring J, Hunter DJ, De Vivo I. Novel breast cancer risk alleles and endometrial cancer risk. Int J Cancer 2008; 123:2961–2964.
36. Beiner ME, Finch A, Rosen B, et al. The risk of endometrial cancer in women with BRCA1 and BRCA2 mutations, a prospective study. Gynecol Oncol 2007; 104:7–10.
37. Thompson D, Easton DF, Breast Cancer Linkage Consortium. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst 2002; 94:1358–1365.
38. The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 1999; 91:1310–1316.
39. Brinton LA, Westhoff CL, Scoccia B, et al. Causes of infertility as predictors of subsequent cancer risk. Epidemiology 2005; 16:500–507.
40. Uharcek P, Mlyncek M, Ravinger J, Matejka M. Prognostic factors in women 45 years of age or younger with endometrial cancer. Int J Gynecol Cancer 2008; 18:324–328.
41. Evans-Metcalf ER, Brooks SE, Reale FR, Baker SP. Profile of women 45 years of age and younger with endometrial cancer. Obstet Gynecol 1998; 91:349–354.
42. Elliott JL, Hosford SL, Demopoulos RI, Perloe M, Sills ES. Endometrial adenocarcinoma and polycystic ovary syndrome: risk factors, management, and prognosis. South Med J 2001; 94:529–531.
43. Pillay OC, Te Fong LF, Crow JC, et al. The association between polycystic ovaries and endometrial cancer. Hum Reprod 2006; 21:924–929.
44. Kulie T, Slattengren A, Redmer J, Counts H, Eglash A, Schrager S. Obesity and Women‘s Health: An Evidence-Based Review. J Am Board Fam Med 2011; 24:75–85.
45. Navaratnarajah R, Pillay OC, Hardiman P. Polycystic ovary syndrome and endometrial cancer. Semin Reprod Med 2008; 26:62–71.
46. Hardiman P, Pillay OC, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet 2003; 361:1810–1812.
47. Althuis MD, Moghissi KS, Westhoff CL, et al. Uterine cancer after use of Clomiphene Citrate to induce ovulation. Am J Epidemiol 2005; 161:607–615.
48. Pfeiffer RM, Mitani A, Landgren O, et al. Timing of births and endometrial cancer risk in Swedish women. Cancer Causes Control 2009; 20:1441–1449.
49. Brinton LA, Sakoda LC, Lissowska J, et al. Reproductive risk factors for endometrial cancer among Polish women. Br J Cancer 2007; 96:1450–1456.
50. Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev 2002; 11:1531–1543.
51. Albrektsen G, Heuch I, Tretli S, Kvåle G. Is the risk of cancer of the corpus-uteri reduced by a recent pregnancy – a prospective study of 765,756 Norwegian women. Int J Cancer 1995; 61:485–490.
52. Jick H, Watkins NW, Hunter JR, et al. Replacement estrogens and endometrial cancer. N Eng J Med 1979; 300:218–222.
53. Henderson, B. E., Ross, R. K., Bernstein, L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res1988; 48:246–253.
54. Weiderpass E, Adami HO, Baron JA, et al. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst 1999; 91:1131–1137.
55. Grady D, Gebretsadik T, Kerlikowske K, Ernster V,Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol 1995; 85:304–313.
56. Whitehead MI, Fraser D. The effects of estrogens and progestogens on the endometrium. Modern approach to treatment. Obstet Gynecol Clin North Am 1987; 14:299–320.
57. Pike MC, Peters RK, Cozen W, et al. Estrogen-progestin replacement therapy and endometrial cancer. J Natl Cancer Inst 1997; 89:1110–1116.
58. Doherty JA, Cushing-Haugen KL, Saltzman BS, et al. Long-term use of postmenopausal estrogen and progestin hormone therapies and the risk of endometrial cancer. Am J Obstet Gynecol 2007; 197:139.e1–7.
59. Newcomb PA, Trentham-Dietz A. Patterns of postmenopausal progestin use with estrogen in relation to endometrial cancer (United States). Cancer Causes Control 2003; 14:195–201.
60. Jain MG, Rohan TE, Howe GR. Hormone replacement therapy and endometrial cancer in Ontario, Canada. J Clin Epidemiol 2000; 53:385–391.
61. Razavi P, Pike MC, Horn-Ross PL, Templeman C,Bernstein L,Ursin G. Long-term postmenopausal hormone therapy and endometrial cancer. Cancer Epidemiol Biomarkers Prev 2010; 19: 75–483.
62. Crosbie EJ, Zwahlen M, Kitchener HC, Egger M,Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2010; 19:3119–3130.
63. Beral V, Bull D, Reeves G. Million Women Study Collaborators. Endometrial cancer and hormone replacement therapy in the Million Women Study. Lancet 2005; 365:1543–1551.
64. Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer 1999; 80:827–841.
65. Terry P, Vainio H, Wolk A, Weiderpass E. Dietary factors in relation to endometrial cancer: a nationwide case–control study in Sweden. Nutr Cancer 2002; 42:25–32.
66. McCann SE, Freudenheim JL, Marshall JR, Brasure JR, Swanson MK, Graham S. Diet in the epidemiology of endometrial cancer in western New York (United States). Cancer Causes Control 2000; 11:965–974.
67. Littman AJ, Beresford SA, White E. The association of dietary fat and plant foods with endometrial cancer (United States). Cancer Causes Control 2001; 12:691–702.
68. Jain MG, Rohan TE, Howe GR, Miller AB. A cohort study of nutritional factors and endometrial cancer. Eur J Epidemiol 2000; 16:899–905.
69. Furberg AS, Thune I. Metabolic abnormalities (hypertension, hyperglycemia and overweight), lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. Int J Cancer 2003; 104:669–676.
70. Nagata C, Takatsuka N, Kawakami N, Shimizu H. Total and monounsaturated fat intake and serum estrogen concentrations in premenopausal Japanese women. Nutr Cancer 2000; 38:37–39.
71. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006; 295:1549–1555.
72. Stoll BA. Western diet, early puberty, and breast cancer risk. Breast Cancer Res Treat 1998; 49:87–93.
73. Lindemann K, Vatten LJ, Ellstrøm-Engh M, Eskild A. Body mass, diabetes and smoking, and endometrial cancer risk: a follow-up study. Br J Cancer 2008; 98:1582–1585.
74. Iemura A, Douchi T, Yamamoto S, Yoshimitsu N,Nagata Y. Body fat distribution as a risk factor of endometrial cancer. J Obstet Gynaecol Res 2000; 26:421–425.
75. Xu WH, Matthews CE, Xiang YB, et al. Effect of adiposity and fat distribution on endometrial cancer risk in Shanghai women. Am J Epidemiol 2005; 161:939–947.
76. Schmeler KM, Soliman PT, Sun CC, Slomovitz BM, Gershenson DM, Lu KH. Endometrial cancer in young, normal-weight women. Gynecol Oncol 2005; 99:388–992.
77. Haidopoulos D, Simou M, Akrivos N, et al. Risk factors in women 40 years of age and younger with endometrial carcinoma. Acta Obstet Gynecol Scand 2010; 89:1326–1330.
78. Trentham-Dietz A, Nichols HB, Hampton JM, Newcomb PA. Weight change and risk of endometrial cancer. Int J Epidemiol 2006; 35:151–158.
79. Charneco E, Ortiz AP, Venegas-Ríos HL, Romaguera J, Umpierre S. Clinic-based case-control study of the association between body mass index and endometrial cancer in Puerto Rican women. P R Health Sci J 2010; 29:272–278.
80. Thomas CC, Wingo PA, Dolan MS, Lee NC, Richardson LC. Endometrial cancer risk among younger, overweight women. Obstet Gynecol 2009; 114:22–27.
81. Chang SC, Lacey JV, Brinton LA, et al. Cancer Epidemiol Biomarkers Prev 2007; 16:723–730.
82. Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007; 335:1134–1145.
83. Chia VM, Newcomb PA, Trentham-Dietz A, Hampton JM. Obesity, diabetes, and other factors in relation to survival after endometrial cancer diagnosis. Int J Gynecol Cancer 2007; 17:441–446.
84. Key T, Appleby P, Barnes I, Reeves G. Endogenous hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 2002; 94:606–616.
85. Ali AT, Ferris WF, Naran NH, Crowther NJ. Insulin resistance in the control of body fat distribution: a new hypothesis. Horm Metab Res 2011; 43:77–80.
86. Soliman PT, Wu D, Tortolero-Luna G, et al. Association between adiponectin , insulin resistance, and endometrial cancer. Cancer 2006; 106:2376–2381.
87. La Vecchia C, Negri E, Franceschi S, D’Avanzo B, Boyle P. A case-control study of diabetes mellitus and cancer risk. Br J Cancer 1994; 70:950–953.
88. Saltzman BS, Doherty JA, Hill DA, et al. Diabetes and endometrial cancer: an evaluation of the modifying effects of other known risk factors. Am J Epidemiol 2008; 167:607–614.
89. Friberg E, Mantzoros CS, Wolk A. Diabetes and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev 2007; 16:276–280.
90. Zendehdel K, Nyrén O, Ostenson CG, Adami HO, Ekbom A, Ye W. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst 2003; 95:1797–1800.
91. Serdar Serin I, Ozcelik B, Basbug M, Ozsahin O, Yilmazsoy A, Erez R. Effects of hypertension and obesity on endometrial thickness. Eur J Obstet Gynecol Reprod Biol 2003; 109:72–75.
92. Jahanzeb M. Reducing the risk for breast cancer recurrence after completion of tamoxifen treatment in postmenopausal women. Clin Ther 2007; 29:1535–1547.
93. Love CD, Muir BB, Scrimgeour JB, Leonard RC, Dillon P, Dixon JM. Investigation of endometrial abnormalities in asymptomatic women treated with tamoxifen and an evaluation of the role of endometrial screening. J Clin Oncol 1999; 17:2050–2054.
94. Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, Van Leeuwen FE. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Lancet 2000; 356:881–887.
95. Mignotte H, Lasset C, Bonadona V, et al. Latrogenic risks of endometrial carcinoma after treatment for breast cancer in a large French case-control study. Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC). Int J Cancer 1998; 76:325–330.
96. Stockwell HG, Lyman GH. Cigarette smoking and the risk of female reproductive cancer. Am J Obstet Gynecol 1987; 157:35–40.
97. Parazzini F, La Vecchia C, Bocciolone L, Franceschi S. The epidemiology of endometrial cancer. Gynecol Oncol 1991; 41:1–16.
98. Parazzini F, La Vecchia C, Negri E, Moroni S, Chatenoud L. Smoking and risk of endometrial cancer: results from an Italian case-control study. Gynecol Oncol 1995; 56:195–199.
99. Terry PD, Rohan TE, Franceschi S, Weiderpass E. Cigarette smoking and the risk of endometrial cancer. Lancet Oncol 2002; 3:470–480.
100. Viswanathan AN, Feskanich D, De Vivo I, et al. Smoking and the risk of endometrial cancer: results from Nurses’ Health Study. Int J Cancer 2005; 114:996–1001.
101. Zhou B, Yang L, Sun Q, et al. Cigarette smoking and the risk of endometrial cancer: a meta-analysis. Am J Med 2008; 121:501–508.
102. Polesel J, Serraino D, Zucchetto A, et al. Cigarette smoking and endometrial cancer risk: the modifying effect of obesity. Eur J Cancer Prev 2009; 18:476–481.
103. Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis 2000; 21:427–433.
104. Brenner RM, Slayden OD, Nayak NR, Baird DT,Critchley HO. A role for the androgen receptor in the endometrial antiproliferative effects of progesterone antagonists. Steroids 2003; 68:1033–1039.
105. Baron JA, La Vecchia C, Levi F. The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol 1990; 162:502–514.
106. Pothuri B, Ramondetta L, Martino M, Alektiar K, Eifel PJ, Deavers MT. Development of endometrial cancer after radiation treatment for cervical carcinoma. Obstet Gynecol 2003; 101:941–945.
107. Pothuri B, Ramondetta L, Eifel P, Deavers MT, Wilton A, Alektiar K. Radiation-associated endometrial cancers are prognostically unfavorable tumors: a clinicopathologic comparison with 527 sporadic endometrial cancers. Gynecol Oncol 2006; 103:948–951.
108. Kumar S, Shah JP, Bryant CS, Seward S, Ali-Fehmi R, Morris RT, Malone JM Jr. Radiation associated-endometrial cancer. Obstet Gynecol 2009; 113:319–325.
109. De Ruyck K, Van Eijkeren M, Claes K, et al. Radiation-induced damage to normal tissues after radiotherapy in patients treated for gynecologic tumors: Association with single nucleotide polymorphisms in genes and chromosomal radio-sensitivity in lymphocytes. Int J Radiat Oncol Biol Phys 2005; 15:1140–1149.
110. Lacey JV Jr., Brinton LA, Lubin JH, Sherman ME,Schatzkin A, Schairer C. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev 2005; 14:1724– 1731.
111. Jemal A, Clegg LX, Ward E, et.al. Annual report to the nation on the status of cancer, 1975–2000, with special feature regarding survival. Cancer 2004; 101:3–27.
112. Hicks ML, Phillips JL, Parham G, et al. The national cancer data base report on endometrial carcinoma in African-American women. Cancer 1998; 83:2629–2637.
113. Kogevinas M, Pearce N, Susser M, Boffetta P. Social inequalities and cancer. IARC Sci. Publ. No. 138. Lyon: IARC; 1997.
114. Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst 2007; 99:1384–1394.
115. Hemminki K, Li X. Level of education and the risk of cancer in Sweden. Cancer Epidemiol Biomarkers Prev 2003; 12:796–802.
116. Mouw T, Koster A, Wright ME, Blank MM, Moore SC, Hollenbeck A, Schatzkin A. Education and risk of cancer in a large cohort of men and women in the United States. PLoS ONE 2008; 3: e3639–e3651.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2013 Issue 5
Most read in this issue
- Incarcerated uterus in pregnancy – pitfalls of diagnosis, clinical course and therapy: two case reports
- Risk factors for endometrial cancer
-
Pregnancy and delivery in a patient with pure 46,XY karyotype
Summary of actual knowledge about XY women - Doctor’s specialty training in obstetrics and gynecology at university medical schools: 2012–2013


