-
Medical journals
- Career
Implementation of One-Stop-Clinic for Risk Assessment of chromosomal abnormalities in the first trimester, evaluating the effectiveness and making it part of our daily practice
Authors: I. Dhaifalah 1; L. Dusek 2; J. Santavy 1
Authors‘ workplace: Department of Genetic and Fetal Medicine, University Hospital, Olomouc, Czech Republic 1; Institut of Biostatistics and Analysis, Masaryk University, Brno, Czech Republic 2
Published in: Ceska Gynekol 2011; 76(4): 292-306
Overview
Objectives:
Implementation and introducing of first trimester screening using nuchal translucency (NT), nasal bone (NB), maternal serum pregnancy associated plasma protein-A and free β-human chorionic gonadotropin (PAPP-A and fß-hCG) as a new method of screening for Down’s Syndrome in a One-Stop-Clinic for Assessment of Risk for chromosomal abnormalities in the Czech Republic. To prove its effectiveness and make it part of our daily practice. Modify and/or withdraw the existing current practice.Methods:
Prospective study of 5010 singleton pregnancies who attended our clinic for prenatal screening for chromosomal and congenital abnormalities between 11-13+6 weeks gestation from January 2005 to December 2007. Pregnancies were followed up to ascertain live birth of Down syndrome babies.Conclusion:
The uptake was 100% for screening and around 95% for invasive testing in the screen positive group. All expected trisomies 21 were detected along with many other aneuploidies for false positive rate of 4.4%. The study demonstrated the feasibility and efficacy of introducing OSCAR for chromosomal abnormalities in a University Hospital setting. The introduction of an examination of nasal bone reduced our false positive results and increased our sensitivity. Women who participated in our screening strategy were no longer advised to undergo the triple test.Key words:
implementation of first trimester screening, one stop clinic (OSCAR), nuchal translucency, nasal bone.INTRODUCTION
It is well recognized that any screening program will confer some benefit and some harm on the population served by that program. Screening programs of poor quality, and those that have been introduced on the basis of insufficient evidence, may produce more harm than good. However, when screening for Down’s syndrome, an assessment of the benefits and costs of the program is further complicated by different people’s understanding of benefit and harm in this situation. Despite inherent difficulties, there should be certain criteria that should be used to assess the performance of screening programs. These should be evidence based and the implementation should ensure equality of access and a uniform standard of care.
When evaluating a screening test for use on a large scale, it is necessary not only to investigate the test, but also determine the extent of the infrastructure required to underpin its implementation. This may involve many different clinical services, departments, and health professionals. For the benefit of health professionals providing the service and the users of that service, it is necessary to develop policies and protocols governing the local screening providers. Coordinating the various services comprising screening at the local level and informing and training the staff who offer these services is mandatory [9, 10, 11, 14, 20].
Audit and monitoring needs to be an integral part of the service, to ensure continuous quality improvement and to provide information from which the demand and need for future service provision within the local population, and its associated costs, can be extrapolated.
First trimester screening which is considered to be the most effective method of screening is used at our centre. It is the method of choice for screening of trisomy 21 (Down’s syndrome) and for other chromosomal abnormalities as well as for routine scanning between 11-13+6 weeks of pregnancy. We use The Fetal Medicine Foundation (FMF), London, UK recommendation as the guidelines for implementing this method [3, 17, 23].
An ultrasound scan examination of the fetus, measurement of the markers for chromosomal abnormalities (NT and NB) and blood sampling to measure free ß-hCG and PAPP-A, is be out on the same day (OSCAR) [5, 16]. The women are informed of their results within 24–48 hours. If a problem is identified, invasive testing is offered during the same visit or immediately the next day. The invasive testing is preceded by genetic counseling to help women to make decisions prior to and after the tests have been provided.
Our main aim is to withdraw the less effective methods used in our current practice and substitute them with more effective method of a one stop manner for the first time in the Czech Republic.
METHODS
The study was conducted at the Medical Genetic and Foetal Medicine Department, University Hospital Olomouc. This department is located in Olomouc, in the Moravian part of the Czech Republic. Palacky University Hospital, Olomouc is one of the twelve hospitals directly managed by the Ministry of Health of the Czech Republic, and is the top medical facility in Olomouc region. The Department provides a full range of services in prenatal diagnosis and genetics. It has an average of 12 000 examinations (ultrasound, genetic consultations and laboratory examinations including cytogenetic and molecular genetic investigations) per year.
Design and sample
It is a prospective study covering singleton pregnant women (5010) booked for screening between 11-13+6 weeks of pregnancy between January 2005 and December 2007.
Women are initially seen in the community by their obstetricians in about 99% of cases. They receive an information leaflet about the one-stop clinic service and the screening tests that are available either from their obstetrician, with their initial booking appointment, or on arrival to our department before the screening test.
On attending the clinic, women have a consultation with the sonographers (obstetricians with a genetic specialization, certified from FMF) concerning the available tests and, if they elect to have prenatal screening for chromosomal anomalies, all relevant clinical information is recorded on the request forms. After pre-test counselling, women opting into the screening program undergo US scanning and at the same time blood samples are taken for serum biochemical screening after signing an informed consent.
Process of screening
Screening in the first trimester is based mainly on ultrasound. If the gestational age is between 11–13+6 weeks; the foetal NT, presence or absence of the NB and crown–rump length (CRL) are investigated and measured. The whole ultrasound process is completed within a 15–20 minute period in 95% of cases. Ultrasound examinations are performed trans-abdominally with the Toshiba Power Vision 6000 (convex probe 3.5–5 MHz) (Toshiba, Tokyo, Japan) or the GE-Voluson 730 Expert (convex probe 3.5–5 MHz) (GE Healthcare Technologies, ZIPf, Austria). The blood samples are sent to the hospital laboratory (situated about 50 meters from our clinic) within one hour. Serum is separated and analysed for free ß-hCG and PAPP-A using B.R.A.H.M. Kryptor, Germany.
When the results are available (within 2 hours) they are logged onto the fetal database and a composite risk report is produced. Patient-specific risks are calculated by a multivariate approach using population’s parameters established by FMF. Women with an increased risk (risk ≥1 in 300) for trisomy 21, 18 and 13 are referred to a genetic counsellor who discusses with the woman the appropriate risk and its management. If the woman agrees she undergoes chorionic villous sampling (or amniocentesis later) and fetal karyotyping in the procedure room of the department.
Molecular and cytogenetic diagnosis of a high-risk/screen-positive result
Rapid methods as quantitative polymerase chain reaction (QF-PCR) and in some cases fluorescent in-situ hybridization (FISH) are used. Results are available within 24–48 hours. These methods are used for the confirmation of trisomy 21, 18, 13 and abnormalities of sex chromosomes. In all cases samples undergo further diagnosis by conventional karyotyping providing the results in three weeks. A woman who has a definitive diagnosis of chromosomal abnormalities is offered genetic consultation and support whilst making a decision on the future of the pregnancy and for planning for future pregnancies. Termination of pregnancy is possible for chromosomal abnormalities in the Czech Republic up to the 24th week of pregnancy and most women with confirmed trisomy 21, 13 or 18 in the fetus elect to terminate the pregnancy.
Staff training
All health professionals responsible for providing information to users of the service are familiar with the various screening tests available and were trained in the use of the database software. Obstetricians performing the scans were trained, and certified by FMF. The training was done by practical training and theoretical courses.
Seminars were organized and information (including information leaflets) were disseminated to all who look after pregnant women (e.g. private obstetricians).
Audit and quality assurance
An internal audit was held on a monthly basis and an external one annually. In an audit, the performance of the obstetricians carrying out the scan was judges as satisfactory in cases in which they were able to demonstrate good performance in measuring the NT through a control of some of their images and a search of their distribution curve. The audit of the centre was based on the detection rate and false positive rates and the performance of the obstetricians performing the scans.
The laboratory providing maternal serum screening tests participated and was successful in internal and external audits of its performance. The internal audit is carried out by the Czech Society of Clinical Biochemistry and the external audit is undertaken by the United Kingdom National External Quality Assessment Service (UKNEQAS).
Internet, e-mail and helpline facilities (www.ulgrs. upol.cz), were created to enable information to be passed directly and quickly to health professionals and to women.
Pilot testing
The method of screening was introduced gradually over a period of one year (January 2004 to December 2004). The risk of trisomy 21 was estimated for 695 singleton pregnancies. The specific risk was calculated using the FMF calculation risk model software by an accredited obstetrician. The Immulite DPC 2000 (Diagnostic Products Corporation, Los Angeles, USA) was used for blood analysis and Toshiba Power Vision 6000 for US examinations. Women with risk of ≥1 in 300 were offered CVS and fetal karyotyping. Only 17% women of the study group were older than 35 years. All cases of trisomy 21 (2) and three cases of other chromosomal abnormalities were detected for a 6% false positive rate. The whole group of women had a second trimester screening test by age and triple test those with positive results had been offered amniocentesis.
OSCAR showed high sensitivity with a low false positive rate in comparison to our current routine practices and we also demonstrated that it could be offered in an efficient manner. Therefore we decided to introduce it to our daily practice, replacing the previously deployed methods.
During the pilot study many modifications and improvements were made. The obstetricians and health professional staff were trained and certified, information leaflets and consent forms were written, we obtained a new high quality ultrasound machine and a new instrument (KRYPTOR) for blood analysis. Network installation of the software risk calculation model and computer facilities were developed by the Department of Information Technology in our hospital in cooperation with the software owners in Germany. Many modifications were made in the software to improve the printouts, the language was changed to fulfill our requirements. We chose the 1 : 300 risk as the cut-off for positive result to overcome inter-observer differences and errors. We advised that women who were tested by our method of screening do not need to be tested with triple test also; instead we recommended an anomaly scan examination for NTD and other organs at the 20–22 week of pregnancy.
DATA COLLECTION AND ANALYSIS
The database software FMF calculation risk model (Astraia, SRN, and Munich, Germany) was used for calculation of risk, data collection, monitoring and audit. QF-PCR and cytogenetic results were recorded also in this database software. The outcome of pregnancies in women included in this study was collected by questionnaires sent by post. In cases where no reply was received, the patients were contacted by phone.
A comprehensive review of completed data was carried out and database information was prepared in Microsoft Excel (Microsoft Corp, USA). Experts from the Biostatistics and Analyses Institute, Faculty of Medicine, Masaryk University, Brno, analyzed the data.
The statistical significance of differences in relative frequency of categories among several groups of patients was analyzed by means of maximum likelihood chi-square test [28]. Statistical significance of differences among categories in continuous variables was analyzed using the Kruskal-Wallis test followed by post hoc multiple comparisons of mean ranks [4].
Adjustment of ultrasonography and blood markers for gestational age based on Crown Rump Length was computed by means of linear regression prior to comparison of patients’ categories [27].
The achieved performance for the screening examination was described using false positive and false negative discovery rates, together with their sensitivity and specificity for 1 : 300 risk „Cut-off“(empirically given). Receiver operating characteristics (ROC curves) were adopted for the visualization of sensitivity and specificity of this standard „Cut-off“ and for the analysis of the relationship between computed continuous risk for trisomy and its specificity and sensitivity for trisomy detection [15, 26, 29].
The area under the ROC curve, its confidence interval and statistical significance was computed and adopted for the evaluation of statistical significance of trisomy screening test performance [2, 12, 13].
Analyses were performed using SPSS 17.0 (SPSS, Inc.) and Statistica 8.0 software for Windows (Statsoft, Inc.).
RESULTS
The screened population was mainly drawn from Olomouc (79.9%), Moravian-Silesian (12.6%) and Zlin (4.4%) districts; the remainder came from the adjacent areas. Over the three-year study period a total of 5010 women with singleton pregnancies were offered first trimester screening in the OSCAR clinic. Most (97.2%) of the screened women were primiparous.
The uptake of first trimester screening was 100%. The ethnic origins of the population studied consisted of 99.9% Caucasian, 0.12% Asian and one Afro-Caribbean. The median maternal age was 29 years (range between 16 to 38), 16.4% women were older than 35 years.
There were 99.9% (5006) normal intrauterine pregnancies and 0,1% (4) cases of missed abortion at the time of the scan. 12.2% (373) of the women received an invasive testing, 94 for age > 35 years, 21 for positive triple test and 56 for other reasons (age with minor ultrasound markers or family history). Table 1 show the basic description of our sample data.
1. Basic description of sample data set 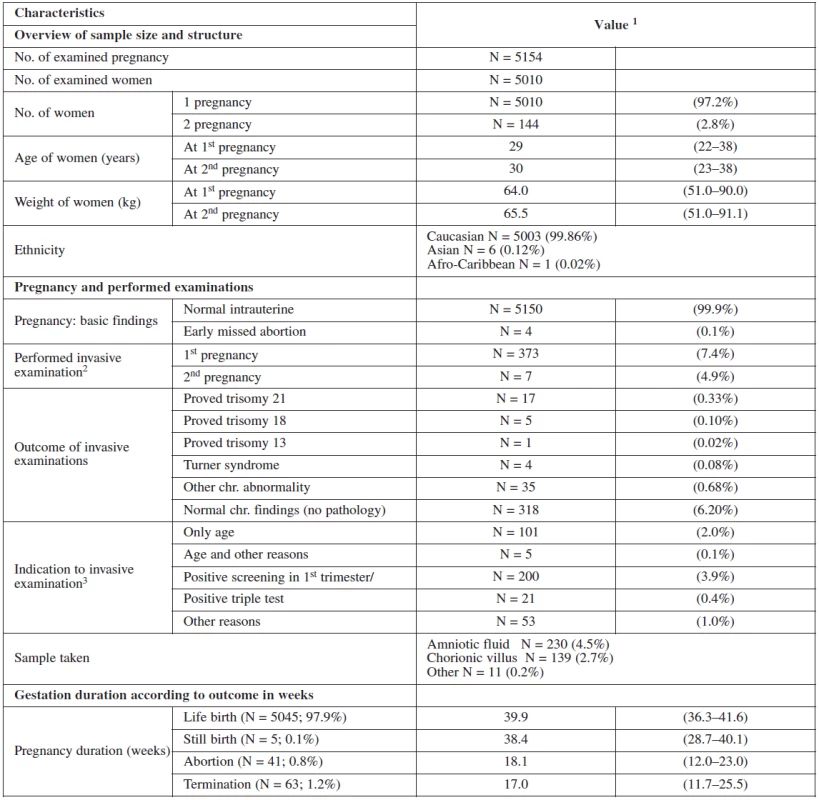
1 Categorical variables are summarized as percentages of categories; continuous variables by median and 5th–95th percentile range 2 Percentage of invasive tests within first and second gestations 3 Based on carried out invasive tests Figure 1 summarizes and structures the screening process of our sample. In the screened population 4613 women had a screen negative result and 221 had a screen positive result (4.4% false positive). 197 of them chose to have the invasive test at our department. Of these, 135 had CVS and 62 had amniocentesis. The rest of the group (24) underwent different management. 14 refused the test, 3 had invasive test at their regional clinic and 7 were advised for further management (mostly having border line risk). In the group that underwent invasive testing at our department, 23 cases of trisomies were detected of which 17 were trisomies 21 and another 17 other chromosomal abnormalities.
Figure 1. Structure of a sample from the viewpoint of the screening process 
In the screen negative group, 176 women (3.5%) opted for invasive testing for other reasons such as age, triple test and family history. Trisomies were detected in only two pregnancies of this group (45,X0, 47,XX +mar) and one case of tuberous sclerosis was detected. In these three cases, family history was the indication, and in each case the parents opted for termination.
Table 2 and table 3 show the outcome of all pregnancies in our sample in which we ascertained the outcome of as well as the difference in the outcome in women with invasive testing and without termination in comparison with the other women. There were 4772 (97.9%) normal live births, 58 (1.2%) had termination for various reasons, 38 (0.8%) aborted and 5 (0.1%) had stillbirths. The non-invasive group had a higher number of normal live births, spontaneous delivery and fewer terminations generally. In the group that underwent invasive testing, if termination was indicated for reasons other than chromosomal aneuploidy, this was earlier in gestation and the women were of higher maternal age.
2. Follow-up findings according to gestation outcome 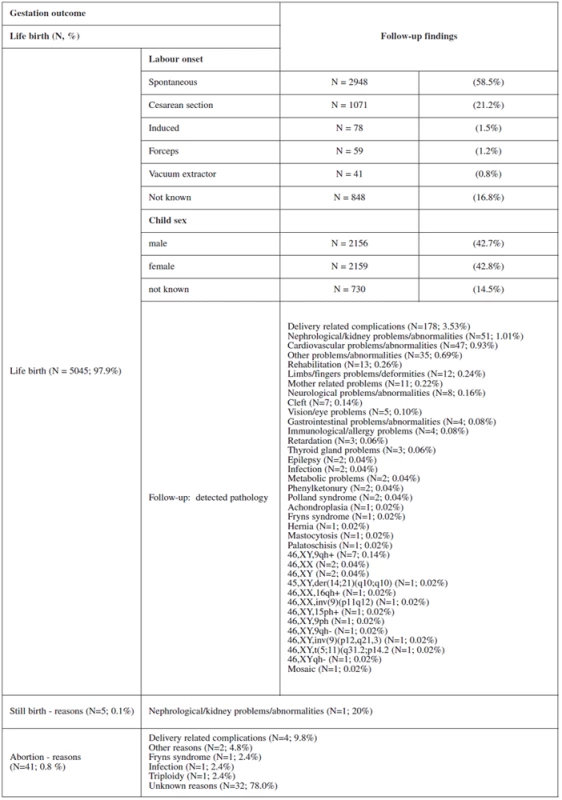
ad.: Table 2. Follow-up findings according to gestation outcome 
3. Gestation outcome in women with invasive testing and without termination – comparison with the other women 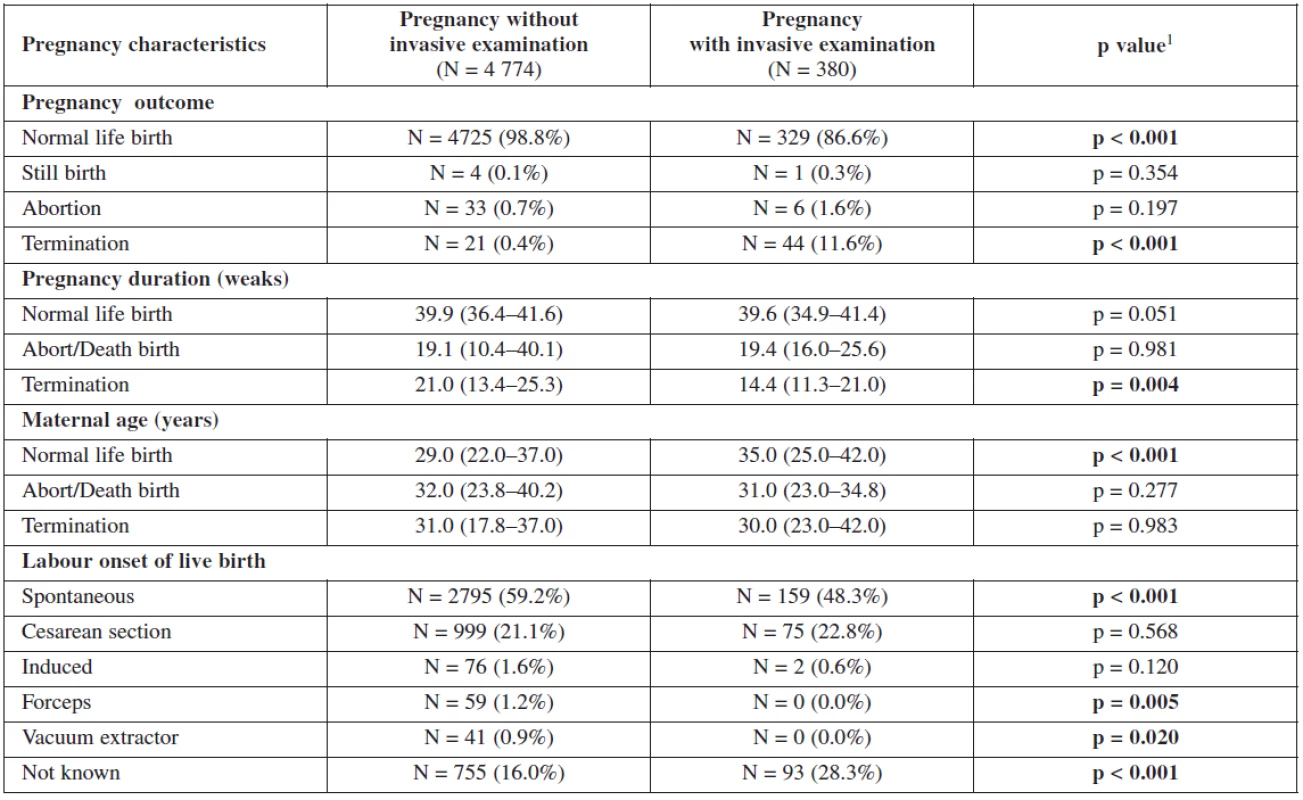
Maternal age is the most important factor in our screening test. It is from where the background coefficient of risk is calculated. The relationship of maternal age, nuchal translucency and biochemical markers, as well as the relationship of these markers with the gestational age is shown in figure 2.
Figure 2. Selected US and blood markers as related to gestational age (crown-rump length of the fetus) and age of the mother 
It can clearly be seen that the age of the mother is independent of all of them and this is why it can be used as a priori risk factor. On the other hand, the gestational age have a correlation with each marker. NT and PAPP-A increase with the increase in the crown-rump length (gestational age) with a peek between 60 and 70 mm, β‑hCG shows early increase and early decrease.
Table 4 and table 5 show the statistical significance of ultrasound and biochemical markers used for screening over the whole of our data sample. Nuchal translucency was highest among terminated cases due to positive screening with an average of 3.7mm. Fetal heart rate was the lowest among live births 158/beats per minute. Nasal bone was absent in 35.4% in positively screened terminated cases, in 3% among cases terminated for other reasons than positive screening and in 7% among aborted cases; and only in 0.5% of live births. The proportion of fetuses with two vessels in the umbilical cord was also the highest among the group of terminated fetuses following a positive screening test. This group also showed significantly low level of PAPP-A in maternal serum with no big difference in the level of β-hCG among different groups.
4. Examined markers in basic overview 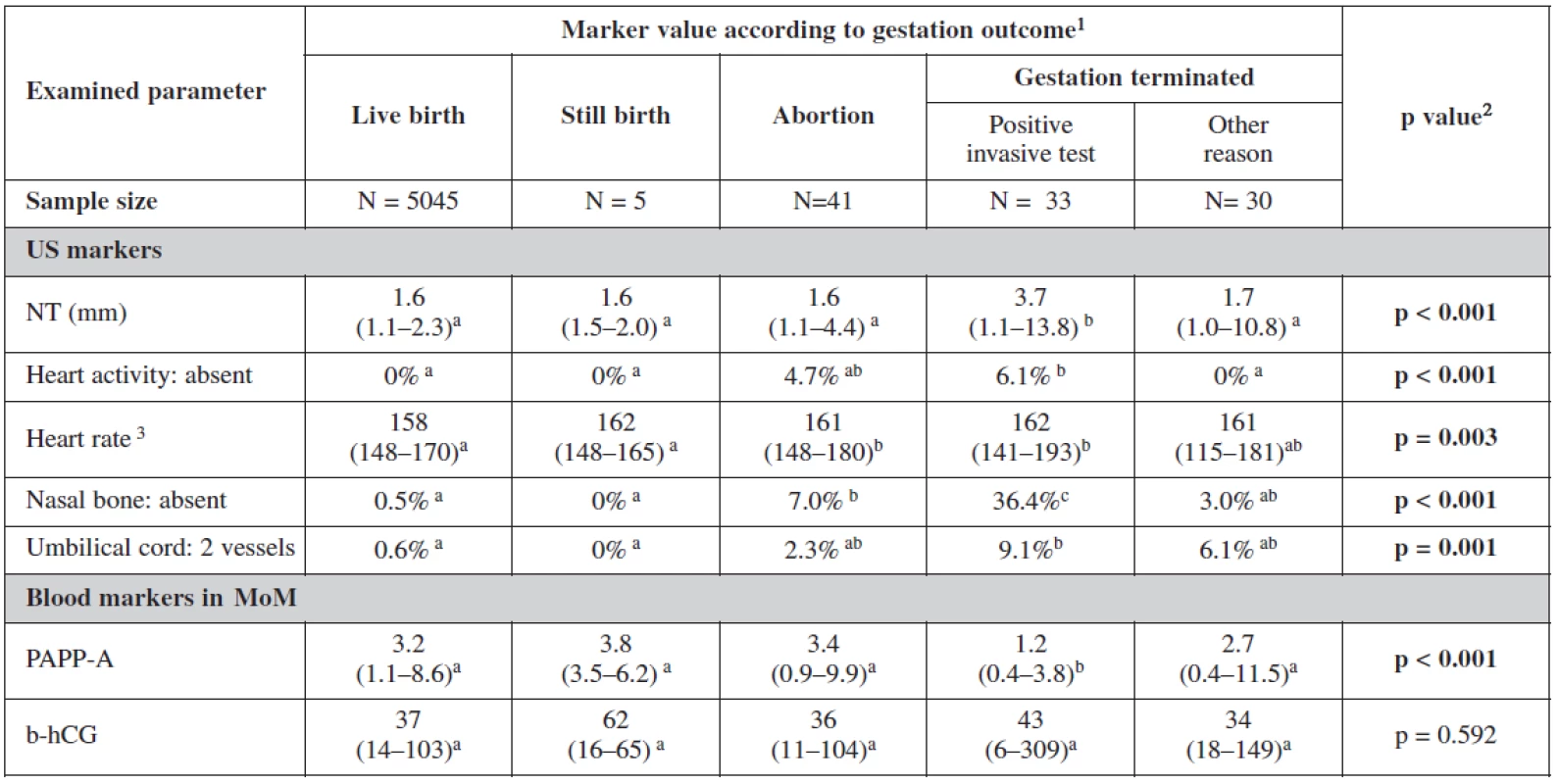
1 Categorical variables are described with percentages of categories; continuous variables by median and 5th and 95th percentile range 2 Statistical significance of relationship between categorical variables was analyzed by means of maximum likelihood chi-square; statistical significance of difference of continuous variables among categories was analyzed using Kruskal-Wallis test 3 Only cases with present fetal heart activity a-c Marks of statistical significance of differences between gestation outcomes: categories marked by different superscript letter are mutually significantly different in given parameter (p < 0.05) 5. Examined markers in relation to screening process 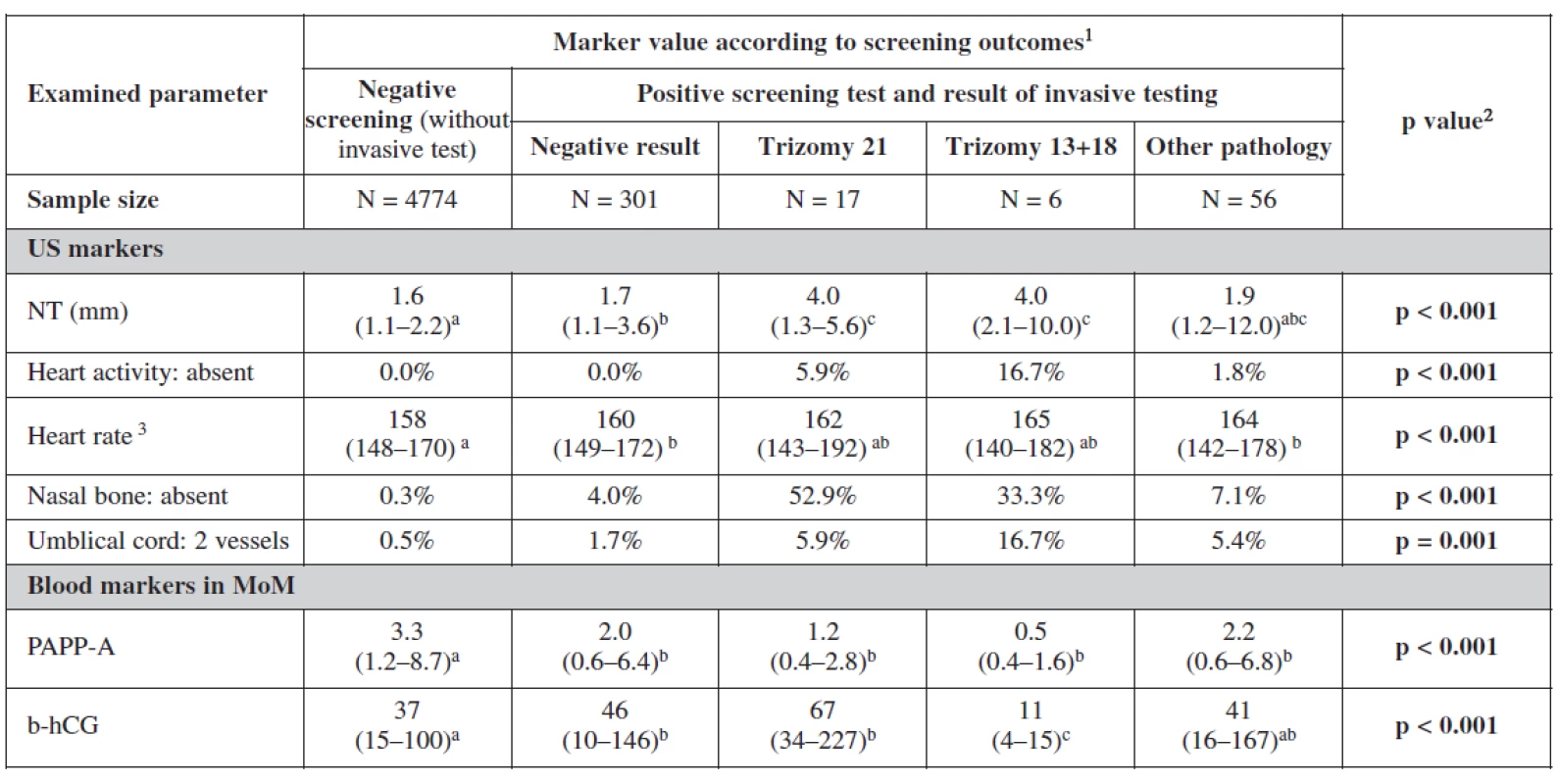
1 Categorical variables are described with percentages of categories; continuous variables by median and 5th and 95th percentile range 2 Statistical significance of relationship between categorical variables was analyzed by means of maximum likelihood chi-square; statistical significance of difference of continuous variables among categories was analyzed using Kruskal-Wallis test 3 Only cases with present fetal heart activity a-ca-c Marks of statistical significance of differences between gestation outcomes: categories marked by different superscript letter are mutually significantly different in given parameter (p < 0.05; multiple comparisons of mean ranks) Analyzing the markers in relation to our screening processs, the relationship between categorical variables was more statistically significant. Nuchal translucency was the most sensitive markers for trisomies 21 and 18+13. Fetal heart rate was lower among negative screened women. In cases with trisomy 18+13 fetal heart rate was significantly higher. The nasal bone was more sensitive indicator for trisomy 21 (52.9%) than trisomy 18+13. Two umbilical vessels were also of statistical significance as expected among trisomy 18+13 (16.7%) and nearly the same for trisomy 21 and other chromosomal abnormalities. Important in this table are the biochemical markers as they showed the lowest level of PAPP-A and free β-hCG in trisomy 18+13. For trisomy 21, PAPP-A was low and free β-hCG was much higher as it was also expected. It is interesting to notice in this analysis the lower level of both markers in positive screen group and those with other pathologies.
The influence of the presence or absence of the nasal bone or even its uncertainty is clearly seen in our test. The risk coefficient decreases when the nasal bone is present for all trisomies, being most sensitive for trisomy 21. On the other hand the risk increases dramatically when the nasal bone is reported as being absent. This has a big influence on our border line risk coefficients (table 6).
6. Influence of nasal bone examination on probabilistic risk scoring 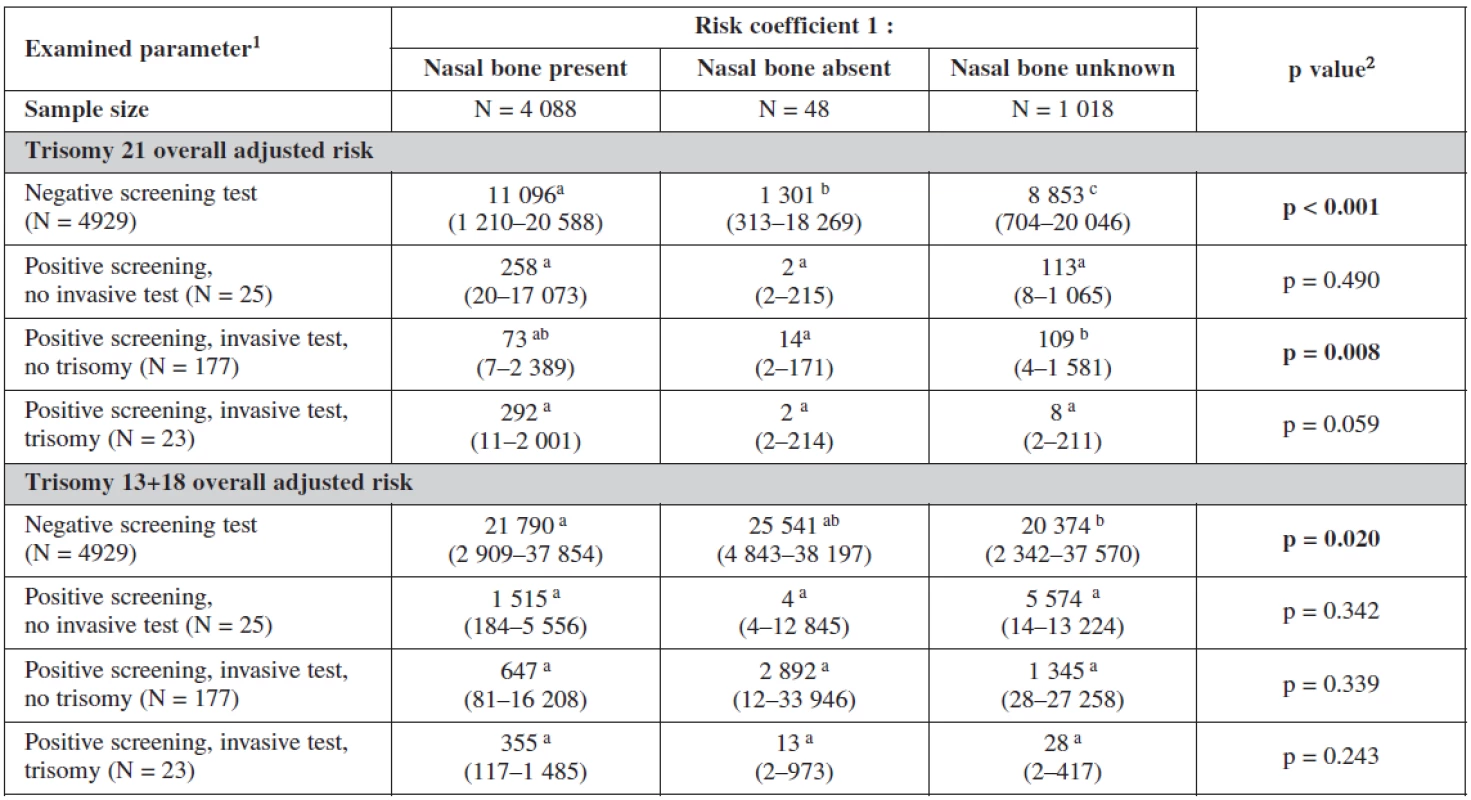
1 median (5th and 95th percentile) 2 Statistical significance of difference of continuous variables among categories was analyzed using Kruskal-Wallis test a-ca-c Marks of statistical significance of differences between gestation outcomes: categories marked by different superscript letter are mutually significantly different in given parameter (p < 0.05; multiple comparisons of mean ranks) The risk probability ratio in relation to the gestational outcome is summarised in table 7. The background risk coefficient was highest among the women with still birth. When the risk for trisomy 21 was 1 : 280 the risk for still birth was as high as 1 : 175. When the risk for trisomy 18+13 was 1 : 489 the background risk for still birth was 1 : 305. Nuchal translucency risk coefficient was highest for trisomy 21 (1 : 24) and for trisomy 18+13 (1 : 58) and was the most sensitive marker for all trisomes.
7. Screening examination: risk probability ratio according to gestation outcome 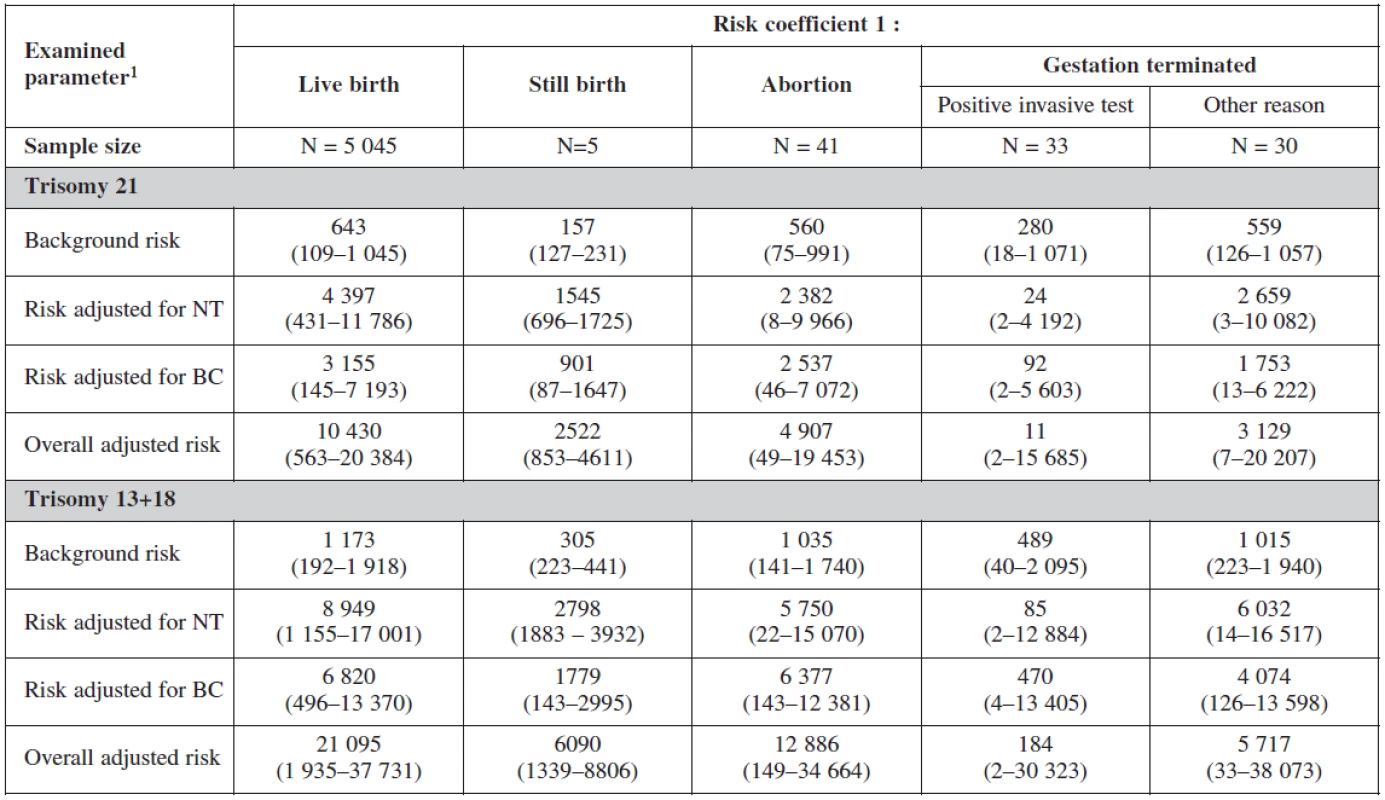
1 median (5th and 95th percentile) For biochemical markers (PAPP-A and β-hCG) risk coefficient was more sensitive for trisomy 21 (1 : 92), trisomy 18+13 (1 : 470) and for still birth (1 : 901) when risk is calculated for trisomy 21.
The overall adjusted risk for the screening test (combining all markers) was most sensitive for trisomy 21 (1 : 11) and less sensitive for trisomes 18+13 (1 : 184).
Interesting is to notice the important of biochemical profiles among women with still birth (they have higher risk on the bases of their biochemical markers (low PAPP-A). The risk probability ratio of the test was most sensitive for trisomies 21, 18, 13 with no difference among the group with positive testing and physiological karyotype (table 8).
8. Screening examination: risk probability ratio according to invasive testing 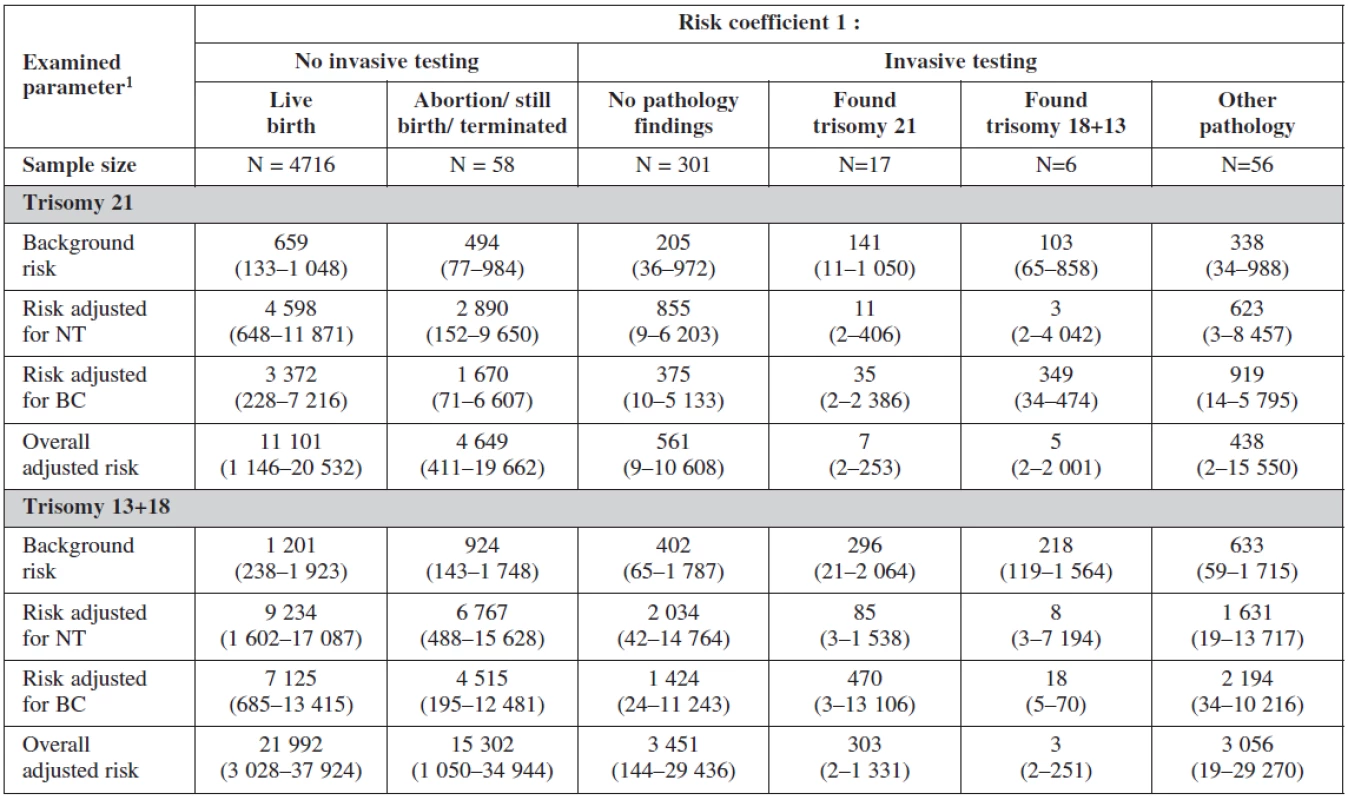
1 median (5th and 95thpercentile) Receiver operative curve describes the attained sensitivity and specificity of the test. A combination of maternal age, foetal nuchal translucency thickness, nasal bone, maternal serum free β-hCG and PAPP-A) showed 100% sensitivity for all trisomies with 3.5% and 1.6% false positives for trisomies 21 and 18+ 13 respectively when the “Cut-off“ risk coefficient is 1 : 300 (figure 3 and 4).
Figure 3. ROC analysis of the results of the screening test 
Figure 4. ROC analysis of the results of the screening test for trisomy using risk cut off 1:150 
If the “Cut-off“ level risk is reduced to 1 : 150, the sensitivity and the false positive rates of the test are reduced (sensitivity; 76.5% and 66.7%, false positive 2.77% and 0.78%) for trisomy 21 and 18+13 respectively.
“Cut-off“ point 1 : 300 seems to be well selected as no case with proven trisomy was detected above this level (figure 5).
Figure 5. Sensitivity and specificity of risk indicating cut-off points in the screening test 
Evaluation of the test and the relationship between probabilistic risk ratios calculated for trisomy 21 and 18+13 are shown in figure 6.
Figure 6. Relationship between probabilistic risk ratios calculated for trizomy 21 and for trizomy 13+18 
Overlap of the coefficients for trisomy 21 and 18+13 is noticed, with greater sensitivity for trisomy 21.
Evaluation of screening test in cross validation 
1 adjusted risks for trisomy 13+18 is higher than 1:300 2 adjusted risks for trisomy 21 is higher than 1:300 3 area under curve Interestingly, the number of abortions and termination is higher within the area under the curve analysis of either trisomy 21 or trisomy 18+13.
The scatterplot documents that screening scoring for trisomy 21 and for trisomy 18+13 is generally significantly correlated and follow the same trend in overall data. Cut-off point 1 : 300 seems to be well located as none case with proved trisomy was detected above this level, neither trisomy 21 nor trisomy 18+13. The level of risk Cut-off should not be decreased as some positively detected cases were found nearby the Cut-off level, both trisomy 21 and trisomy 18+13.
However, it is apparent that the cut-off itself has some intrinsic variability which necessarily leads to some inconclusive areas where the risk is indicated for relatively many cases, with subsequent negative outcome of the screening test. Therefore, the “Cut-off“ level should be addressed by uncertainty analysis and the confidence limits should be supplied as additional support for the clinical decision making.
DISCUSSION AND CONCLUSION
The aim of antenatal screening for Down syndrome is to offer women during pregnancy a screening test which can identify those women at higher risk of having a child with Down’s syndrome. The group at increased risk is then offered diagnostic tests. With high quality counseling, parents can then make an informed choice about how to proceed. In the Czech Republic the state supports and finances the prenatal screening and diagnosis for all of its pregnant population. But the intention to ensure that women across the country receive a high standard of screening and care is still missing.
The study has demonstrated the feasibility and efficacy of introducing OSCAR for chromosomal abnormalities in a university hospital. All cases of trisomy 21 had been detected for a 4.4% false positive rate, the prevalence of trisomy 21 and other aneuploidies is considerably greater than at term or in the second trimester. Our prevalence of 21 cases per 501 women is consistent with that expected from the maternal age profile population and the risk of trisomy 21 at 12 weeks of gestation [22].
The findings of the study confirm the prediction that a combination of maternal serum free β-hCG and PAPP-A with fetal nuchal translucency thickness at 11–14 weeks of gestation would identify about 90% of trisomy 21 pregnancies for a 5% false positive rate [8]. The introduction of the nasal bone as an ultrasound marker has reduced our false positive results from 6% in our pilot study to 4.4% [8] and dramatically increased our detection rate. This is far superior to the average sensitivity of 65% achievable by second trimester biochemical screening [30].
Using the combined first trimester OSCAR screening method had reduced the invasive procedures from 16.5 % on base of age and 18% on the base of current practice all over the country to 4.4 % in our population [24, 25].
Many other congenital abnormalities were identified and managed accordingly in the first trimester. Shifting our practice from screening in the second trimester to the first trimester had to be accompanied by the anomaly scan at 20 weeks of pregnancy. Women who undergo our screening strategy are no longer advised to take the triple test. The anomaly scan at 20 to 22 weeks of pregnancy was a sufficient step in our strategy to detect any case of neural tube defects or abnormality that could be seen only during the later stages of pregnancy.
In view of the evidence in favor of first trimester screening, this method has been widely adopted in many European countries. In the Czech Republic, however, the Czech Society of Gynecology and Obstetrics and the Ministry of Health are recommending a National Screening Program based on the second trimester triple test and age at present. This strategy results in a high number of invasive procedures in the country (18–19%) for 80% detection rate.
The aim of our OSCAR clinic is to improve patient satisfaction by reducing the number of visits and minimizing travel costs, patient anxiety and patient stress, while offering maximum utilization of hospital outpatient resources [1, 6, 7, 18, 19, 21].
The setting up of our one-stop clinic required little change to our normal prenatal care practice. Since the beginning of 2005, our first trimester ultrasound scans has been part of the every-day routine scanning and the introduction of fetal nuchal translucency thickness scanning was carried out by reorganizing scanning times and required only minimal additional resources.
Ishraq Dhaifalah, MD, Ph.D.
Department of Medical Genetic and Fetal Medicine
University Hospital
I. P. Pavlova 6
557 52 Olomouc
Czech Republic
ishraq.dhaifalah@gmail.com
Sources
1. Bindra, R., Heath, V., Liao, A., et al.One Stop Clinic for Assessment of Risk for Trisomy 21 at 11-14 weeks: a prospective study of 15,030 pregnancies. Ultrasound Obstet Gynec, 2002, 20, p. 219-225.
2. Cheng, E., Luthy, D., Zebelman, A., et al. A prospective evaluation of a second-trimester screening test for fetal Down syndrome using maternal serum alpha-fetoprotein, hCG, and unconjugated estriol. Obstet Gynecol, 1993, 81, p. 72-77.
3. Cicero, S., Curcio, P., Papageorghiou, A., et al. Absence of nasal bone in fetuses with trisomy 21 at 11-14 weeks of gestation: an observational study. Lancet, 2001, 358, p. 1665-1667.
4. Cuckle, H., Lith, J. Appropriate biochemical parameters in first trimester screening for trisomy 21. Prenat Diagn, 1999, 19, p. 505-512.
5. Dhaifalah, I., Vrbicka, D., Santavy, J. OSCAR (one-stop clinic for assessment of fetal risk): our experience with first trimester screening for chromosomal abnormalities. Ces Gynek, 2006, 71, p. 363-336.
6. Dhaifalah, I., Mičkova, I., Vrbická, D., et al. Advanced maternal age an indication for invasive procedures. Ces Gynek, 2007, 72, p. 181-184.
7. Dhaifalah, I., Santavý, J., Mičková, I., et al. Effectivity of the nasal bone as an ultrasound marker for down syndrom at 11 to 13+6 weeks gestation. Ces Gynek, 2007, 72, p. 19-23.
8. Economides, DL., Whitlow, BJ., Kadir, R., et al. First trimester sonographic detection of chromosomal abnormalities in an unselected population. BJOG, 1998, 105, p. 58-62.
9. Eisenberg, VH., Schenker, JJ. The moral aspects of prenatal diagnosis. Eur J Obstet Gynec Reprod Biol, 1997, 72, p. 35-45.
10. Fost, N. Ethical issues in genetics. Pediat Clin North Amer, 1992, 39, p. 79-89.
11. Gordon, JW. The morality of prenatal diagnosis. Hum Reprod, 1995, 10, p. 767-768.
12. Kellner, L., Weiss, R, Weiner, Z, et al. The advantages of using triple-marker screening for chromosomal abnormalities. Am J Obstet Gynecol, 1995, 172, p. 831-836.
13. MacDonald, M., Wagner, R., Slotnick, R. Sensitivity and specificity of screening for Down syndrome with alpha-fetoprotein, hCG, unconjugated estriol, and maternal age. Obstet Gynec, 1991, 77, p. 63-68.
14. Mori, M., Brambati, B., Tului, L. The current ethical controversy over reproductive medicine: prenatal diagnosis. Hum Reprod, 1995, 10, p. 765-766.
15. Mujezinovic, F., Alfirevic, Z. Procedure-related complications of amniocentesis and chorionic villous sampling: a systematic review. Obstet Gynecol, 2007, 110, p. 687-694.
16. Nicolaides, KH., Cicero, S., Liao, AW. One-stop clinic for assessment of risk of chromosomal defects at 12 weeks of gestation. Prenat Neonat Med, 2005, 5, p.145-154.
17. Pandya, PP., Goldberg, H., Walton, B., et al. The implementation of first-trimester scanning at 10–13 weeks’ gestation and the measurement of fetal nuchal translucency thickness in two maternity units. Ultrasound Obstet Gynecol,1995, 5, p. 20-25.
18. Papageorghiou, AT., Avgidou, K., Bindra, R., et al. One stop clinic for assessment of risk (OSCAR) for trisomy 21 in singleton pregnancies at 11 to 13 + 6 weeks of gestation. Ultrasound in Obstet Gynecol, 2005, 26, p. 375
19. Said, S., Geary, M., Malone, FD. Women’s satisfaction with the OSCAR (One-Stop Clinic Assessment of Risk) model of first-trimester combined screening. Ultrasound Obstet Gynecol, 2007, p. 377-377.
20. Schafer, D., Arnemann, J., Brude, E., Baumann, R. Society must decide about prenatal diagnosis. Hum Reprod, 1995, 10, p. 768-769.
21. Schuchter, K., Hafner, E., Stangl, G., et al. The first trimester ‘combined test’ for the detection of Down syndrome pregnancies in 4939 unselected pregnancies. Prenat Diagn, 2002, 22, p. 211‑215.
22. Snijders, DJM., Noble, P., Sebire, N., et al. UK multicenter project on assessment of risk of trisomy 21 by maternal age and fetal nuchal translucency thickness at 10–14 weeks of gestation. Lancet, 1998, 351, p. 353-356.
23. Snijders, RJ., Thom, EA., Zachary, JM., et al. First-trimester trisomy screening: nuchal translucency measurement training and quality assurance to correct and unify technique. Ultrasound Obstet Gynecol, 2002, 19, p. 353-359.
24. Spencer, K., Souter, V., Tul, N., et al. A screening program for trisomy 21 at 10–14 weeks using fetal nuchal translucency, maternal serum free ß-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol, 1999, 13, p. 231-237.
25. Spencer, K., Spencer, CE., Power, M., et al. Screening for chromosomal abnormalities in the first trimester using ultrasound and maternal serum biochemistry in a one stop clinic: A review of three years prospective experience. Br J Obstet Gynaecol, 2003, 110, p. 281-286.
26. Tabor, A., Vestergaard, CH., Lidegaard, Q. Fetal loss rate after chorionic villus sampling and amniocentesis: an 11-year national registry study. Ultrasound Obstet Gynecol, 2009, 34, p. 19-24.
27. Torloni, MR., Vedmedovska, N., Merialdi, M., et al. Safety of ultrasonography in pregnancy: WHO systematic review of the literature and meta-analysis. ISUOG-WHO Fetal Growth Study Group.Ultrasound Obstet Gynecol, 2009, 33, p. 599-608.
28. Wald, N., Kennard, A., Hackshaw, A., McGuire, A. Antenatal screening for Down’s syndrome. Health Technol Assess, 1998, 2, p. 1-112.
29. Wald, NJ., Cuckle, HS., Densem, JW., et al. Maternal serum screening for Down’s syndrome: the effect of routine ultrasound scan determination of gestational age and adjustment for maternal weight. Br J Obstet Gynaecol, 1992, 99, p. 144-149.
30. Whitlow, BJ., Chatzipapas, IK., Lazanakis, ML., et al. The value of sonography in early pregnancy for the detection of fetal abnormalities in an unselected population. BJOG, 1999, 106, p. 929-936.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inCzech Gynaecology

2011 Issue 4-
All articles in this issue
- Transabdominal ultrasound examination in gynecology
- Ultrasound-guided minimally invasive interventions in gynecologic oncology
- Application of SNP array method in prenatal diagnosis
- The impact of exogenous luteinizing hormone on IVF/ICSI cycles parameters
- Methylation of selected tumor-supressor genes in benign and malignant ovarian tumors
- Current classification of malignant tumours in gynecological oncology – part I
- Biochemical aspects of fetal hypoxia
- Fetal tricuspid regurgitation
- Is it necessary to revise the guidelines on prevention of thrombembolic disease in pregnancy?
- Risk of the prolapse „de novo“ in primary unaffected compartment by using the syntethic mesh in the surgery treatment of the pelvic organe prolapse
- Implementation of One-Stop-Clinic for Risk Assessment of chromosomal abnormalities in the first trimester, evaluating the effectiveness and making it part of our daily practice
- Czech Gynaecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Fetal tricuspid regurgitation
- Application of SNP array method in prenatal diagnosis
- Transabdominal ultrasound examination in gynecology
- Current classification of malignant tumours in gynecological oncology – part I
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

