-
Medical journals
- Career
Implementation and applications of robotic surgery within gynecologic oncology and gynecology; analysis of the first thousand cases
Authors: C. Lönnerfors; J. Persson
Authors‘ workplace: Lund and Lund University, Sweden, Head of Department Andreas Herbst, MD, PhD, Associate Professor ; Department of Obstetrics and Gynecology, Skåne University Hospital
Published in: Ceska Gynekol 2013; 78(1): 12-19
Overview
Objective:
To describe the effects of implementing a robotic surgical program within gynecologic oncology and gynecology at a tertiary referral hospital.Design:
Prospective study.Setting:
University Hospital, Lund and Lund University, Sweden.Methods:
Based on prospective data retrieved for all patients, we evaluated intraoperative times, estimated blood loss, rate of conversion and rate of intraoperative complications in the first 1000 robotic surgeries to describe the effects of implementing the technology and the applications of robotic surgery over time.Results:
Between the years 2005–2011, 606 women were operated on for a malignant disease and 394 for a benign disorder. The median age was 49 years (14–89 years) and the median BMI 25,2 kg/m2 (14.7–61.7 kg/m2). The median blood loss was 100 ml (range 0-1300 ml). Nineteen (1.9%) intraoperative complications occurred. Conversion to laparotomy was necessary in 37 patients (3.7%) primarily due to excessive adhesions (n = 15) or unexpected disseminated cancer (n = 11). The proportion of minimally invasive surgery for cervical, endometrial, and early ovarian cancer increased from 26% in 2005 to 81% in 2011. A wide variety of procedures including rare and very complex surgery, otherwise operated by laparotomy, were gradually introduced. Overall, the total OR time per procedure decreased by 159 minutes between the first and last two-year periods, mainly due to a reduction in surgical time by 132 minutes, mainly seen in oncology cases.Conclusion:
Robotic surgery enables minimally invasive surgery for a larger proportion of patients with malignant and select benign gynecological disorders with low rates of conversions and intraoperative complications. The effort and time needed to build a successful robotic team should not be underestimated. An adequate surgical volume with a large proportion of complex cases is necessary to motivate a robotic program.Keywords:
robotic surgery – surgical applicationsINTRODUCTION
Laparoscopic surgery is advantageous compared to open surgery in terms of reduced blood loss, complications and length of hospital stay. However, laparoscopy is technically challenging which has somewhat hindered its adaptation for complex procedures within gynecology and gynecologic oncology [1, 2]. The Lap-2 trial showed that laparoscopy in comparison to laparotomy had an improved safety profile with fewer serious complications, shorter hospital stay, and significant improvement in quality of life but a high overall conversion rate of 25.8% [3]. Traditional laparoscopy has been underutilized whereas the introduction and development of robotic surgery with its associated advantages of wristed instruments allowing better surgical precision and dexterity, movement downgrading, tremor elimination, a stable three-dimensional view, and improved surgeon ergonomics has lead to an increased number of patients being offered a minimal invasive approach.
Robot-assisted laparoscopic surgery using the da Vinci surgical system (da Vinci Surgical system, Intuitive Surgical Inc, CA, USA) for gynecological procedures received United States Food and Drug Administration (FDA) approval in April 2005. Initial reports found the technique to be feasible for a variety of procedures within gynecology and gynecologic oncology [4, 5, 6].
Given the limitations of traditional laparoscopy in performing first and foremost gynecologic oncology procedures and the potential improvement in patient outcomes a standard da Vinci surgical robot was introduced at Skåne University Hospital, Lund, Sweden in October 2005. Initially, the surgical system was shared with the departments of Urology, Pediatric Surgery and General Surgery limiting access to first one, and after the installation of an additional da Vinci-S system in 2007, to two days a week. From June 2010 a da Vinci Si system was introduced for the sole use within gynecologic oncology and gynecology. The annual caseload of patients suitable for robotic surgery was estimated to be 300, with two-thirds for gynecologic oncology. The estimate was based on incidence of cervical, endometrial and early ovarian cancers and a recruitment area of 1.7 million inhabitants for gynecological cancers and rare/complex benign cases and 400 000 for routine gynecological surgery.
Prior to the implementation of the robotic surgery program, traditional laparoscopic surgery was regularly used for hysterectomies and select pelvic and/or paraaortic lymphadenectomies whereas only a few radical hysterectomies had been performed laparoscopically. However, mainly due to a limited number of skilled laparoscopic surgeons in combination with time-consuming procedures, a majority of gynecological cancer surgery at our department was performed by laparotomy. In 2005, 79% of patients with cervical cancer and 68% of patients with endometrial cancer underwent laparotomy whereas only 10.5% and 24% respectively were operated by traditional laparoscopy. The robotic program was instigated in order to expand the patient population being offered a minimally invasive approach.
A prospective protocol for all robotic procedures performed at our institution was used from the beginning in order to evaluate this mode of surgery. The aim of this report is to outline our experience following implementation of a robotic program.
MATERIALS AND METHODS
We retrieved perioperative clinical and surgical data from the first 1000 consecutive women planned for robot assisted surgery between October 2005 to December 2011 to analyse time of surgery, sub-times during surgery, hospital stay, intraoperative complications and conversions to open surgery over time. An intention to treat approach was used for the analysis. We also retrieved data on type of surgical procedure for an overview of applications.
In general, all patients previously scheduled for a laparotomy were considered for robot-assisted surgery and in the case of traditional laparoscopic procedures, when there was a potential for quicker and higher quality surgery. Initially, this applied mainly to routine procedures in patients with cancer of the cervix, uterus or early ovarian cancer. Simple hysterectomies for various benign conditions were performed robotically to gain experience, as a step-in procedure for new surgeons and in order to fully utilize the capacity of the surgical robot. In addition, benign surgery was performed primarily for select myomectomies, women with endometriosis or an unclear adnexal mass in order to proceed with full staging if applicable. Women with significant comorbidities, known advanced disease, multiple prior abdominal surgeries or large tumor sizes not allowing for gentle retrieval were initially not offered a robotic approach. However, with increasing experience morbidly obese women, women with known or expected intraabdominal adhesions, multiple prior abdominal surgery or advanced endometriosis were primarily considered for robotic surgery. The indications were further expanded to include rare, more advanced procedures such as robotic radical fertility sparing trachelectomy, surgery for pelvic recurrences including pelvic exenterations, and removal of bulky pelvic and paraaortic nodes prior to radiotherapy [7, 8]. Various surgical procedures during pregnancy and in women with uterine malformations and for obstetric indications such as Cesarean scar pregnancy and scar dehiscence and, abdominal cervical cerclage were also performed [9–11]. All women gave their informed consent.
The robotic surgical technique used for different procedures has been previously described [7–17] and was modified depending on the procedure performed. In general, a central docking between the patient’s legs was used and for most cases all four robot arms were utilized, the fourth arm usually placed in the right iliac fossa. Initially, two assistant trocars were used; one in the right upper quadrant and one in the left lower quadrant. With increasing experience the lower assistant trocar was abandoned. Various fornix presenters, but not a uterine manipulator, were used for patients with cancer necessitating a hysterectomy.
An initial team of three gynecological surgeons, and two operating room nurses underwent a training programme including a “dry-lab” and a porcine lab training and, after about 30 procedures performed, visited one of few existing centres of excellence worldwide at that time (Mayo Clinic, Scottsdale Arizona). Three additional surgeons (introduced in 2006, 2009 and 2011 respectively) and additional nurses have thereafter gradually been introduced after local training programs. At the moment, the team consists of five consol surgeons and six operating room nurses. The anesthesiological personnel are not consistent.
An Institutional Review Board approval was obtained. A prospective protocol was used for retrieval of clinical and surgical data on all robotic procedures and continuously transcribed into a prospective STATVIEW database (SAS Institute Inc., Cary, NC, USA) where further data such as short-term and long-term follow-up data were continuously transferred.
For analysis of difference over time we subdivided the material into two-year periods (from onset to December 2007, from 2008 to December 2009 and from 2010 to December 2011).
For statistical analysis the Chi square, Mann--Whitneys, Kruskall-Wallis or Fishers exact test was used as applicable. A Bonferroni correction was applied in three tailed comparisons. A value of p<0.05 was considered significant.
RESULTS
Of the 1000 women included, 165 were operated prior to December 2007, 329 between 2008 and December 2009 and 506 from 2010 until December 2011.
The three most experienced surgeons performed 359, 222 and 173 procedures respectively, whereas during the last period 36% of the procedures were performed by surgeons introduced at a later date. For all patients the median age was 49 years (14–89 years) and the median BMI 25,2 kg/m2 (14.7–62.4 kg/m2). 361 (36.1%) women had at least one previous laparotomy and in 199 cases a traditional laparoscopic adhesiolysis was necessary before finalizing port placement and docking of the robot. In an additional 41 patients (4.1%) an adhesiolysis was performed after the robot was docked adding a median of 15 minutes (range 1–106 minutes) to surgical time.
Sixty-one percent of the patients operated had a malignant disorder and the most common indications for surgery were endometrial (n=294) and cervical (n=220) cancer (table 1), which during the last time period constituted 85% of women with cervical cancer and 76% of women with endometrial cancer undergoing surgery at our department. The different surgical procedures performed are shown in table 2. In all, 776 patients had a colpotomy performed and 335 patients underwent a pelvic and/or paraaortic lymph node dissection as part of a staging procedure.
1. Summary of diagnoses on 1000 consecutive patients planned for robot assisted laparoscopic surgery for gynecological benign or malign disease at a tertiary referral teaching hospital 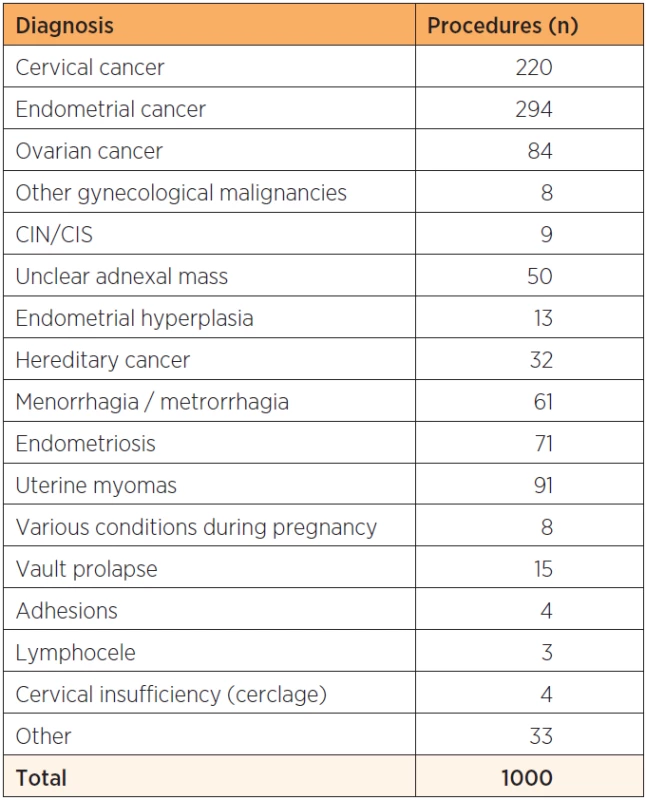
2. Summary of types of robot assisted laparoscopic procedures performed for cervical, endometrial and ovarian cancer at a tertiary referral teaching hospital 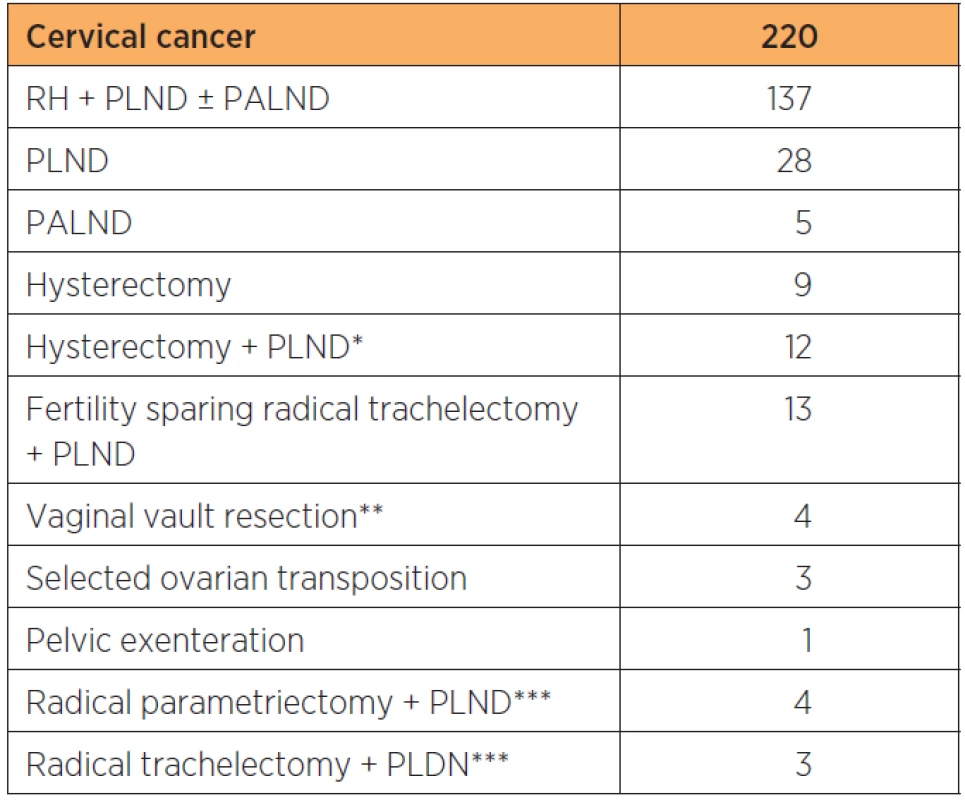

RH: radical hysterectomy, BSOE: bilateral salpingoophorectomy, PLND: pelvic lymphadenectomy, PALND: paraaortic lymphadenectomy, H: hysterectomy, *including bulky node resection, ** for Isolated cuff recurrencies, *** following previous non-radical surgery for unknown cancer Overall, total time for OR use per procedure decreased by 159 minutes between the first and last two-year periods. This was mainly due to a decrease in time for the surgery itself which was in average shortened by 132 minutes and, to a smaller extent due to shortening of preparation time, induction and reversal of anesthesia which in total decreased by 27 minutes (table 3).
3. OR times, use of robotic instruments and post operative hospital stay associated with robotic surgery within gynecology and gynecologic oncology at a tertiary referral teaching hospital in 1000 consecutive patients 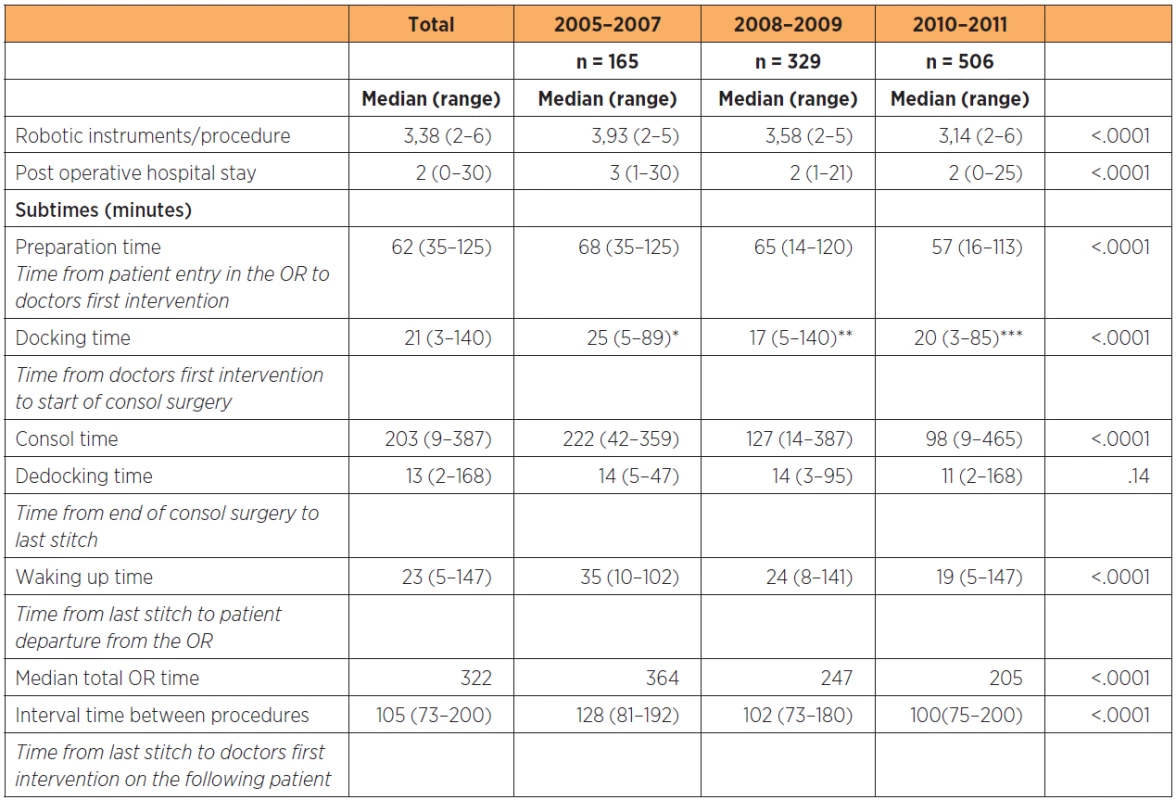
Data subdivided by two-year periods from onset of the robotic program. *including adhesiolysis in 28 patients, **including adhesiolysis in 76 patients, ***including adhesiolysis in 96 patients The average time from the last stitch on the first patient to the first intervention by the surgeon on the following procedure was 105 minutes and decreased with 28 minutes between the first and last year groups (p<0.0001). The average number of robot instruments used per procedure decreased from 3.93 to 3.14 (p<0.0001).
Operating times for well-defined procedures decreased with time and a significant reduction was seen for benign hysterectomies and radical hysterectomy including pelvic lymphadenectomy for cervical cancer (table 4). Even a significant decrease in post-operative hospital stay was seen between groups (table 3).
4. Demographics and surgical time for benign hysterectomy, staging for stage 1 ovarian cancer, staging for endometrial cancer, and radical hysterectomy including pelvic lymphadenectomy for cervical cancer on consecutive patients at a tertiary referral teaching hospital 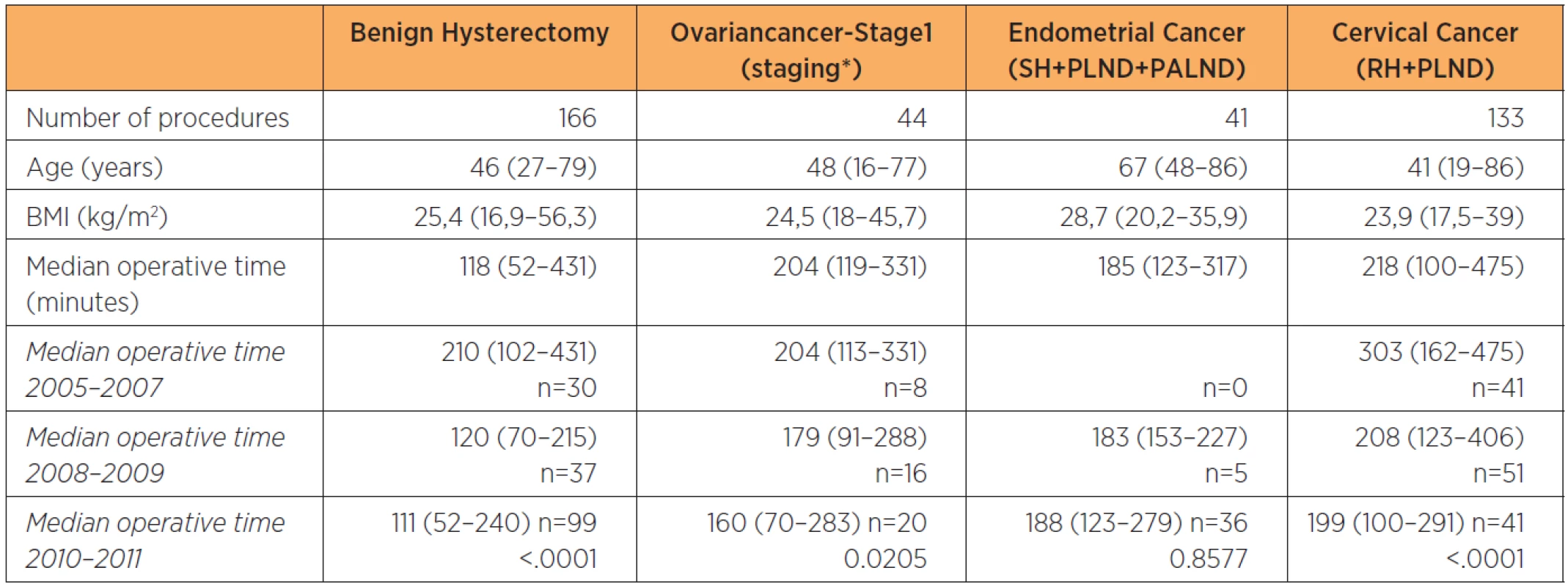
Data subdivided by two-year periods from onset of the robotic program. H: hysterectomy, BSOE: bilateral salpingoophorectomy, PLND: pelvic lymphadenectomy, PALND: paraaortic lymphadenectomy, RH: radical hysterectomy, *PLND + PALND ± omentectomy ± H± SOE ± appendectomy Thirty-seven patients (3.7%) were converted to open surgery, most commonly due to intraabdominal adhesions (n=15) or previously unanticipated cancer spread (n=11). Other reasons were bleeding (n=1), technical problems with the robot (n=6) and anestesiological reasons (n=2). The remaining two women were converted by a general surgeon and an urologist respectively; one patient due to a previously unknown tumor in the colon and the other for constructing a neobladder following a total robotic exenteration.
The median blood loss was 100 ml (range 0–1300 ml). Fifteen patients had bleeding equal to or exceeding 500 ml (1.5%). Two patients had an intraoperative bleeding of more than 1000 ml (0.2%), one in which a conversion to a laparotomy was necessary.
An intraoperative complication occurred in 19 patients (1.9%), three of which occurred during the initiation of the procedure. In eight patients an accidental cystotomy happened during surgery and was promptly repaired. One injury to the ureter occurred during surgery and a reanastomosis after the insertion of a splint was performed robotically. In four women damage to the intestine occurred and was repaired robotically, in one woman a damage to the intestine happened when entering the abdomen with the first trocar and an immediate laparotomy was performed. Injury to a major vessel was seen in four patients; in three patients the damage was repaired robotically and involved the epigastric artery, the external iliac artery and the external iliac vein respectively. In the fourth patient a bleeding from the ovarian vein necessitated a conversion to a laparotomy. This patient was later found to have undetected cirrhosis of the liver and venous hypertension.
One patient (number 799) developed compartment syndrome shortly after the introduction of a new OR table with new leg stirrups. This patient with cervical cancer underwent a radical hysterectomy including pelvic lymph node dissection and the operating time was 279 minutes.
Median BMI increased significantly between groups (23.0 compared with 25.9 between first and last groups, p<0.0001) as high BMI was gradually considered an indication by itself. 215 women with a BMI between 30 and 40 and 31 women with BMI over 40 have undergone surgery with the highest BMI of 62.4 kg/m2. Nine conversions (4.2%) occurred in the obese women, two due to adhesions, four due to intraabdominal tumor spread, two due to technical problems with the robot and one was converted by an urologist for the construction of a neobladder. Two conversions (6,5%) occurred in the morbidly obese women, one due to intraabdominal adhesions in a patient with extensive endometriosis and one due to technical malfunction of the robot. Four women who were obese (1.9%) and none of the morbidly obese women experienced an intraoperative complication. The rate of conversions and intraoperative complications in obese and morbidly obese women did not differ from the normal weight women (p=0.68 (BMI 30–40 kg/m2), p=0.09 (BMI>40 kg/m2) and p=1.00 (BMI 30–40 kg/m2),and p=0.46 (BMI>40 kg/m2) respectively).
DISCUSSION
Introducing robotic surgery allowed our institution to dramatically expand our minimal invasive surgical program as demonstrated by the decrease in the patients with cervical cancer and endometrial cancer operated by laparotomy from 79% and 68% respectively in 2005 to 9% and 23% respectively in 2010. Analysis of our first 1000 patients demonstrated the feasibility of incorporating robotic surgery at our department with low conversion rates, a low rate of intraoperative complications, low blood loss and diminishing surgical times. With increasing experience the technology was found to be feasible for a wide variety of indications. However, there will be limitations to further progress in shortening of OR times and surgical results due to the inevitable introduction of new surgeons and team members within an institution over time as well as expanding the application of technique to more challenging patients and procedures.
The fact that non-surgical time constituted 26.0% and 37.1 % of the total OR times (table 3) for the first and last year groups implicates that shorter, less complex surgical procedures will likely not be efficient, neither from a OR use perspective nor from an overall economic perspective unless compensated by a substantial decrease in short and long term complication rates. Comparative data between robotic surgery and traditional laparoscopic surgery is mostly available for staging procedures for endometrial cancer where robotic surgery has been associated with shorter operative time, less blood loss and lower conversion rate [16–19]. Our seven-year experience with the da Vinci system suggests that in order for a robotic program to be potentially cost efficient an adequate volume of complex procedures usually performed by laparotomy i.e radical or simple hysterectomies with pelvic and/or paraaortic lymphadenetomy, various procedures in obese patients and surgery for advanced endometriosis should form the basis of the surgical population. In concordance with Rocco et al. this is also important to obtain and sustain the continuity of the learning curve, and to ascertain the quality of patient outcome [20]. In addition, the properties of robotic surgery, enables a variety of low-frequency complex procedures traditionally performed by laparotomy to be performed by minimal invasive surgery. For economic reasons, the robot may be shared with another speciality. However based on our initial experience, low access to the robot within a speciality makes it difficult to get over the thresh-hold to efficiate the use and substantially prolong the learning curve for surgeons and teams.
The effort and time needed for implementing a successful robotic program should not be underestimated. As any other advanced tool the robot is only as good as the person using it and it is highly important to know the equipment and to possess a certain skill for it to reach its full potential [21]. Dry-lab studies have shown that the robot is more advantageous compared to traditional laparoscopy when the procedure becomes technically more challenging regardless of the level of the surgeons experience [22]. Converting this knowledge from dry-labs to surgery on humans however requires knowledge on optimal port placement, availability of the proper instrumentation, knowledge and application of different sources of energy, use of the correct robotic arms, docking technique and proper patient positioning.
We find that in order to be successful a dedicated team is a necessity and educating all staff at the department is highly important. A new program for patient care involving not only the operating room staff but also the caretakers on the ward was necessary as patients with gynecological cancers who were previously operated by laparotomy were now undergoing minimal invasive surgery.
The surgical times for staging procedures for gynecological cancer are similar to times reported by other authors whereas the surgical time for benign hysterectomies is quite long [1, 2, 16, 23, 24]. The latter reflects the procedure being used as a step-in procedure for new surgeons and otherwise performed mostly in complex cases for example in patients with multiple adhesions, extensive endometriosis or large uteri.
Further reduction in the total OR time could probably be obtained if simultaneous reversal of anestesia in the first patient in the OR and induction of anestesia on the following patient in a preparation room could be performed and by reducing training of anestesia and OR personnel frequently taking place at our OR. The former is not possible logistically and for medicolegal reasons and the latter is neither possible nor desirable at a teaching hospital.
Conversions to laparotomy were low at 3.7% and are comparable to other studies [1, 2, 18, 19]. No significant increase in conversions was seen with increasing BMI, which coincides with findings by Paley et al. [1). Increasing BMI did not coincide with an increase in complications and in concordance with other authors the complication rate among obese and morbidly obese women was low [1, 18, 25].
The rate of conversions and intraoperative complications is low compared with results for traditional laparoscopy, in particular taking into account the high proportion of advanced procedures [3, 26]. However, the rate of intraoperative complications and conversions did not decrease with time. This was probably due to the expansion of the indications for robotic surgery to more complex patients and the gradual introduction of more consol surgeons.
Seventy percent of the patients converted to laparotomy had intraabdominal adhesions (p=0.050) or previously unknown cancer spread and 65% of patients with an intraoperative complication had major intraabdominal adhesions (p<0.0001). Still, even in the patients with adhesions (n=240) the conversion and intraoperative complication rate was low (5.8% and 5.4%). Supported by the fact that intraoperative complications occurring during port placement were rare (0.3%) we now consider known or suspected adhesions to be an indication rather than a contraindication to robotic surgery.
Strengths of this study are a high number of consecutive robotic procedures performed at a single institution and the prospective data collection. A weakness is the lack of comparison to laparotomy and traditional laparoscopic surgery. Due to a low rate of gynecological cancer surgery not performed by robotic surgery and the majority of complex benign cases being performed by robotic surgery a comparison with other surgical modalities is difficult due to selection bias. We have therefore abstained from making this comparison.
CONCLUSION
A surgical robot provides the possibility to offer minimally invasive surgery to a larger patient population for both malignant and selected benign gynecological disorders with acceptable surgical times, low conversion rates and low rates of intraoperative complications. It is important to be realistic, expect, accept and overcome obstacles, and have a dedicated well-trained team with thorough knowledge of the system and its properties. An adequate surgical volume with a large percentage of complex cases is necessary in order for a program to be potentially successful both with regards to patient outcome and economic feasibility.
Jan Persson, MD, PhD, Associate Professor
Department of Obstetrics and Gynecology
Skåne University Hospital
Lund and Lund University Sweden
SE-22185 Lund
Sweden
e-mail: jan.persson@med.lu.se
Sources
1. Paley, PJ., Veljovich, DS., Shah, CA., et al. Surgical outcomes in gynecologic oncology in the era of robotics: analysis of first 1000 cases. Am J Obstet Gynecol, 2011, 204, p. 551.e1–9.
2. Vejlovich, DS., Paley, PJ., Drescher, CW., et al. Robotic surgery in gynecologic oncology: program initiation and outcomes after the first year with comparison with laparotomy for endometrial cancer staging. Am J Obstet Gynecol, 2008, 198, p. 679.e1–10.
3. Walker, JL., Piedmonte, MR., Spirtos, NM., et al. Laparoscopy compared with laparotomy for comprehensive staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol, 2009, 27(32), p. 5331–5336.
4. Advincula, AP., Reynolds, RK. The use of robot-assisted laparoscopic hysterectomy in the patient with a scarred or obliterated anterior cul-de-sac. JSLS, 2005, 9(3), p. 287–291.
5. Advincula, AP., Song, A., Burke, W., Reynolds, RK. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc, 2004, 11(4), p. 511–518.
6. Reynolds, RK., Burke, WM., Advincula, AP. Preliminary experience with robot-assisted laparoscopic staging of gynecological malignancies. JSLS, 2005, 9(2), p. 149–158.
7. Persson, J., Kannisto, P., Bossmar, T. Robot-assisted abdominal laparoscopic vesselsparing radical trachelectomy. Gynecol Oncol, 2008, 111(3), p. 564–567. Epub July 2008.
8. Persson, J., Imboden, S., Reynisson, P., et al. Reproducibility and accuracy of robot-assisted laparoscopic fertility sparing radical trachelectomy. Gynecol Oncol, 2012, 127(3), p. 484–488. Epub 2012 Aug 28.
9. Anderberg, M., Bossmar, T., Arnbjornson, E., et al. Robot assisted hemihysterectomy for a rare genitourinary malformation with associated vessel anomalies. Case report. Eur J Pediatr Surg, 2010, 20(3), p. 206–208. Epub 2009 Dec 16.
10. Persson, J., Bossmar, T., Teleman, P. Robot assisted laparoscopic surgery for a rudimentary uterine horn with two non-communicating cavities. J Rob Surg, 2010, 4(2), p. 137–140.
11. Persson, J., Gunnarsson, G., Lindahl, B. Robot assisted surgery of a 12-week scar pregnancy with temporary occlusion of the uterine blood supply. J Rob Surg, 2009, 3, p. 53–55.
12. Persson, J., Reynisson, P., Borgfeldt, C., et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol, 2009, 113(2), p. 185–190.
13. Lönnerfors, C., Persson, J. Robot assisted laparoscopic myomectomy for unfavourably situated myomas. Acta Obstet Gynecol Scand, 2009, 88(9), p. 994–999.
14. Persson, J., Reynisson, P., Måsbäck, A., et al. Histopathology indicates lymphatic spread of a pelvic retroperitoneal ectopic pregnancy removed by robot assisted laparoscopy with temporary occlusion of the blood supply. Acta Obstet Gynecol Scand, 2010, 89(6), p. 835–859.
15. Geppert, B., Lönnerfors, C., Persson, J. Robot assisted hysterectomy in obese and morbidly obese women: surgical technique and comparison with open surgery. Acta Obst et Gynecol Scand, 2011, 90, p. 1210–1217.
16. Boggess, JF., Gehrig, PA., Cantrell, L. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol, 2008, 199, p. 357.e1–7.
17. Magrina, JF., Kho, RM., Weaver, A., et al. Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol, 2008, 109, p. 86–91.
18. Gehrig, PA., Cantrell, LA., Shafer, A., et al. What is the optimal minimally staging in the obese and morbidly obese woman? Gynecol. Oncol, 2008, 111, p. 41–45.
19. DeNardis, SA., Holloway, RW., Bigsby IV, GE., et al. Robotically assisted laparoscopic hysterectomy versus total abdominal hysterectomy and lymphadenectomy for endometrial cancer. Gynecol Oncol, 2008, 111, p. 412–417.
20. Rocco, B., Lorusso, A., Coelho, RF., et al. Building a robotic program. Scand J Surg 2009, 98, p. 72–75.
21. Mendivil, A., Holloway, RW., Boggess, JF. Emergence of robotic assisted surgery in gynecologic oncology: American perspective. Gynecol Oncol, 2009, 114, p. 24–31.
22. Kaul, S., Shah, NL., Menon, M. Learning curve using robotic surgery. Curr Urol Rep, 2006, 7(2), p. 125–129.
23. Payne, TN., Dauterive, FR. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol, 2008, 15(3), p. 286–291.
24. Visco, A., Advincula, AP. Robotic gynecologic surgery. Obstet Gynecol, 2008, 112(6), p. 1369–1384.
25. Seamon, LG., Bryant, SA., Rheaume, PS., et al. Compre-hensive surgical staging for endometrial cancer in obese patients. Obstet Gynecol, 2009, 114, p. 16–21.
26. Chi, DS., Abu-Rustum, NR., Sonoda, Y., et al. Ten year experience with laparoscopy in gynecologic oncology service: analysis of risk factors for complications and conversions to laparotomy. Am J Obstet Gynecol, 2004, 191, p. 1138–1145.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inCzech Gynaecology

2013 Issue 1-
All articles in this issue
- History of Department of Obstetrics and Gynecology in Olomouc
- “Learning curve” robotic radical hysterectomycompared to standardized laparoscopy assisted radical vaginal and open radical hysterectomy
- Sacrospinous fixation for vaginal vault prolapse after hysterectomy sec. Miyazaki – longterm results
- RHD genotyping from cell-free fetal DNA circulating in pregnant women peripheral blood and sensitivity assessment of innovated diagnostic approaches for introduction into the clinical practice
- Births of children of low and very low weight at the University Hospital in Olomouc (1993–2011)
- Incidence of erythrocyte alloimmunization in pregnant women in olomouc region
- Epidemiology and management of thyroid disorders in pregnancy
- Influence of the length of cultivation of no early cleavage embryos on the IVF success rate
- The occurence of genetic trombophilic markers in patients evaluated for infertility
- Objective assessment of the tissue trauma in surgery of endometrial cancer
- Bleeding disorders in pregnancy
- Erythrocyte alloimmunization in pregnant women, clinical importance and laboratory diagnostics
- Disorders of the thyroid gland in pregnancy
- Status of bone mineral density after the long-standing application of contraception Depo-Provera
- Implementation and applications of robotic surgery within gynecologic oncology and gynecology; analysis of the first thousand cases
- Czech Gynaecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Influence of the length of cultivation of no early cleavage embryos on the IVF success rate
- Sacrospinous fixation for vaginal vault prolapse after hysterectomy sec. Miyazaki – longterm results
- Erythrocyte alloimmunization in pregnant women, clinical importance and laboratory diagnostics
- Bleeding disorders in pregnancy
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


