-
Medical journals
- Career
The effect of Aronia melanocarpa extract on the phospholipid composition of the rat myocardium during stress
Authors: Volodymyr Shvets; Hanna Maslak; Vadim Davydov; Halyna Berest; Inna Nosulenko
Published in: Čes. slov. Farm., 2022; 71, 98-102
Category: Original Article
doi: https://doi.org/https://doi.org/10.5817/CSF2022-3-98Overview
The research was performed on 80 male rats of the Wistar line. Animals of two age groups were used: adults (10–12 months) and old (22–25 months). The obtained data show that the development of immobilization stress in adult and aged rats are accompanied by the formation of a characteristic complex of changes in the phospholipid composition of the myocardium. Intraperitoneal injection of the chokeberry extract (Aronia melanocarpa) at a dose of 0.2 g/kg 60 minutes before the immobilization has limited stress modulation of myocardial phospholipid composition in aged animals. Thus, the extract of Aronia melanocarpa increases the myocardial resistance to the injury effect of stress.
Keywords:
Myocardium – aging – immobilization – Aronia melanocarpa
Introduction
Stress is an important etiological factor in the development of cardiovascular diseases1–4). It is associated with myocardial ischemia, arrhythmias, coronary, and cardiosclerosis, etc. Therefore, the search for drugs with marked antistress and cardioprotective effects is highly relevant. Herbal drugs are their promising representatives, including medications from Aronia melanocarpa extract. Extracts from this plant contain a wide range of biologically active substances, including bioflavonoids and their representatives – anthocyanidins5–7).
The biological activity of bioflavonoids appears through their significant antioxidant, cardioprotective and anti-inflammatory effects8–12). At the same time, the mechanism of their cardiotropic effects is still far from a final understanding. It can be assumed that it is associated with their influence on the structure and function of biological membranes and, in particular, on the state of the lipid bilayer of cardiomyocyte membranes. Considering the pronounced agedependent nature of the stress damaging effect on the heart, the work aimed to study the impact of Aronia melanocarpa extract on the phospholipid composition of the different age rat myocardium during immobilization stress.
Experimental part
Eighty Wistar male rats were used in the study. Animals were kept in constant environmental conditions (20 °C, 12-h light/dark cycle) and were on a standard laboratory diet. Animals of two age groups were adults (10–12 months) and old (22–25 months). We divided both age groups of animals into four subgroups:
1 – intact rats
2 – control rats that were affected by immobilization stress by fixing them in a dorsal position for 30 minutes
3 – rats that were intraperitoneally administered with the A. melanocarpa extract, 0.2 g/kg, 60 minutes before the immobilization9, 13)
4 – rats that were intraperitoneally administered the dimethyl sulfoxide (DMSO), 175 mg/kg, 60 minutes before the immobilization14)
The effectiveness of stress was controlled pathomorphologically and by measuring the level of glucocorticoid hormones (11-hydroxycorticosteroids) in the blood by the fluorimetric method using a spectrofluorimeter Hitachi MPF-4 (Japan)15).
The study was conducted in accordance with the requirements of the European Council Directive of November 24, 1986, for Care and Use of Laboratory Animals (86/609/EEC)16), and according to the general ethical principles of experiments on animals adopted by the First National Congress of Ukraine on Bioethics (2001), as well as other international agreements and legislation of Ukraine in this area (Protocol No.10, approved 15.10. 2019 by Bioethics commission of Zaporizhzhia state medical university).
Immediately after immobilization, animals were decapitated by guillotine under ether anaesthesia. The heart was removed and washed from the blood. The left ventricular myocardium was isolated and homogenized with 0.1 M sodium phosphate buffer (pH 7.5). 10% of homogenates were used for lipid extraction17). Lipid extracts were fractionated by the thin-layer chromatography (TLC) technique using a CAMAG TLC Scanner 3 (Camag, Switzerland).
Separation of the lipid homogenate fractions was performed using one-dimensional thin-layer chromatography in a hexane : diethyl ether : acetic acid (79.2% : 19.8% : 1%) system on glass plates (10 × 15 cm) with a thin silica gel layer (Laane Kalur, Estonia). According to this division technique, the spot of the phospholipids states at the start.
Phospholipids (PLs) of the lipid extract were fractionated using two-dimensional TLC on “Kieselgel-60” plates (7 × 7 cm) (Merck). We used two different solvent systems for separation – chloroform : methanol : benzene : NH4OH, 25% (58.5% : 27% : 9% : 5.5%) and chloroform : methanol : benzene : acetone : acetic acid – water (58.3% : 25% : 8.3% : 4,2% : 3.3% : 0.9%).
Identification of separated PLs was performed by Rf index that was calculated using CAMAG TLC Scanner 3 software and through using there various dying17).
Isolated PLs were scraped off the plates and extracted with Folch reagent17). The extracts were evaporated and mineralized with HClO4 for 20 min at 180 °C. Inorganic phosphorus in the samples was determined using the Vaskovsky reagent18). The protein concentration in homogenates was estimated by Lowry19).
Statistical analyses were performed using Student’s t-test.
Results and discussion
Figure 1 shows that immobilization does not significantly affect the total amount of phospholipids in the heart homogenates of adult rats.
Fig. 1. The effect of Aronia melanocarpa extract on the total amount of PLs in myocardial homogenates of different age group rats during immobilization stress, μg Pinorg/mg protein (М ± m) 
However, the content of phosphatidylcholine (PC) reduces by 29%, and the contents of lysophosphatidylcholine (LPC) and phosphatidylserine + phosphatidylinositol (PS + PI) were increased by 158%, and 122%, respectively, compared to intact animals (Table 1).
1. Individual phospholipids in the heart homogenates of different age group rats, mcg Pinorg/mg protein (М ± m) 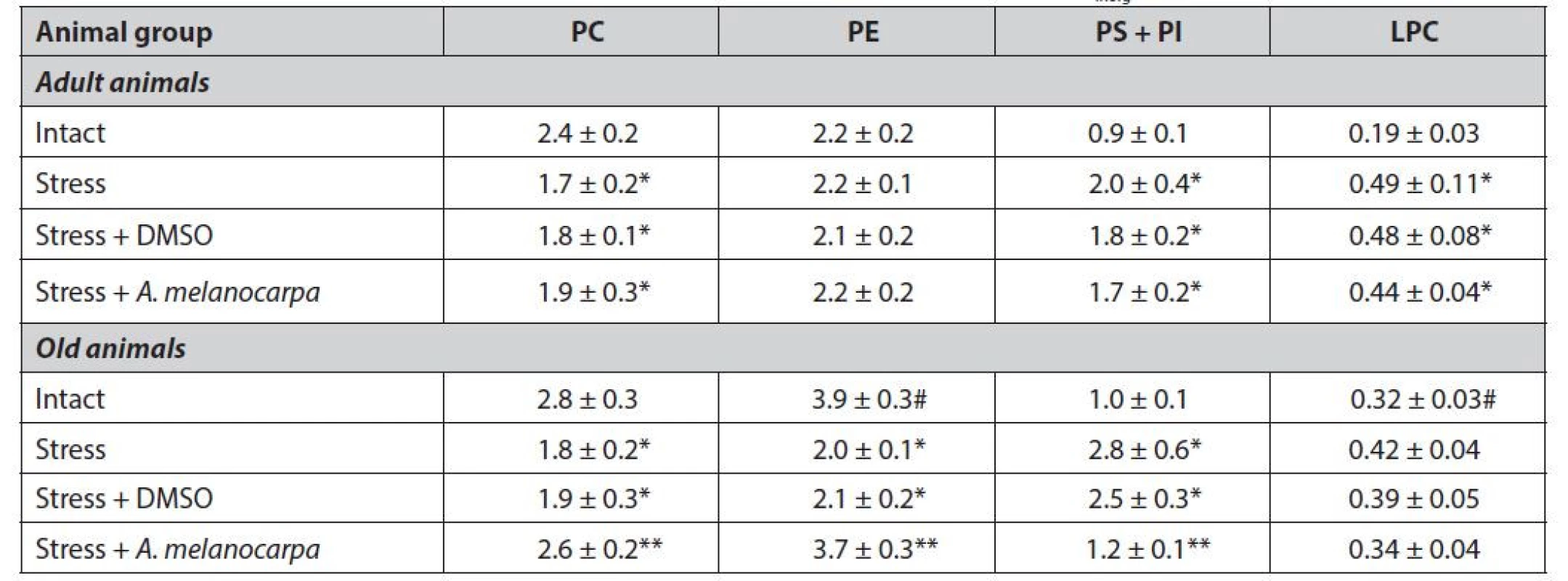
*significantly different relative to the intact group (P < 0.05)
**significantly different relative to the control group (P < 0.05)
#significantly different relative to the adult animals (P < 0.05)The total PLs in the heart homogenates of old animals after immobilization were reduced by 15%, compared to initial values. PC and phosphatidylethanolamine (PE) content was decreased simultaneously by 36% and 49%, respectively, compared with unstressed rats. The content of PS and PI increased 2.8 times in the heart homogenates.
The study results showed that immobilization stress is accompanied by modifying the myocardial phospholipid structure. This modification appeared more in the old animal group.
While assessing a possible reason for the phospholipid composition modification of old rat hearts during stress, it is assumed that the inhibition of PE synthesis is closely linked in the process associated with the use of ATP and PC – by limiting the speed of PE methylation20, 21). The above-mentioned fact can be explained by the age-dependent decreasing of ATP-level in the myocardium of immobilized rats4). In this case, an alternative phospholipids biosynthesis pathway, associated with phosphatidylserine synthesis and the use of CDP-glycerol as the precursor, is activated20, 21). Another cause for the decrease in the level of PC in cardiomyocytes can be an increase in its hydrolysis in the reaction catalyzed by phospholipase A2 22), resulting from an enhanced secretion of adrenaline in the adrenal glands during immobilization stress23–25).
In adult immobilized animals, the occurred modification in the phospholipid composition manifested only by an increase in the hydrolysis of PC in the reaction catalyzed by phospholipase A2 due to hypersecretion of catecholamines during stress. It can be proved by a prominent accumulation of LPC in the heart homogenates.
Preliminary administration of Aronia melanocarpa extract 60 minutes before the immobilization prevented the occurrence of all changes from the PL composition of the old rat heart homogenates and prevised a decrease in the total PLs amount. Administration of A. melanocarpa extract did not have a similar effect on the PL composition of the adult rat myocardium.
It can be assumed that the cardioprotective activity and positive effects on the myocardium phospholipids composition during stress may be associated with the antioxidant properties of A. melanocarpa extract9, 13, 26–27).
To verify that assumption, we assay the effect of preliminary administration of DMSO on the PL composition of the rat myocardium during stress.
Studies pointed out that the myocardial phospholipid composition of both age groups of animals, which were administered DMSO 60 minutes before the immobilization, does not differ from the control group animals. It is an important argument in favor of the fact that changes in the myocardial phospholipid composition of both age group animals during stress were not associated with the stimulation of free radical processes in the myocardium. Therefore, the protective effect of A. melanocarpa extract during stress is not associated with its antioxidant properties9, 13, 26–27).
Thus, the cardioprotective13, 16) effect of A. melanocarpa extract is age-dependent and associated with its action on the phospholipid structure of cardiomyocyte membranes.
One of the possible mechanisms of that effect can be associated with the inhibition of phospholipids hydrolysis in the reaction catalyzed by phospholipase A2. It may also be based on the effect of A. melanocarpa extract components on the secretion of catecholamines and intracellular calcium balance. Moreover, we propose the possibility of a direct effect of A. melanocarpa extract on the synthesis of cardiomyocyte phospholipids. We assume that the direct effect may be associated with the impact of extract components on the biosynthesis of cardiomyocyte phospholipids or with the increase in the efficiency of its energy supplement. However, the detailed mechanism of the protective effect of A. melanocarpa extract on the heart during stress is still not clear. We will focus on their explorations in the future.
Conclusions
The development of immobilization stress in rats is accompanied by appears of age specific changes in the phospholipid composition of the myocardium. Intraperitoneal injection of the chokeberry extract (Aronia melanocarpa) 60 minutes before immobilization at a dose of 0.2 g/kg restricts the stress modulation of myocardial phospholipid composition in old animals.
Conflict of interest: none.
Received November 3, 2021 / Accepted April 27, 2022
Volodymyr Mykolayovuch Shvets
Zaporizhzhya State Medical University Department of Biochemistry Mayakovsky 26, 69035 Zaporizhzhya, Ukraine
e-mail: v.n.shvets@gmail.com
H. Maslak
Dnipropetrovsk Medical Academy of the Ministry of Health of Ukraine Department of Biochemistry and Medical Chemistry, Dnieper, Ukraine
V. Davydov
Pirogov Russian National Research Medical University Department of Biochemistry and Molecular Biology, Moscow, Russia
H. Berest
Zaporizhzhya State Medical University Department of Management and Economics of Pharmacy and Drug Technology, Zaporizhzhya, Ukraine
I. Nosulenko
Zaporizhzhya State Medical University Department of Pharmacognosy, Pharmacology, and Botany, Zaporizhzhya, Ukraine
Sources
1. Song H., Fang F., Arnberg F., Mataix-Cols D., Cruz L., Almqvist C., Fall K., Lichtenstein P., Thorgeirsson G., Valdimarsdóttir U. A. Stress-related disorders and risk of cardiovascular disease: population based, sibling controlled cohort study. BMJ 2019; 365, 370–374.
2. Kivimaki M., Steptoe A. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2018; 15, 215–229.
3. Fujino Y., Tanabe N., Honjo K., Suzuki S., Shirai K., Iso H., Tamakoshi A. A prospective cohort study of neighborhood stress and ischemic heart disease in Japan: a multilevel analysis using the JACC study data. BMC Public Health 2011; 11, 398–404.
4. Davydov V., Shvets V. Adenine nucleotide and creatine phosphate pool in adult and old rat heart during immobilization stress. Gerontology 2002; 48, 81–83.
5. Krga I., Milenkovich D. Anthocyanins: from source and bioavailability to cardiovascular – health benefits and molecular mechanisms of action. J. Agric. Food. Chem. 2019; 67, 1771–1783.
6. Cvetanovic A., Zengin G., Zekovic Z., Svarc-Gajic J., Razic S., Damjanovic A., Mašković P., Mitić M. Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia Melanocarpa’s extract obtained by subcritical water extraction. Food. Chem. Toxical. 2018; 121, 458–466.
7. Staszowska-Karkut M., Materska M. Phenolic composition, mineral content, and beneficial bioactivities of leaf extracts from Black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020; 12, 463–469.
8. Toufektsian M., Lorgeril M., Nagy N., Salen P., Donati M., Giordno L., Mock H., Silke Peterek S., Matros A., Petroni K., Pilu R., Rotilio D., Tonelli C., de Leiris J., Boucher F., Martin C. Chronic dietary intake of plantderived anthocyanins protects the rat heart against ischemia - reperfusion injury. J. Nutr. 2008; 138, 747–752.
9. Cuvorova I. N., Davydov V. V., Prozorovskii V. N., Shvets V. N Peculiarity of the antioxidant action of the extract from Aronia melanocarpa leaves antioxidant on the brain. Biomeditsinskaya Khimiya 2005; 51(1), 66–71.
10. Vendrame S., Klimis-Zacas D. Potential factors influencing the effects of anthocyanins on blood pressure regulation in humans: a review. Nutrients 2019; 11, 1431 – 1439.
11. Middleton E., Kandaswami C., Theoharides T. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000; 52, 673–751.
12. Benavente-Garcia O., Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food. Chem. 2008; 56, 6185–6205.
13. Ipatova O. M., Prozorovskaya N. N., Prozorovski V. N., et al. Chokeberry leaf extract, which has a biological activity, and method of its preparing. Russian Federation patent 2171111; 2001 July 27.
14. Davydov V., Shvets V. The effect of 1,2,4-thiotriazolyl 5-mercaptoacetic acid new derivatives on lipid peroxidation in the heart from adult and old rats during stress. Exp. Gerontol. 2002; 37, 571–573.
15. De Moor P., Steeno O., Raskin M., Hendrikx A. Fluorimetric determination of free plasma 11-hydroxycorticosteroids in man. Acta Endocrinol. 1960; 33, 297–307.
16. European convention for the protection of vertebrate animals used for experimental and other scientific purposes. European Treaty Series – No. 123. Strasbourg: Council of Europe 1986.
17. Kates M. Techniques of lipidology: isolation, analysis and identification of lipids. Elsevier 1986.
18. Martin I., Grotewiel M. Oxidative damage and age-related functional declines. Mech. Ageing. Dev. 2006; 127, 411–423.
19. Lowry O., Rosebrough N., Randall R., Farr A. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951; 193, 265–275.
20. Gibellini F., Smith T. K. The Kennedy pathway – De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010; 62, 414–428.
21. Mc Master CR. From yeast to humans – roles of the Kennedy pathway for phosphatidylcholine synthesis. FEBS Lett 2018; 592, 1256–1272.
22. Pabbidi M. R., Ji X., Maxwell J. T., Mignery G. A., Samarel A. M., Lipsius S. L. Inhibition of cAMP-Dependent PKA Activates β2-Adrenergic Receptor Stimulation of Cytosolic Phospholipase A2 via Raf-1/MEK/ERK and IP - 3-Dependent Ca2+ Signaling in Atrial Myocytes. PLoS One 2016; 15, e0168505.
23. Qteishat A. A., Gabriyanchik M. A., Bokov D. O. Changes in parameters of biochemical and oxidative stress in university students during and after examinations. Cell Stress Chaperones 2021; 26, 811–817.
24. Selye H. The story of the adaptation syndrome. Medgiz 1960.
25. Rudko N. P., Davydov V. V., Shvets V. N, Panasenko A. I. The effect of 1,2,4-thiotriazolyl-5-mercaptoacetic acid on cathecholamine level in the blood of adult and old rats under stress. Exp. Clin. Med. 2001; 11–13.
26. Xu X., Chen X., Huang Z., Chen D., Yu B., Chen H., He J., Luo Y., Zheng P., Yu J., Luo J. Dietary apple polyphenols supplementation enhances antioxidant capacity and improves lipid metabolism in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2019; 103, 1512–1520.
27. Nhuan D., Hwang E. Bioactive compound contents and antioxidant activity in Aronia (Aronia melanocarpa) leaves collected at different growth stages. Prev. Nutr. Food Sci. 2014; 19, 204–212.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2022 Issue 3-
All articles in this issue
- Metronomic therapy in the treatment of cancer
- RNDr. PhMr. Ernest Alt – in memoriam
- Za prof. Ing. Petrom Kovácsom, DrSc. – in memoriam
- Pulsed electric field energy calculation to damage red galangal (Alpinia purpurata, K. Scumm) rhizome slices and its essential oil yield and quality with hydrodistillation
- Study of possible sex features of ramipril and candesartan treatment under experimental arterial hypertension in rats
- The effect of Aronia melanocarpa extract on the phospholipid composition of the rat myocardium during stress
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Metronomic therapy in the treatment of cancer
- The effect of Aronia melanocarpa extract on the phospholipid composition of the rat myocardium during stress
- Study of possible sex features of ramipril and candesartan treatment under experimental arterial hypertension in rats
- Pulsed electric field energy calculation to damage red galangal (Alpinia purpurata, K. Scumm) rhizome slices and its essential oil yield and quality with hydrodistillation
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

