-
Medical journals
- Career
The amino acid and carbohydrate composition of the herb and roots of Smallanthus sonchifolius
Authors: Olga V. Demeshko 1; Elena V. Krivoruchko 1; Victoria I. Volochai 1; Victoria A. Samoilova 1; Vladimir V. Kovalev 2
Authors‘ workplace: National University of Pharmacy, Department of Pharmacy, str. Valentynivska 4, 61168 Kharkiv, Ukraine 1; National University of Pharmacy, Department of Technology of Drug, Kharkiv, Ukraine 2
Published in: Čes. slov. Farm., 2020; 69, 48-51
Category: Short communication
Overview
The free and protein-bound amino acid composition of the herb and roots of Smallanthus sonchifolius was analyzed by HPLC method. Fourteen free and fifteen protein-bound amino acids were determined in yacon herb, and three free and fourteen protein-bound amino acids in the roots. Among the free amino acids, proline (0.44 µg/mg) and aspartic acid (0.12 µg/mg) were dominant in the herb and proline (0.28 µg/mg) in the roots. Among the protein-bound amino acids, aspartic acid (18.58 µg/mg), glutamic acid (16.33 µg/mg) and proline (14.52 µg/mg) prevailed in the herb, and proline (3.14 μg/mg) in the roots. Fructose, sucrose and arabinose were identified in free form in the herb of S. sonchifolius applying gas chromatography-mass spectrometry (GC-MS). The polysaccharide complex was obtained from yacon herb, its yield was 5.13 ± 0.09%. Fructose (3.11 µg/mg) was the only monosaccharide identified in the hydrolysate of the obtained complex.
Keywords:
Smallanthus sonchifolius (yacon) – Amino acids – Carbohydrates – GC-MS HPLC methods
Introduction
The rapidly increasing incidence of diabetes throughout the world1) has led to the expansion of the search for natural sources of biologically active substances to improve the quality of life and enrich the diet of this category of patients. One of the most promising plants in this case is Smallanthus sonchifolius (yacon). This species is the herbaceous perennial plant of the genus Smallanthus (Polymnia) in the Asteraceae family. Yacon, which is a distantly related species to the common sunflower (Helianthus annus) and Jerusalem artichoke (Helianthus tuberosus), was traditionally grown in Andean highland regions of Central and Western America. This vegetable and medicinal plant is introduced in many European countries2). In recent years hypoglycemic and antioxidant activities of yacon3–6) have been investigated by scientists from different countries.
The tuberous roots of yacon have a sweet and crunchy flesh taste. Yacon roots sweetness is caused by free fructose that is predominant monosaccharide in the underground storage organs. The tuberous roots contain 70–80% saccharides, mainly fructooligosaccharides. The underground storage organs of yacon accumulate over 60% (on dry basis) of inulin type (2→1) fructans, mainly oligomers (GF2–GF16). The tuberous roots contain only 0.3–3.7% of protein. Also, the free amino acid composition of yacon roots was studied. The quantity of total free amino acids in the yacon ranged from 147.9 to 341.1 mg/100 g and it differed among the samples from different producing districts. The main free amino acids in yacon were arginine and glutamine in all cultivars7). The herb of S. sonchifolius is a by-product in the harvesting of yacon roots and additionally has a large phytomass that makes the phytochemical study of biologically active substances of this raw material promising. In contrast to the underground part, amino acids and carbohydrates of leaves and stems are not studied enough. In the literature, there are data that the aerial part of this plant contains 15.97% of protein (on dry basis)8).
Previously, we reported about the carboxylic acid composition of the herb and roots of S. sonchifolius9). For further comprehensive research of the biologically active substances of yacon, it is expedient to study the amino acid and carbohydrate composition of these raw materials. It is important to note that amino acids and carbohydrates are substances of the primary biosynthesis of plants and influence the occurrence and development of the general pharmacological effect.
The aim of this study was to evaluate the content of free and protein-bound amino acids in the herb and roots of S. sonchifolius and to determine the qualitative and quantitative content of carbohydrates in the herb of this species.
Experimental part
Plant materials
The objects of the research were yacon herb and root crops harvested at the end of the growing season in the Merefa district of the Kharkiv region in the experimental farm of the Ukrainian Academy of Agrarian Sciences in 2018.
Determination of amino acids
Free protein-forming amino acids in the plant raw material were determined quantitatively after extraction of free amino acids from the plant raw material, and protein-bound amino acids were determined after acid hydrolysis of the preparations, followed by HPLC analysis of the hydrolysates, using pre-column derivatization by 9-fluorenylmethoxycarbonyl chloride (FMOC) and o-phthalaldehyde (OPA) and a fluorescence detector. Each analysis used five determinations.
Samples of the plant raw material were prepared and analyzed as follows:
a) free amino acids: 100 mg of powdered preparation was placed into a vial, treated with 2 ml of 1 N aqueous HCl solution, and held at 50 °C for 3 h in an ultrasonic bath.
b) total amino acids: 100 mg of preparation was placed into a vial, treated with 2 ml of 6 N aqueous HCl solution, and placed into a thermostatic chamber at 110 °C for 24 h.
Then, 0.5 ml of the centrifuged extract/hydrolysate was evaporated in a rotary vacuum evaporator, rinsed three times with distilled H2O to remove HCl, re-suspended in 0.5 ml of distilled H2O, and filtered through a 0.2 μm regenerated cellulose membrane. Amino acids were identified by comparing their retention times with a mixture of amino acid standards (Agilent 5061-3334). The contents of bound amino acids were determined by subtracting the contents of free amino acids from their total contents10–12, 14).
Chromatographic separation was performed on an Agilent 1200 liquid chromatograph (Agilent Technologies, USA) using a Zorbax AAA column (150 mm × 4.6 mm, 3 μm) and mobile phases A (Na2HPO4, 40 mm, pH 7.8) and B (AcCN–MeOH– H2O, 45 : 45 : 10, v/v/v) in gradient mode at a constant flow rate of 1.5 ml/min. The column was thermostated at 40 °C. The pre-column derivatization was carried out in automated programmed mode using FMOC (Agilent 5061-3337) and OPA (Agilent 5061-3335). Derivatized amino acids were detected using a fluorescence detector. The results of studies of amino acids in the herb and roots of yacon are presented in Table 1.
1. The amino acid content in the herb and roots of S. sonchifolius, µg/mg 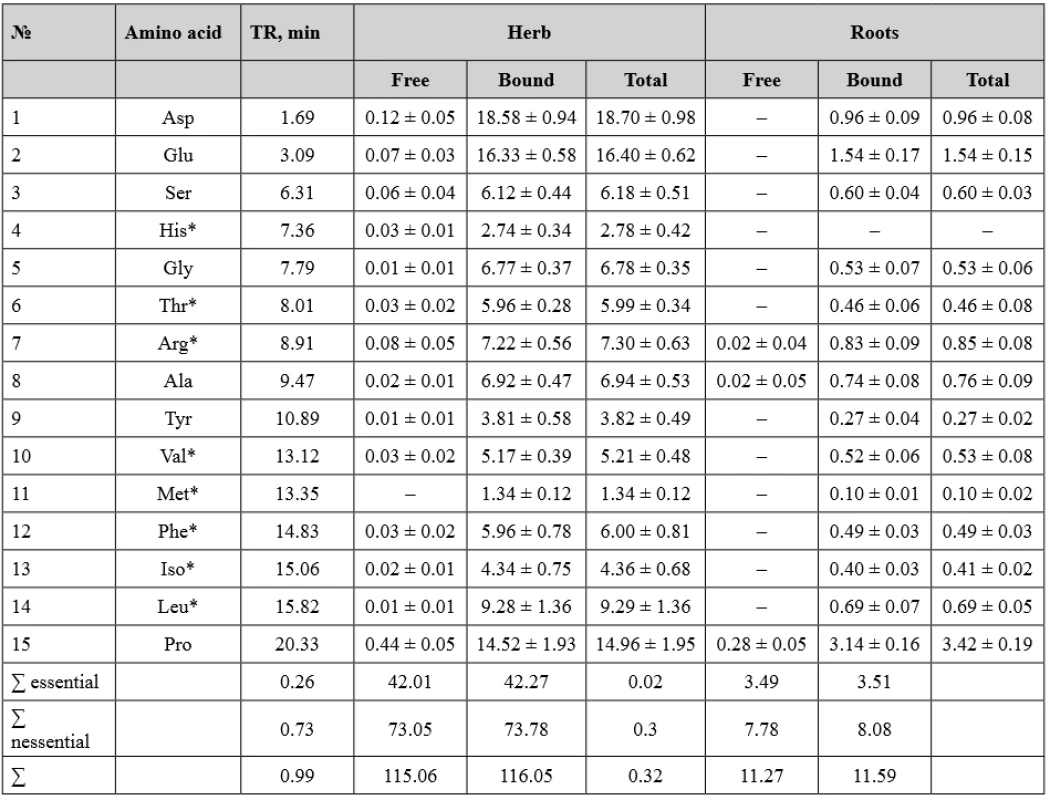
* essential amino acids Determination of the carbohydrates
Obtaining polysaccharides
20.0 g of powdered dry herb of S. sonchifolius was poured with 200 ml of water and heated under reflux in the water bath for 1 hour. The extraction was performed twice. The extracts were combined, concentrated to 20 ml and precipitated with four times the volume of 96% ethanol. The precipitate was separated, washed and dried.
Primary screening of monosaccharides and oligosaccharides, free monosaccharides and oligosaccharides
The vacuum dried supernatant obtained during the centrifugation of polysaccharides precipitate was used for identification of free mono - and oligosaccharides. The dry residue was dissolved in 0.5 ml of 96% ethanol and studied by descending paper chromatography in the acetone-n-butanol-water solvent system (7 : 2 : 1). The monosaccharides and oligosaccharides were detected by spraying the aniline hydrogen phthalate solution followed by heating in a drying cabinet up to 105–110 °C for 5–7 minutes. For identification of monosaccharides, reference solutions of standard samples were used.
The bound monosaccharides and oligosaccharides in polysaccharides
0.2 g of polysaccharides of yacon herb was dissolved in 0.72 ml of a mixture of water and ethanol (1 : 1) and hydrolyzed with the same volume of 20% sulfuric acid in a water bath, controlling the degree of hydrolysis by paper chromatography. The duration of the complete hydrolysis was 5 hours. The hydrolysate was neutralized, vacuum dried. The dry residue was dissolved in 0.5 ml of 96% ethanol and studied by the method described above.
HPLC conditions for qualitative and quantitative analysis
The qualitative and quantitative composition of sugars in plant material were determined by GC/MS based on the extraction of free sugars from plant material and full acid hydrolysis of herbal preparations to determine the total monosaccharide composition, followed by obtaining acetates of their aldonitrile derivatives and their analysis. Each analysis used five determinations.
Sample preparation and analysis of plant raw materials
a) free monosaccharides: 0.5 g of powdered dry plant material was placed in a vial and 5 ml of 80% ethanol was added. Extraction of free monosaccharides was performed in an ultrasonic bath at 80 °C for 4 h. Then, 2 ml of the extract were collected, evaporated to dryness, and re-suspended with 2 ml of an aqueous solution of the internal standard (2.5 mg per sample);
b) monosaccharide composition after hydrolysis of plant raw material: 5 ml of 2 M trifluoroacetic acid was added to 0.5 g of powdered dry raw material; hydrolysis held at 110 °C for 6 h. Then, 2 ml of hydrolyzate was collected, evaporated, and washed with water to remove trifluoroacetic acid. The hydrolysate was then re-suspended with 2 ml of an aqueous solution of the internal standard (2.5 mg per sample).
Chromatographic separation was performed on a chromatograph Agilent 6890N/5973inert (Agilent technologies, USA) using a capillary column HP-5 ms (3 mm × 0.25 mm × 0.25 μm, Agilent technologies, USA). Evaporator temperature was 250 °C, the interface temperature 280 °C. Separation was carried out in the programming mode of the temperature: initial temperature of 160 °C was maintained for 8 min, then raised with a gradient of 5 °C/min to 240 °C. The final temperature was held for 6 min. A sample of 1 μl was injected in a split flow mode 1 : 50. Detection was in the SCAN mode in a range of 38–400 m/z. The flow rate of carrier gas through the column was 1.2 ml/min. Identification was carried by retention time of monosaccharide standards and by the library of mass spectra NIST 02. Quantitative analysis was carried out by adding a solution of internal standard to the test sample.
The following mixture of standard samples was used: monosaccharides: Rhamnose, Arabinose, Xylose, Fucose, Mannose, Glucose, Galactose, Fructose; disaccharide: Sucrose. For the internal standard solution Sorbitol was used13–15). The study of carbohydrates in the herb of the S. sonchifolius is presented in Table 2.
2. Carbohydrates of the herb of S. sonchifolius, mg/kg 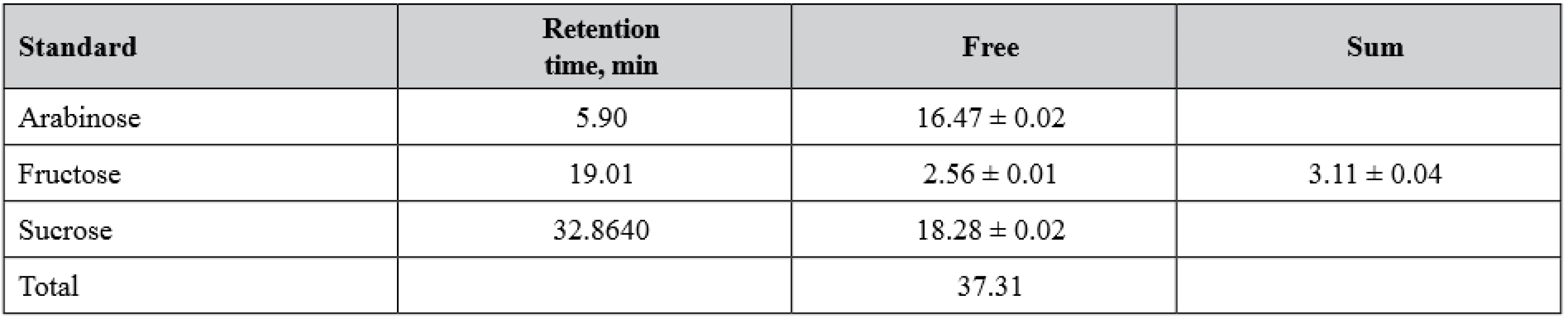
Results and discussion
As a result of the HPLC analysis, the content of 14 free and 15 protein-bound amino acids was determined in S. sonchifolius herb, and 3 free and 14 protein-bound amino acids were detected in roots. Among the free amino acids, Pro (0.44 µg/mg), Asp (0.12 µg/mg) were dominant in the herb. Asp (18.58 µg/mg), Glu (16.33 µg/mg), Pro (14.52 µg/mg) prevailed among the protein-bound amino acids in this raw material. In yacon roots, Pro was found in the highest quantity in free and bound forms.
Primary screening of the monosaccharides and oligosaccharides by paper chromatography showed the presence of fructose, sucrose and arabinose in the free form in the herb of S. sonchifolius. The polysaccharide complex was obtained from the yacon herb, its yield being 5.13 ± 0.09%. Fructose was the only monosaccharide identified during the pre-screening in the hydrolysate of the complex studied.
The chromatography-mass-spectrometry results confirmed the data obtained during primary screening. Sucrose, arabinose, and fructose were determined among free monosaccharides and oligosaccharides of the yacon herb. Sucrose was dominant in this raw material in the free form. In the hydrolysate of the polysaccharide complex, only fructose was detected (3.11 µg/mg).
Conflict of interest: none.
Assoc. prof. Olga V. Demeshko, PhD (∗) • E. V. Krivoruchko • V. I. Volochai • V. A. Samoilova
National University of Pharmacy, Department of Pharmacy
str. Valentynivska 4, 61168 Kharkiv, Ukraine
e-mail: olgademeshko@gmail.com
V. V. Kovalev
National University of Pharmacy, Department of Technology of Drug, Kharkiv, Ukraine
Sources
1. Cho N., Shaw J. E., Karuranga S., Huang Y., da Rocha Fernandes J. D., Ohlrogge A. W., Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018; 138, 271–281.
2. Valentová K., Stejskal D., Bartek J., Dvořáčková S., Křen V., Ulrichová, J., Šimánek V. Maca (Lepidium meyenii) and yacon (Smallanthus sonchifolius) in combination with silymarin as food supplements: in vivo safety assessment. Food Chem. Toxicol. 2008; 46(3), 1006–1013.
3. Baroni S., da Rocha B. A., de Melo J. O., Comar J. F., Caparroz-Assef S. M., Bersani-Amado C. A. Hydroethanolic extract of Smallanthus sonchifolius leaves improves hyperglycemia of streptozotocin induced neonatal diabetic rats. Asian Pac. J. Trop. Med. 2016; 9(5), 432–436.
4. Sousa S., Pinto J., Pereira C., Malcata F. X., Pacheco M. B., Gomes A. M., Pintado M. In vitro evaluation of yacon (Smallanthus sonchifolius) tuber flour prebiotic potential. Food Bioprod. Process. 2015; 95, 96–105.
5. Sousa S., Pinto J., Rodrigues C., Gião M., Pereira C., Tavaria F., Malcata F. X., Gomes A., Bertoldo Pacheco M. T., Pintado M. Antioxidant properties of sterilized yacon (Smallanthus sonchifolius) tuber flour. Food Сhem. 2015; 188, 504–509.
6. Carlsen M. H., Halvorsen B. L., Holte K., Bøhn S. K., Dragland S., Sampson L., Barikmo I. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010; 9(1), 3.
7. Tokiko Mizuno, Koji Yamada. Free amino acid composition in the root of Yacon (Smallanthus sonchifolius). J. Integr. Stud. Diet. Habit. 2007; 18(3), 283–287.
8. Lachman J., Fernández E. C., Orsák M. Yacon (Smallanthus sonchifolius (Poepp. et Endl.) H. Robinson) chemical composition and use-a review. Plant Soil Environ. 2003; 49(6), 283–290.
9. Demeshko O. V, Krivoruchko E. V, Samoilova V. A, Romanova S. V. Gas chromatography – mass spectrometry studies of the component composition of carboxylic acids of the herb and roots crops of Smallanthus sonchifolius. Ces. slov. Farm. 2018; 67(4), 160–163.
10. Demeshko O. V, Kovalyov V. N, Kovalyov S. V. The study of amino acid composition in the herb of Оnobrychis arenaria. Ukr. Biopharm. J. 2015; 2, 75–78.
11. Henderson J. W., Ricker R. D., Bidlingmeyer B. A., Woodward C. Agilent1100 HPLC. Technic. Note 1999; 5980(1193E), 10.
12. Molnár-Perl I. Advancement in the derivatizations of the amino groups with the o-phthaldehyde-thiol and with the 9-fluorenylmethyloxycarbonyl chloride reagents. J. Chromatogr. B. 2011; 879(17–18), 1241–1269.
13. Jambor A., Molnar-Perl I. Quantitation of amino acids in plasma by high performance liquid chromatography: simultaneous deproteinization and derivatization with 9-fluorenylmethyloxycarbonyl chloride study. J. Chromatogr. A. 2009; 1216(34), 6218–6223.
14. Karpyuk U. V., Kislichenko V. S., Gur’eva I. G. HPLC Determination of free and bound amino acids in Bryonia alba. Chem. Nat. Compd. 2015; 51(2), 399–400.
15. Otter D. E. Standardised methods for amino acid analysis of food. Br. J. Nutr. 2012; 108(2), 230–237.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2020 Issue 1-
All articles in this issue
- Metal complexes in medicine and pharmacy – the past and the present II
- K životnému jubileu pani doc. RNDr. Zuzany Vitkovej, CSc.
- Životné jubileum prof. RNDr. Daniela Grančaia, CSc.
- Zomrel Alois Borovanský
- Pharmacokinetic aspects of beta-lactam antibiotic therapy in intensive care unit patients: A one-center experience with TDM
- Double-coated pellets with semipermeable ethylcellulose coating for detection of cholinesterase inhibitors
- Development and uniformity evaluation of low-dose medicated chewing gums prepared by compression method
- Investigation of thioctic acid, magnesium stearate and pyridoxine hydrochloride compatibility
- The amino acid and carbohydrate composition of the herb and roots of Smallanthus sonchifolius
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Metal complexes in medicine and pharmacy – the past and the present II
- Pharmacokinetic aspects of beta-lactam antibiotic therapy in intensive care unit patients: A one-center experience with TDM
- Investigation of thioctic acid, magnesium stearate and pyridoxine hydrochloride compatibility
- Zomrel Alois Borovanský
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

