-
Medical journals
- Career
A clinical, radiographic and histologic observation of human immature permanent tooth after failed revitalization treatment and subsequent root canal treatment
Authors: Š. Belák 1; R. Žižka 1,2; J. Šedý 3,4; M. Přibyl 1; D. Černý 1,5; K. Čížková 6; Z. Tauber 6
Authors‘ workplace: Klinika zubního lékařství, Lékařská fakulta Univerzity Palackého a Fakultní nemocnice, Olomouc 1; Czech Educational and Dental Research Innovative Group, Brno 2; Privátní stomatologická praxe, Praha 3; Ústav normální anatomie, Lékařská fakulta Univerzity Palackého, Olomouc 4; Privátní stomatologická praxe, Hradec Králové 5; Ústav histologie a embryologie, Lékařská fakulta Univerzity Palackého, Olomouc 6
Published in: Česká stomatologie / Praktické zubní lékařství, ročník 120, 2020, 2, s. 37-41
Category: Case Report
Overview
Introduction and goal of casuistic: Regenerative endodontics and hence maturogenesis is currently one of the possible alternatives of permanent teeth treatment with unfinished development. In the case of the success of therapy and the formation of newly produced mineralized tissue, we have limited information on the histological character of such tissue. This case report brings clinical, radiological and histological evaluation of unsuccessful maturogenesis.
Self-observation: The patient who was treated with unsuccessful maturogenesis and subsequent endodontic treatment of the root canal of the upper left permanent middle incisor appeared after 21 months with a horizontal root fracture. The tooth was extracted and examined histologically.
Discussion: Radiological and histological results of previously published case reports are discussed together with possible factors that influence the character and amount of newly produced mineralized tissue.
Conclusion: Newly produced mineralized tissue is similar to cement or bone tissue.
Keywords:
histology – apical periodontitis – immature permanent tooth – revascularization – revitalization – maturogenesis
INTRODUCTION
In the last decade revitalization became popular treatment modality for management of immature permanent teeth with necrotic pulp. In the contrary to calcium hydroxide apexification and calcium silicate apical plug, the revitalization treatment can solve the problem of the cessation of root development and its consequences, mainly the persistent thin and fragile root canal wall [1].
Since introduction of this novel treatment by Iwaya et al. [2] and Banchs and Trope [3], many successful cases have been reported [4]. They showed that a human necrotic immature tooth after revitalization procedure can achieve radiographic apical closure, increase its root length, and thicken the root canal wall. Despite this radiographic gain of newly produced mineralized tissue and root maturation which gave the impression of pulp regeneration, the histological outcome in animal studies showed rather reparation with tissue consisted of cementum, bone or periodontal ligament than regeneration [5]. Even though pulp-like tissue was described in several cases after revitalization [6–8]. It must be emphasized that in these cases the reason for revitalization treatment was irreversible pulpitis [7, 8] or pulp necrosis after immediate replantation due to accidental extraction of immature tooth [6]. In mentioned cases the pulp may survived at least at the apical part. The rest of histological reports of human teeth concur with the animal studies, independent of the revitalization protocol used [9–13]. The gain of newly produced mineralized tissue is part of clinician-based outcomes of revitalization treatment. The primary goals of revitalization treatment are resolving signs and symptoms of disease, survival of functional tooth in mouth and acceptable aesthetic outcome. It has been described that even though the primary goals of treatment have not been achieved in the long term, the continuous maturation has occurred [14, 15]. A case of continued maturation, even without any treatment, has been described in literature [16].
Recently, Žižka et al. [14] have published a case report of failed revitalization treatment where continued maturation has occurred. The patient was 8-year-old girl whose maxillary left central incisor became symptomatic after dental trauma. The tooth was diagnosed with pulp necrosis, symptomatic chronical apical periodontitis and maxillary swelling. The tooth 21 was treated in two visits according to American Association of Endodontists Clinical Consideration for Regenerative Endodontics, current at the particular time. The tooth become symptomatic one year after revitalization treatment and root canal treatment (RCT) has been performed. Three months after RCT, the tooth was asymptomatic, and the periapical lesion diminished. The purpose of this case report was to present a clinical, radiographic, and histological findings of this case 18 months later.
CASE REPORT
The 11-year-old patient came for recall to the Institute of Dental Medicine Faculty of Medicine University Palacky in Olomouc 21 months after the treatment, i. e. the root canal treatment after failed revitalization treatment. The patient has not returned for follow-up since recall three months after root canal treatment, even though the patient and her parents were educated to do so. The patient´s guardian reported that „she was hit during a game by a friend’s hand one month ago and now the tooth is little bit painful.“ The patient´s chief complaint was the presence of slight pain in attached gingiva of maxillary left central incisor (tooth 21). Clinical examination revealed presence of distinct crown discoloration of tooth 21, swelling of attached gingiva and presence of fistula at the same place (Fig. 1A). Tooth 21 was slightly sensitive to percussion and palpation. A subtle mobility of crown of tooth 21 in comparison to adjacent teeth was present. Periodontal probing depths were within normal limits on palatal side of tooth 21 and adjacent teeth, but buccally it was 5 mm. Radiographic examination of tooth 21 revealed complete resolution of the periapical lesion and mild radiolucency around cervical part of tooth 21 (Fig. 1B). Based on our clinical and radiographic findings, a diagnosis of horizontal root fracture at the cervical level was made, and the tooth was evaluated as non-restorable. The tooth 21 was extracted, the fracture was verified (Fig. 1C) and tooth was processed for histological examination.
1. A Clinical photograph taken during follow-up 21 month after root canal treatment; grayish discoloration of tooth 21 is clearly visible;
B intraoral radiograph taken during follow-up 21 month after root canal treatment;
C extracted tooth 21 with oblique fracture,
D aligned preoperative intraoral radiograph of tooth 21;
E aligned postoperative intraoral radiograph of tooth 21 before root canal treatment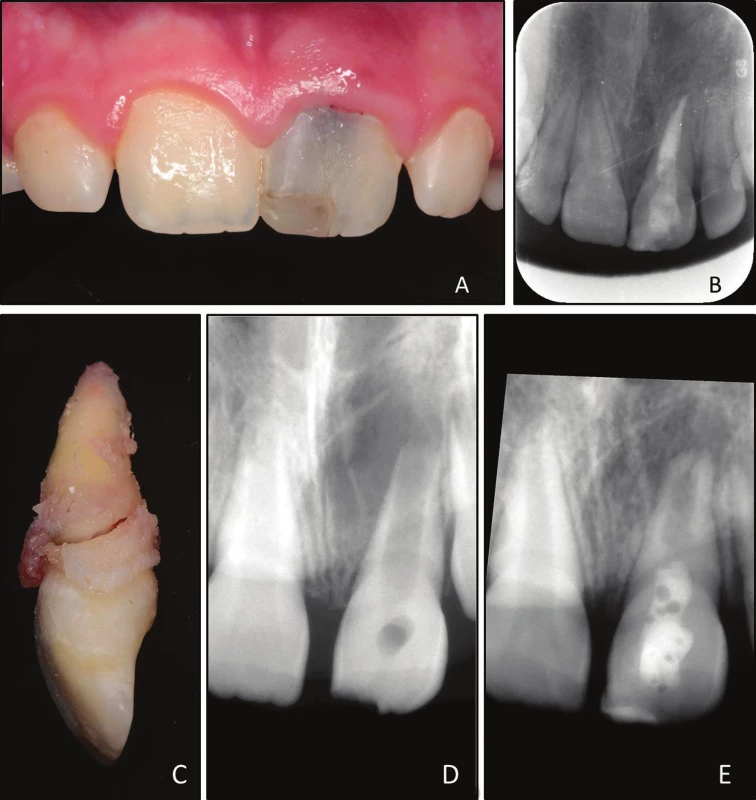
MATERIALS AND METHODS
Radiographic assesment of changes in the root lenght, width and area after revitalization treatment
Images were analysed using ImageJ (National Institutes of Health, Bethesda, MD). The preoperative (Fig. 1D) and 12-month recall after revitalization (Fig. 1E) films were aligned using the TurboReg Plug-In tool. The root length was measured by drawing a line from the most mesial aspect of the cemento-enamel junction (CEJ) as well as the most distal aspect of the CEJ to the centre of the most apical portion of the root canal space. The dentinal wall thickness was measured method described by Bose [17]. The root and pulp space width in apical third was measured, and the remaining dentin thickness was calculated by subtraction of the pulp space from root width. The radiographic root area (RRA) was measured by method described by Flake [18]. Briefly, the RRA is calculated as the difference between total root area and the root canal space. All measurements were done five times, and the values were averaged. The percentage change in the measurements from the preoperative to the recall film was then calculated.
Histological observations
The specimen was immediately immersed in a 10% neutral buffered formalin solution Demineralization was performed in an aqueous solution consisting of a mixture of 22.5% formic acid and 10% sodium citrate for 4 weeks. The endpoint was determined radiographically. At the end of the demineralization process, the root was separated to apical and coronal part with a sharp razor blade circa three mm from apical tip. The radicular segments were washed in running water for 48 hours, dehydrated in ascending grades of ethanol, cleared in xylene, infiltrated, and embedded separately in paraffin according to standard procedures.
With the microtome set at 4–5 μm, longitudinal serial sections were cut on a buccolingual plane for the apical portion until the specimen was exhausted. Special precautions were undertaken to obtain sections passing through the main apical opening. For the coronal portion cross-cut sections were taken in a horizontal plane. Every fifth slide was stained with hematoxylin-eosin for screening purposes and an assessment of tissues formed in the canals. Selected slides were stained with the Taylor modified Brown and Brenn technique for bacteria. Slides were examined under a light microscope.
RESULTS
A comparison of the preoperative and postoperative films showed that a quantifiable increase in radiographic dimensions of the root was observed subsequent to the revitalization treatment. At the 12-month recall, we observed the change in radiographic width of 43.7%, radiographic length of 8.8% and radiographic root area of 44.5%.
Histological observations
Histologically, in the apical third of the root was abundant deposition of newly produced mineralized tissue (Fig. 2). The newly produced mineralized tissue was well mineralized and appeared to resemble cementum-like tissue with occasional lacunae. Only rarely there was present the appearance of tubular dentin, which was not continuous with primary canal dentin. The transition between the canal dentin and newly produced tissue was demarcated by obvious basophilic demarcation line and subsequent absence of dentinal tubules (Fig. 3). The increased root length and thickness were caused by deposition of newly produced mineralized tissue with no signs of tissue ingrowth from periapical area. There were no stainable bacteria in middle and apical third but there was accumulation of inflammatory cells in the soft tissue surrounding the extruded material, probably because of bacterial toxin leaking from the fracture site.
2. Longitudinal histological section of apical part stained with hematoxylin-eozin; there is abundant deposition of newly produced mineralized tissue with no tubular structure which is demarcated by basophilic layer; arrows point to areas of resorption filled with necrotic tissue (magnification 16×) 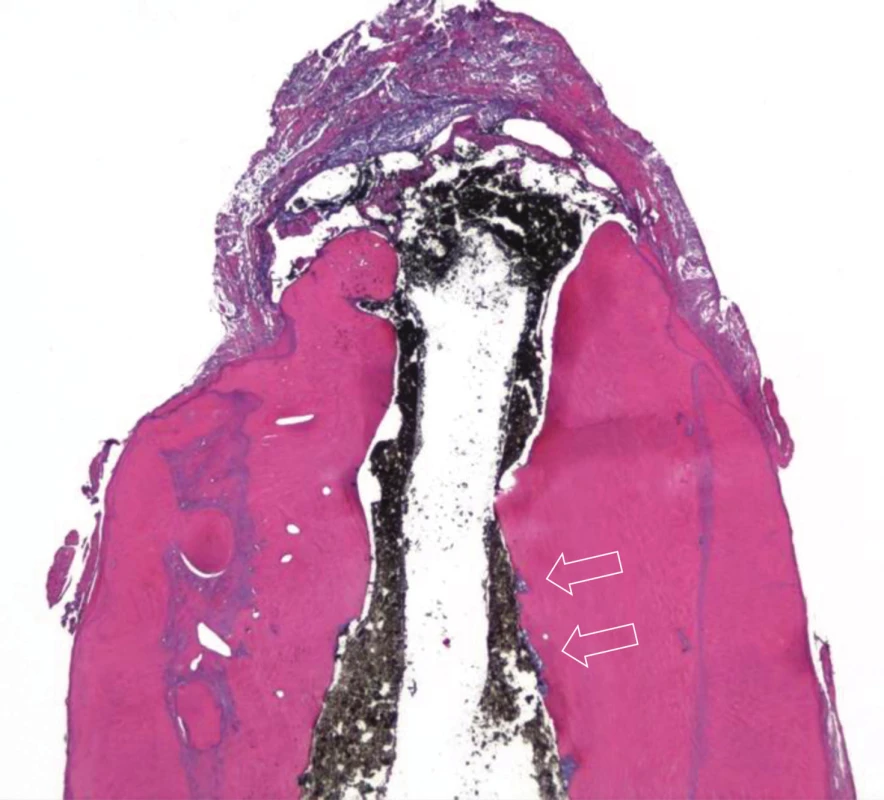
3. Basophilic transition line between dentin (up) and newly produced mineralized tissue with no tubular structure (down) (magnification 400×) 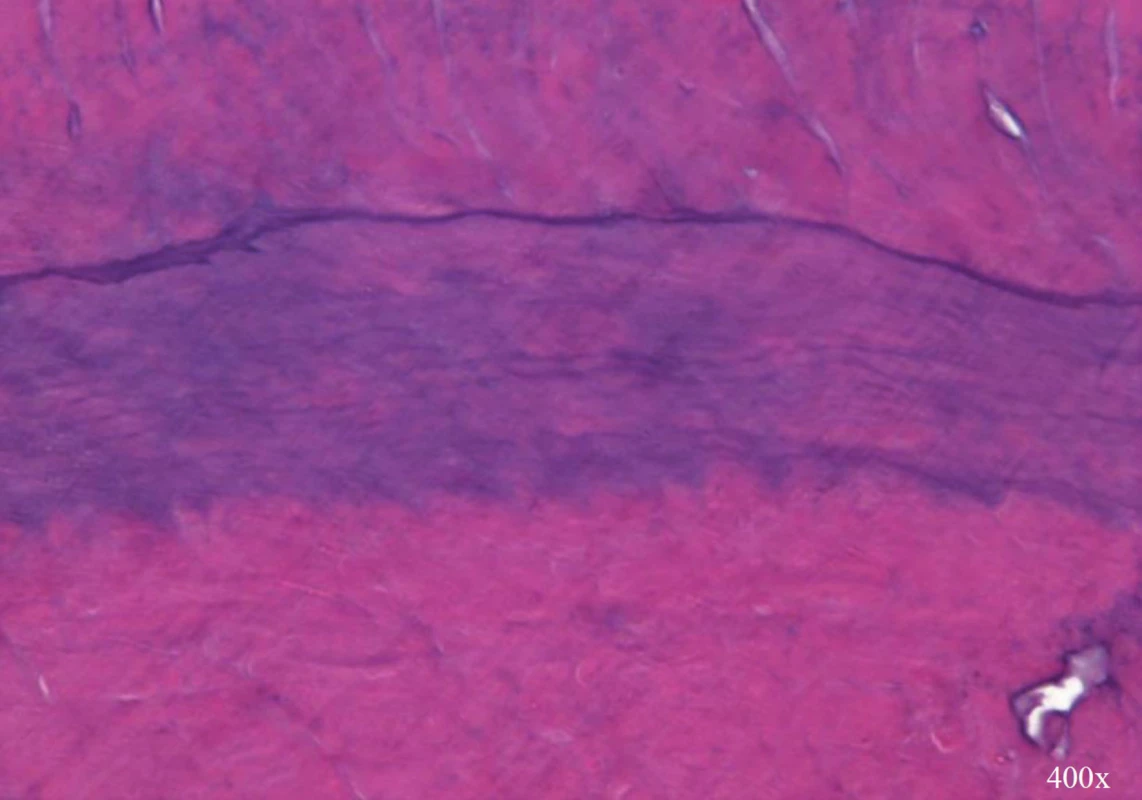
DISCUSSION
The outcome of revitalization treatment have been evaluated clinically and radiographically and only limited amount of histological case reports of a human teeth which underwent revitalization treatment [4, 6–10, 12, 13]. In two case reports the treated teeth were diagnosed with irreversible pulpitis [7, 8] and in one case only the soft tissue was histologically processed [6]. Intracanal calcification was observed in most of the studies [8–13] and were in agreement with animal studies. The etiology of most cases was development anomaly or caries. Only in two cases the etiological factor was dental trauma [7, 10]. It has been showed that revitalization of treated teeth after dental trauma achieve smaller apposition of newly produced mineralized tissue on root canal wall [19, 20]. It was an assumption that it may be caused by damage to Hertwig epithelial root sheath (HERS) cells, which might prevent differentiation of stem cells into desired cementoblasts/odontoblasts. The unpredictable degree of damage to HERS is therefore likely to affect the results of revitalization treatment in terms of deposition of newly produced mineralized tissue [21]. Previous animal studies have showed that the periapical immune response to root canal infection can take place long before the pulp become completely necrotic [22]. Therefore, some teeth with apical periodontitis may have the apical portion of pulp still vital [23, 24]. It has been described that if is validated presence of vital tissue in root canal of immature permanent teeth with apical periodontitis, the treatment of deep pulpotomy can be performed with favourable outcomes [25].
In the apical part of the root canal, there was an abundant apposition of newly produced mineralized tissue. When observed in some area tubules, they are formed by some odontoblasts surviving the trauma and successive procedures. By revitalization treatment we established environment suitable for further development of survived vital tissue. For establishment of this suitable environment the adequate disinfection of root canal is necessary [9]. In literature we can find case reports where repeated revitalization with modified treatment protocol can lead to successful clinical outcome and deposition of newly produced mineralized tissue [26, 27].
The radiographic changes of dimensions were evaluated in two histologic studies [10, 11]. In study by Shimizu et al. only 3-month follow-up X-ray could be used for comparison and change in radiographic length was 2.3% and in width 10.5%. The increased root length was caused by apical deposition of cementum-like tissue [10]. In study by Meschi et al., 11-months follow-up X-ray was assessed and change in radiographic length was 9.5% and a mean increase of 29.1% in root width. At 12-month follow-up X-ray, we assessed a mean increase of 8.8% in root length, 43.7% in root width and 44.5% radiographic root area [11] . In comparison to available studies the amount of newly produced mineralized tissue is rather small [17]. This may be caused by limited time during which the newly produced mineralized tissue was produced.
The failure of retreated central tooth was due to repeated dental trauma. It has been reported that multiple dental trauma episodes affecting same tooth range up to 45%. The risk of sustaining multiple dental injuries is more probable if the first injury occurs before the age of 9 [28] as it happened in our case. We are not able to prevent completely repeated dental trauma, but we should reconstruct tooth with highest fracture resistance as possible. The main issue with reconstruction of immature non-vital teeth would be the large radicular space and weakened walls to cope with shrinkage stress and repeated trauma. There are laboratory studies which indicate that usage of adhesively luted fibre reinforced composite post are improving fracture resistance of immature teeth [29–31] which are corroborated by retrospective study by Ree and Schwartz [32]. All these studies are dealing with adhesive reconstruction of immature teeth with no prior revascularization procedure. No evidence exists about adhesion to the new tissue gained via revascularization procedure. It can only be speculated about histological similarity to root cement.
CONCLUSION
Despite promising radiographic signs of noticeable deposition of mineralized tissue, the tissues formed in root canal space are similar to cementum or bone-like tissue. This tissue may have formed even in the presence of residual bacteria if the local environment allows it. The reconstruction of immature teeth should be done with awareness to possible multiple dental trauma.
ACKNOWLEDGMENTS
The authors deny any conflict of interest related to this study. The authors would like to thank to dr. Domenico Ricucci for preparation of specimen for histological observation.
Corresponding author
MDDr. Radovan Žižka, Ph.D.
Institute of Dental Medicine, Faculty of Medicine, University Palacky and General University Hospital
Palackého 12
772 00 Olomouc
Czech Republic
e-mail: loupaczech@gmail.com
Sources
1. Žižka R, Šedý J. Paradigm shift from stem cells to cell-free regenerative endodontic procedures: a critical review. Stem Cells Dev. 2017; 26(3): 147–153.
2. Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 200; 17(4): 185–187.
3. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004; 30(4): 196–200.
4. Conde MCM, Chisini LA, Sarkis-Onofre R, Schuch HS, Nor JE, Demarco FF. A scoping review of root canal revascularization: relevant aspects for clinical success and tissue formation. Int Endod J. 201; 50(9): 860–874.
5. Altaii M, Richards L, Rossi-Fedele G. Histological assessment of regenerative endodontic treatment in animal studies with different scaffolds: A systematic review. Dent Traumatol. 2017; 33(4): 235–244.
6. Torabinejad M, Faras H. A clinical and histological report of a tooth with an open apex treated with regenerative endodontics using platelet-rich plasma. J Endod. 2012; 38(6): 864–868.
7. Shimizu E, Jong G, Partridge N, Rosenberg PA, Lin LM. Histologic observation of a human immature permanent tooth with irreversible pulpitis after revascularization/regeneration procedure. J Endod. 201; 38(9): 1293–1297.
8. Peng C, Zhao Y, Wang W, Yang Y, Qin M, Ge L. Histologic findings of a human immature revascularized/regenerated tooth with symptomatic irreversible pulpitis. J Endod. 2017; 43(6): 905–909.
9. Lin LM, Shimizu E, Gibbs JL, Loghin S, Ricucci D. Histologic and histobacteriologic observations of failed revascularization/revitalization therapy: a case report. J Endod. 201; 40(2): 291–295.
10. Shimizu E, Ricucci D, Albert J, Alobaid AS, Gibbs JL, Huang GT-J. Clinical, radiographic, and histological observation of a human immature permanent tooth with chronic apical abscess after revitalization treatment. J Endod. 2013; 39(8): 1078–1083.
11. Meschi N, Hilkens P, Lambrichts I, Van den Eynde K, Mavridou A, Strijbos O. Regenerative endodontic procedure of an infected immature permanent human tooth: an immunohistological study. Clin Oral Investig. 2016; 20(4): 807–814.
12. Martin G, Ricucci D, Gibbs JL, Lin LM. Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J Endod. 2013; 39(1): 138–144.
13. Becerra P, Ricucci D, Loghin S, Gibbs JL, Lin LM. Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J Endod. 2014; 40(1): 133–139.
14. Žižka R, Buchta T, Voborná I, Harvan L, Šedý J. Root maturation in teeth treated by unsuccessful revitalization: 2 case reports. J Endod. 2016; 42(5): 724–729.
15. Lin LM, Kim SG, Martin G, Kahler B. Continued root maturation despite persistent apical periodontitis of immature permanent teeth after failed regenerative endodontic therapy. Aust Endod J. 2018; 44(3): 292–299.
16. Whittle M. Apexification of an infected untreated immature tooth. J Endod. 2000; 26(4): 245–247.
17. Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009; 35(10): 1343–1349.
18. Flake NM, Gibbs JL, Diogenes A, Hargreaves KM, Khan AA. A standardized novel method to measure radiographic root changes after endodontic therapy in immature teeth. J Endod. 2014; 40(1): 46–50.
19. Nagata JY, Gomes BPF de A, Rocha Lima TF, Murakami LS, de Faria DE, Campos GR. Traumatized immature teeth treated with 2 protocols of pulp revascularization. J Endod. 2014; 40(5): 606–612.
20. Saoud TMA, Zaazou A, Nabil A, Moussa S, Lin LM, Gibbs JL. Clinical and radiographic outcomes of traumatized immature permanent necrotic teeth after revascularization/revitalization therapy. J Endod. 2014; 40(12): 1946–1952.
21. Nazzal H, Duggal MS. Regenerative endodontics: a true paradigm shift or a bandwagon about to be derailed? Eur Arch Paediatr Dent. 2017; 18(1): 3–15.
22. Yamasaki M, Kumazawa M, Kohsaka T, Nakamura H, Kameyama Y. Pulpal and periapical tissue reactions after experimental pulpal exposure in rats. J Endod. 1994; 20(1): 13–17.
23. Lin LM, Skribner J. Why teeth associated with inflammatory periapical lesions can have a vital response. Clin Prev Dent. 1990; 12(1): 3–4.
24. Russo MC, Holland R, de Souza V. Radiographic and histological evaluation of the treatment of inflamed dental pulps. Int Endod J. 1982; 15(3): 137–142.
25. Tsukiboshi M, Ricucci D, Siqueira JFJ. Mandibular premolars with immature roots and apical periodontitis lesions treated with pulpotomy: Report of 3 cases. J Endod. 2017; 43(9S): 65–74.
26. Chaniotis A. Treatment options for failing regenerative endodontic procedures: Report of 3 cases. J Endod. 2017; 43(9): 1472–1478.
27. Žižka R, Šedý J, Voborná I. Retreatment of failed revascularization/revitalization of immature permanent tooth – A case report. J Clin Exp Dent. 2018; 10(2): 185–188.
28. Glendor U. Epidemiology of traumatic dental injuries – a 12 year review of the literature. Dent Traumatol. 2008; 24(6): 603–611.
29. Dikbas I, Tanalp J, Koksal T, Yalniz A, Gungor T. Investigation of the effect of different prefabricated intracanal posts on fracture resistance of simulated immature teeth. Dent Traumatol. 2014; 30(1): 49–54.
30. Seto B, Chung KH, Johnson J, Paranjpe A. Fracture resistance of simulated immature maxillary anterior teeth restored with fiber posts and composite to varying depths. Dent Traumatol. 2013; 29(5): 394–398.
31. Tanalp J, Dikbas I, Malkondu O, Ersev H, Gungor T, Bayirli G. Comparison of the fracture resistance of simulated immature permanent teeth using various canal filling materials and fiber posts. Dent Traumatol. 2012; 28(6): 457–464.
32. Ree MH, Schwartz RS. Long-term success of nonvital, immature permanent incisors treated with a mineral trioxide aggregate plug and adhesive restorations: a case series from a private endodontic practice. J Endod. 2017; 43(8): 1370–1377.
Labels
Maxillofacial surgery Orthodontics Dental medicine
Article was published inCzech Dental Journal

2020 Issue 2-
All articles in this issue
- Editorial
- International association of paediatric dentistry (iapd) – 50. Výročí založení
- A clinical, radiographic and histologic observation of human immature permanent tooth after failed revitalization treatment and subsequent root canal treatment
- Stability of orthognatic surgery in cleft lip and palate patients
- Healing of endodontic periodontal lesion after non-surgical treatment
- Vagueness and Exactiveness in class II, division 2 diagnostics
- Stomatologie v éře COVID-19
- Doporučená ochrana před přenosem virových infekčních onemocnění v době epidemií, nyní zejména SARS-CoV-2/COVID-19
- Early Childhood Caries: IAPD Bangkok Declaration
- Czech Dental Journal
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Healing of endodontic periodontal lesion after non-surgical treatment
- Stability of orthognatic surgery in cleft lip and palate patients
- Doporučená ochrana před přenosem virových infekčních onemocnění v době epidemií, nyní zejména SARS-CoV-2/COVID-19
- Vagueness and Exactiveness in class II, division 2 diagnostics
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

