-
Medical journals
- Career
(Meth)acrylate copolymers of Eudragit® type in oral tablet technology
Authors: Martina Naiserová 1; Kateřina Kubová 1; Jakub Vysloužil 1; Jurga Bernatoniene 2; Iosif Brokalakis 1; David Vetchý 1
Authors‘ workplace: Department of Pharmaceutics, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences Brno 1; Department of Drug Technology and Social Pharmacy, Faculty of Pharmacy, Lithuanian University of Health Sciences, Kaunas, Lithuania 2
Published in: Čes. slov. Farm., 2019; 68, 183-197
Category: Rewiev article
Overview
This review focuses on the characterization of (meth)acrylate copolymers – Eudragit®, describing their thermal treatment behaviour, possible interactions between cationic and anionic polymers, incompatibilities related to Eudragits® and their use in the pharmaceutical technology of oral tablets. In summary, Eudragit® copolymers are divided into soluble ones, insoluble ones and a combination of these two types. The combination of soluble and insoluble poly(meth)acrylate gave a new type of polymer, Eudragit® FL. In oral tablet technology, Eudragits® are widely used in matrix tablets, either alone or in combination, where they mainly provide sustained drug release. To a lesser extent, Eudragits® are used in gastroretentive systems. Moreover, Eudragits® are also of great importance in coated tablets technology, where these enteric polymers provide specific drug targeting to certain parts of the digestive tract, mainly to the small intestine or colon. Important systems such as CODESTM and MMX® technology are mentioned. Last but not least an overview table of currently available oral medicinal products on the Czech market, where at least one of the Eudragits® was used as a film-forming agent, is included.
Keywords:
Eudragit® – matrix tablets – floating tablets – film-coating tablets – acidoresistant tablets – burst effect – prolonged drug release – colon drug delivery
Introduction
Eudragit® was a trademark of Rohm GmbH & Co. KG. in Darmstadt in Germany, which was first marketed in 19531). The name Eudragit® was derived from the Greek word “Εύ” (meaning good) and the German word “dragieren” (meaning sugar coating), combined together to indicate an “excellent functional coating”2). In 1955, the first poly(meth)acrylates for pH-controlled release became commercially available (Eudragit® L and Eudragit® S as organic solutions in isopropyl alcohol). These were followed in 1959 by a polymer with protective and masking function intended for immediate-release applications (Eudragit® E). Only ten years later poly(meth)acrylates for time-controlled drug release were launched on the market. Eudragit® research and manufacturing today takes place at the Evonik Industries sites in Darmstadt, Weiterstadt and Worms1).
Eudragits® are synthetic acrylic copolymers that are derived from esters of acrylic and methacrylic acid, by free radical polymerization3, 4). Their physicochemical properties are determined by their functional groups (Fig. 1), and their solubility in the digestive tract results from monomer variations and polymerization reaction3). Eudragits®, owing to their stability in the presence of digestive enzymes and body fluids, are known as non-biodegradable polymers. Originally, they had been made as solutions of organic solvents, however in the course of years the product offer was expanded to a wide range of different physical forms. They are available as aqueous dispersions (marked D), granules (100), powders (PO) and organic solutions. Water dispersions greatly contribute to reducing the burden on the environment5, 6).
1. Various Eudragit® copolymers, their respective groups (R) and properties1) 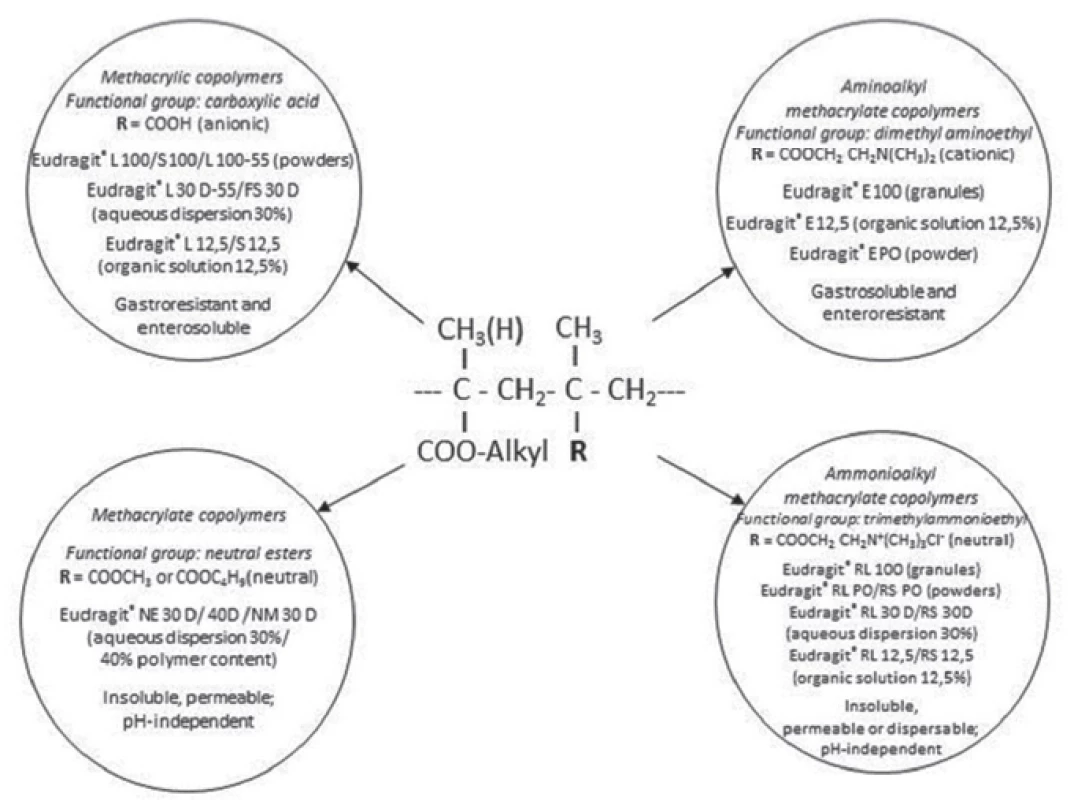
Eudragit® polymers have excellent film-forming properties, which are combined with high flexibility, low water vapour transition rates, high pigment uptake and a wide variety of available products4, 7). Due to the above, Eudragit® main application in the pharmaceutical industry is for the development and manufacturing of solid dosage forms that achieve therapeutically appropriate release profiles (modified release applications); protection of the drug from surrounding influences such as humidity or light; and for the conventional film coating3, 4). Furthermore, they are used to avoid interactions between the core material and the coating as well as to increase a patient’s compliance4).
Characterisation and types of Eudragit® polymers
(Meth)acrylate copolymers can be subdivided into the soluble pH-dependent type and the insoluble pH-independent type. The polymers with pH-dependent behaviour, such as anionic copolymers Eudragit® L, S and FS, are mainly used in gastro-resistant dosage forms, whereas the pH-independent types, such as Eudragit® NE, NM, RL and RS, are widely used for sustained release dosage forms8, 9). All Eudragit® polymers, except the acid soluble Eudragit® E, provide modified release effects10).
Soluble pH-dependent poly(meth)acrylates
Soluble poly(meth)acrylates (Eudragit® E, L, S, FS) exhibit a certain solubility in digestive fluids by salt formation. These polymers have acidic or alkaline groups which enable a pH - dependent release of the API (Active Pharmaceutical Ingredient)4). They are applied in taste masking, enteric formulations as well as in controlled drug release in all sections of the intestine. Namely, Eudragit® L and S are suitable for enteric coating, whereas FS is appropriate rather for colon delivery. Eudragit® E polymer is gastrosoluble and enteroresistent1).
Eudragit® E
This type consists of dimethylaminoethyl methacrylate, methyl methacrylate and butyl methacrylate in a 2 : 1 : 1 ratio. Introduction of the cationic monomer ensures its solubility below pH 5 by salt formation with anions present in gastric fluid2).
Eudragit® E is provided as a micronized powder for aqueous dispersion named Eudragit® E PO, in a granule form for organic solution preparation called Eudragit® E 1009), and in a 12.5% organic solution named Eudragit® E 12.511). Eudragit® E is naturally amorphous with a glass transition temperature of approximately 48.6 °C, and it is perfectly applicable in hot melt extrusion for the preparation of solid dispersions due to being thermoplastic12). Originally, Eudragit® E was widely used for taste and odour masking6). Later, Eudragit® E was reported to be effective in enhancing the dissolution rate of poorly water-soluble drugs and improving the physical stability of amorphous solid dispersions against humidity stress13–15).
Eudragit® L, S, FS
Due to the presence of enterosoluble carboxylic groups, Eudragit® L, S, and FS are anionic pH-dependent polymers suitable for gastro-resistant formulations1, 16) (Table 1). These polymers are soluble at pH above 5.5. Although dissolution pH is mainly determined by the percentage of methacrylic acid, the presence of ester comonomers also contributes11, 17). As coating polymers, they ensure the site-specific drug release in the gastrointestinal tract (GIT), enabling the targeting to specific enteric areas, such as the upper intestine, the ileum, and the colon18).
1. Eudragit® polymers used for GIT targeted drug delivery and their respective properties19) 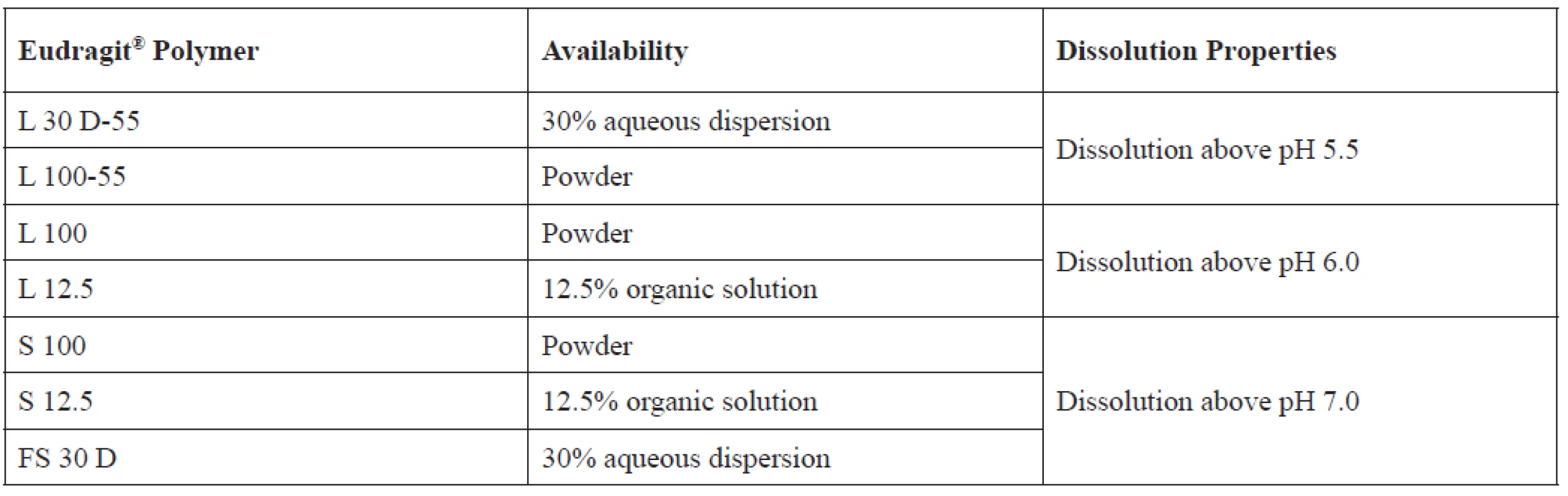
Insoluble pH-independent poly(meth)acrylates
Insoluble but permeable neutral or cationic copolymers, by contrast, enable prolonged release applications through pH-independent swelling and diffusion-controlled dissolution4). These polymers include Eudragit® NE and NM, which have neutral groups (neutral ester groups) and Eudragit® RL and RS, which have alkaline functional groups (trimethylammonioethyl groups)20). Eudragit® NE and NM have an average permeability whereas RL and RS are polymers with a high and a low permeability, respectively2). They are commonly used for the development of delayed and sustained drug release formulations (Table 2)19). In coating technology, Eudragit® NE and NM polymers do not require addition of plasticizer19).
2. Eudragit® polymers used in sustained release formulations and their respective properties19) 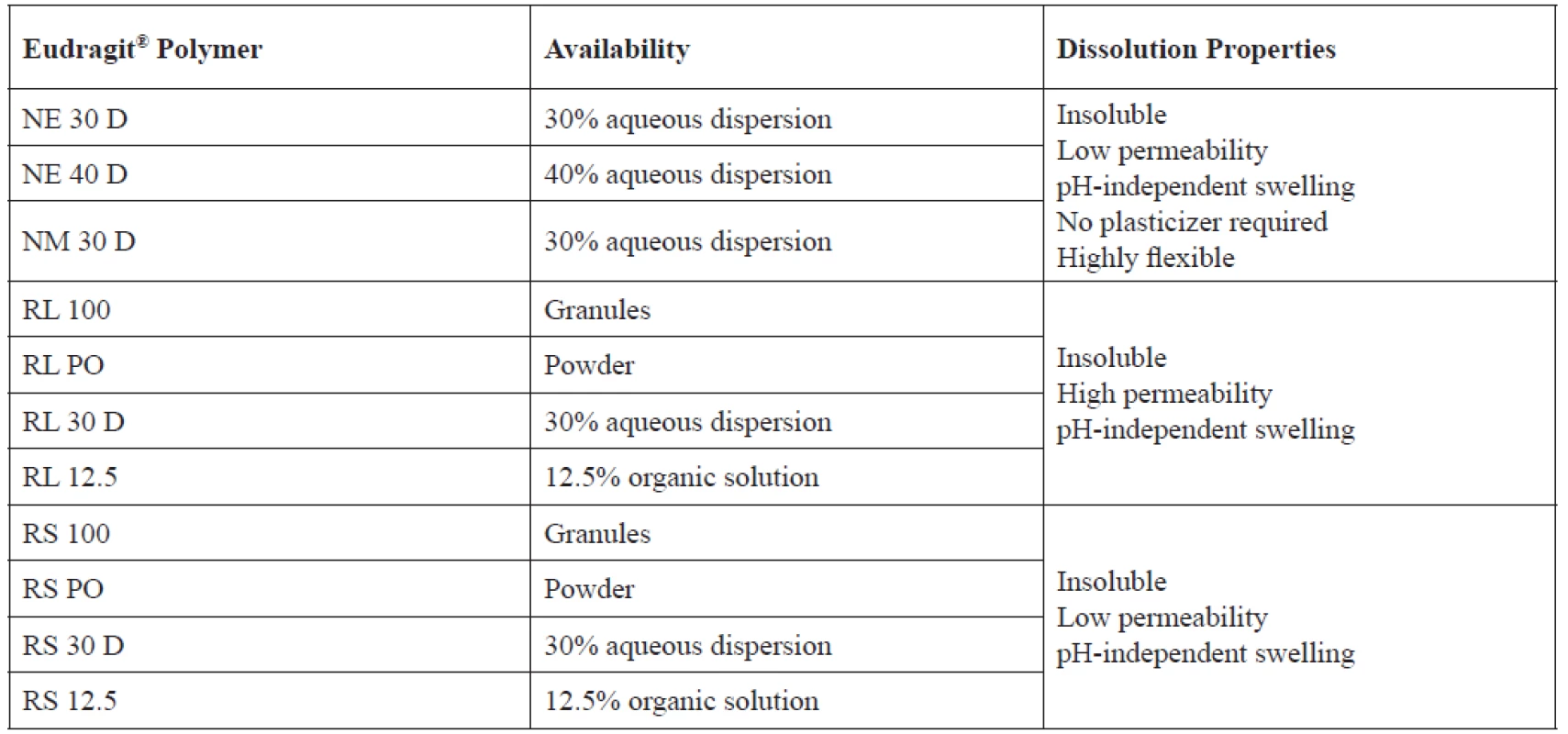
Eudragit® NE, NM
These polymers have no reactive functional group, since all carboxylic groups are esterified. They are mainly used in coating preparations in which the drug release is controlled by the film thickness. Both of these polymers have a minimum film forming temperature of 5 °C11). Depending on the drug solubility, 5–20% of dry polymer substance based on tablet weight is usually sufficient to control drug dissolution and release over a 6–8 h period4). Due to insolubility, they can be utilized in the formulation of inert matrix systems. Their release mechanism is controlled by diffusion and gives straight lines in the plot of dissolved drug versus square root of time2). Table 3 summarizes some important characteristics of the Eudragit® NE and NM water dispersions.
3. Characteristics of Eudragit® NE 30 D and NM 30 D1) 
EA – ethyl acrylate, MA – methyl acrylate Eudragit® RL, RS
Cationic poly(meth)acrylates typically consist of ethyl acrylate, methyl methacrylate, and a low content of methacrylic acid ester with quaternary ammonium groups. The ammonium groups are present as salts, which increase the polymer swelling in aqueous media, thereby making the polymers more permeable20). Quaternary ammonium groups dissociate completely at pH 1–8. Since both are insoluble during gastrointestinal transit, these polymers are employed in matrix formulations in which drug release is driven only by diffusion. Eudragit® RL is highly permeable, while Eudragit® RS is only slightly permeable. They can be mixed in any ratio to adjust the intermediate permeability and obtain a specific release pattern2). Since Eudragit® RL contains more quaternary ammonium groups, it is more hydrophilic and permeable, resulting in accelerated drug release, its features are dominant in these combinations4). Due to the above, the quantity of Eudragit® RS is usually much higher in order to achieve extended release effects8). Typical RS : RL ratios are 95 : 5, 90 : 10 or 80 : 202). Variations in polymer ratio and coating quantity result in maximum flexibility and ability to design the desired formulation. The combination of Eudragit® RS PO with Eudragit® RL PO has been shown to have improved release rates of drug from tablets compared to formulations with Eudragit® RS PO only21–23).
Combination of soluble and insoluble poly(meth)acrylate
Eudragit® FL
Eudragit® FL is a new product combining the advantages of two widely used polymers (Eudragit® L and Eudragit® NM). Due to its dominating gastro-resistant property, Eudragit® L 30 D-55 serves as the lead polymer, while Eudragit® NM 30 D provides higher levels of flexibility. Eudragit® FL exhibits a very low acid value, which makes it highly compatible with a broad range of APIs such as acid-sensitive small molecules or peptides. It enables plasticizer-free formulations, making it ideal for the compression of coated multiparticulate systems. It can be sprayed to form a smooth coat with excellent adhesion. The advantages of the coating process include time reduction (by up to 70%), lowering manufacturing costs, total required polymer amount, avoidance of sticking, and increased drug loading24).
IPEC
Inter-Poly-Electrolyte Complex (IPEC, IPC or PEC) results from the interaction of the cationic polymer (chitosan25)) with the anionic polymer (carbomer26), sodium alginate27)). The preparation of IPEC is a lengthy process. The complex is formed by precipitation in aqueous or buffer solution, followed by isolation, drying, grinding and sifting (to the desired particle size) to obtain a mixture intended for direct compression of the tablets. Some of Eudragits® can be considered as polycations (Eudragit® types E, RL, RS) and others as polyanions (Eudragit® types L, S) (Fig. 2)27, 28). It is known that the stoichiometry of both components in binary IPEC depends on the pH values of the media, ionic strength, concentration, and sometimes on the order of mixing. IPECs can extend drug release either by reducing swelling and erosion of polymeric matrix or by modulating microenvironmental pH. The advantage of this complex is a slow release of a wide range of drugs due to ion-hydrogen interactions within the system. The swelling of the matrix IPEC system is controlled by the relative ratio and composition of the individual components determining the amount of hydrophilic and hydrophobic parts in the IPEC structure27).
2. Interpolyelectrolyte complex between Eudragit® E and L29) 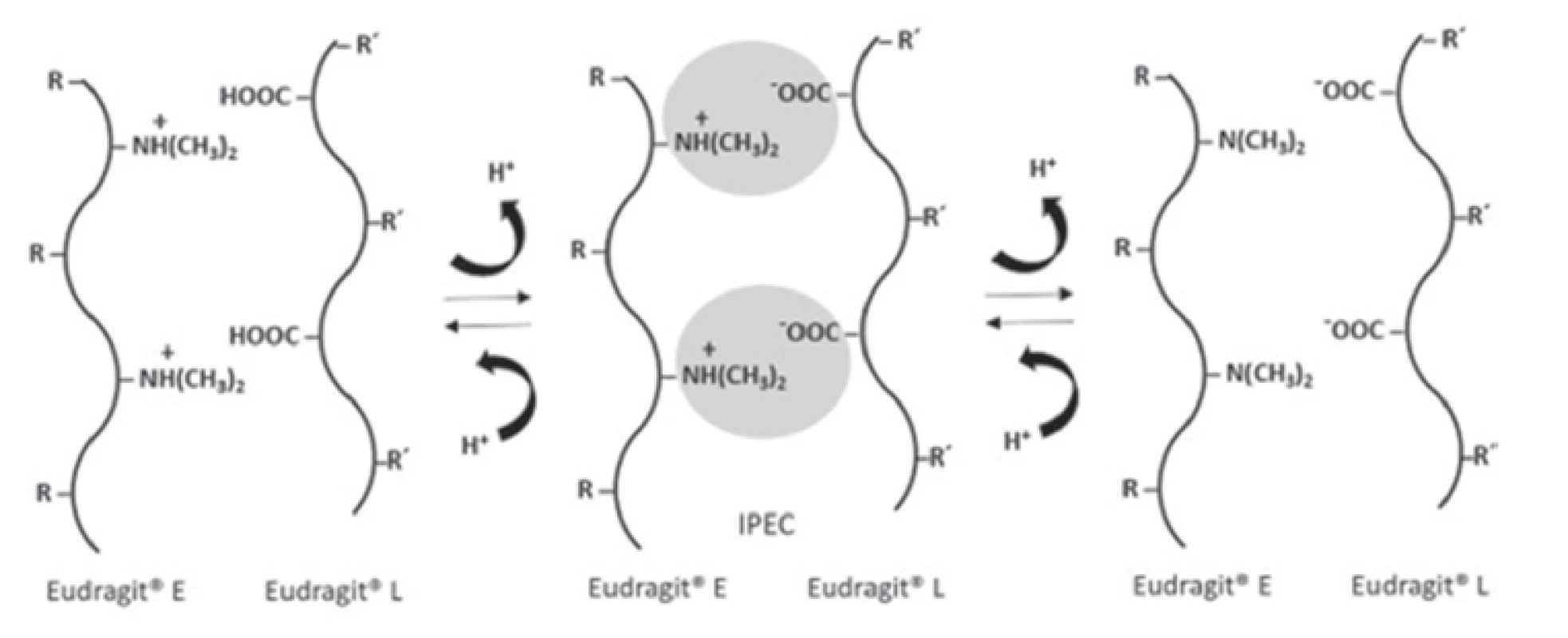
Ofokansi et al.30) prepared ibuprofen tablets from IPECs formed between Eudragit® RL 100 and chitosan. An electrostatic interaction between the carbonyl (–CO–) group of Eudragit® RL 100 and amino group of chitosan of the tablets formulated with the IPECs was capable of preventing drug release in the stomach and small intestine and helped colon-targeted drug delivery of ibuprofen in the treatment of inflammatory bowel diseases. Kinetic analysis of drug release profiles showed that the systems predominantly released ibuprofen in a zero-order manner.
Another approach can be seen in utilizing physical mixtures of polymers in controlling drug release. Li et al.28) evaluated the combination of chitosan as a cationic and Eudragit® L as an anionic polymer. They utilized physical mixture of these polymers and observed the in situ formation of an IPEC-based film on the tablet surface. This novel structure could be only formed under specific dissolution conditions.
Thermal treatment of Eudragit® polymers
The term “thermal treatment means” a process in which the polymer is heated to a certain temperature for a specified time period (curing time). Thermal treatment of amorphous polymers usually requires the heating of a polymer to temperatures above the glass transition temperature (Tg) (Table 4)31). The glass transition temperature, a fundamental property of an amorphous polymer, is the temperature at which a polymer undergoes a change from a hard, brittle glassy state to a soft, flexible rubbery state. It is well known that excipients that lower the Tg, such as plasticizers, make polymers less brittle and more flexible. The thermal treatment often influences the mechanical properties of polymers32).
4. Glass transition intervals of Eudragit® different grades17) 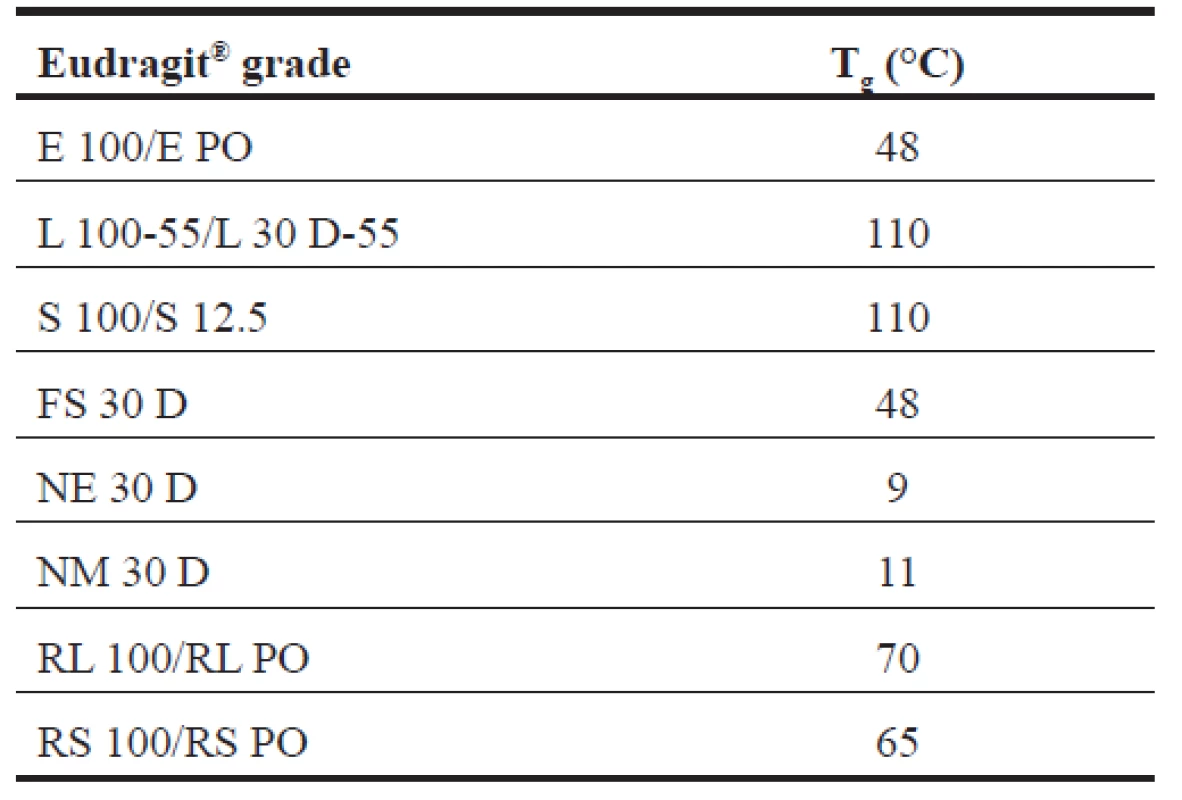
Thermal treatment of the polymeric matrices above the Tg could significantly alter the drug release. The duration of this process is also an important factor31). The effects of thermal treatment of tablets on the drug release rate were attributed to the polymer chain movement and inter-diffusion of the Eudragit® polymer chains in the tablet matrix, which causes a better coalescence of the polymer particles to form a fine network and a matrix with lower porosity and higher tortuosity. In this way, the drug is surrounded and entangled by the polymer network, resulting in a restricted leaching of the drug33).
Azarmi et al.31) evaluated thermal treatment as a process to extend drug release from Eudragit® RL, RS based matrix tablets. A prolonged release was observed when the heat treatment was above the Tg of the polymers. The duration of the heat treatment came up to be an important factor and the results showed that an increase in the duration of the heat treatment resulted in a reduced drug release. The heating of the matrices over 24 hours had no significant effect on the release rate of indomethacin. Furthermore, heat treatment of the matrices above the glass transition temperature of the polymer had no significant effect on the tensile strength of tablets33). In a subsequent study, the same scientific group concluded that the prolonged release of API was due to the movement and redistribution of Eudragit® RS polymer chains in the matrix tablet structure.
On the other hand, the study of Dave et al.34) brought different results. The thermal treatment at 75 °C for 5 hours increased breaking force and decreased the drug release rate only for theophylline/Eudragit® RS PO matrices. For matrix tablets containing Eudragit® RL PO or a mixture of RL PO/RS PO it failed to impart an extended-release property to the tablets. This can be attributed to the relatively more hydrophilic nature of Eudragit® RL compared to Eudragit® RS. Although thermal treatment may result in the formation of a strong polymer matrix structure, the high level of permeability to aqueous media resulted in the rapid disintegration of the tablets and releasing of the drug. For the tablets containing Eudragit® RS PO, theophylline release decreased proportionately with an increase in the thermal treatment temperature and concentration of plasticizer in the matrix.
Several studies have described the thermal treatment of insoluble Eudragit® polymers usually resulting in extended drug release from Eudragit® tablets due to a decrease in matrix porosity. It cannot be forgotten that the copolymer incorporated into a commercial product has a different Tg value than, for example, a blend with other matrix tablet components. Therefore, the thermal treatment of Eudragit® NE and NM matrices having Tg values very low is also important35).
Incompatibilities associated with Eudragits
Although Eudragit® polymers are considered as chemically stable, there are studies documenting polymers incompatibilities with some substances. The interactions of Eudragit® E with histamine-H2 receptor antagonists, Eudragit® L with proton pump inhibitors, and Eudragit® RL, RS with nonsteroidal anti-inflammatory drugs (NSAIDs) are known.
In Sarisuta et al.37) the effect of polymeric Eudragit® E 100 film on coated tablets with ranitidine hydrochloride was investigated. Due to the mild interaction, such as hydrogen bonding, between protonated tertiary amino group of ranitidine hydrochloride and functional group of the Eudragit® E 100, the adhesive force between the film and the tablet surface decreased36). The interaction between Eudragit® L and proton pump inhibitor omeprazole is also known. The acidic nature of the polymer leads to the degradation of omeprazole. A possible solution is to use an inert subcoating layer, which separates the omeprazole containing core from the enteric coating.
Pignatello et al.38) studied the mechanisms of interactions between Eudragit® RL 100 and RS 100 polymers with three NSAIDs: diflunisal, flurbiprofen, and piroxicam. In particular, the incorporation and release of NSAIDs from Eudragit® RL and RS polymers was shown to be strongly dependent on the acidic nature of these drugs, which allows chemical interactions, physical interactions, or both to occur (zwitterionic adducts, ion pairs, ion-exchange resin behaviour) with the ammonium group of polymers.
Physical and chemical interaction of ibuprofen with Eudragit® RL has been reported. Incompatibility occurs due to the electrostatic interactions and/or hydrogen bonding (carboxylic group of ibuprofen) with the quaternary ammonium groups in Eudragit® RL. This probably inhibits uniform dispersion of the drug in the polymer network and ultimately affects the drug loading and release profile in vitro and in vivo39).
Incompatibilities may also occur with some poly(meth)acrylate dispersions depending on the ionic and physical properties of the polymer and solvent. For example, coagulation may occur due to soluble electrolytes, some organic solvents, pH changes and extremes of temperatures. It is known that water dispersions of Eudragit® L, RL and RS are not compatible with magnesium stearate (thickening or coagulation). However, the magnesium stearate contained in the tablets does not affect the properties of the polymer film. Aqueous dispersions show more incompatibilities than organic solutions or solid poly(meth)acrylates6).
Matrix tablets with Eudragits
Matrix tablets involve a homogeneous dispersion of a drug into an excipient or a mixture of excipients which are able to form a matrix structure. Such excipients are termed as matrix carriers and are usually of polymeric origin. Interaction between a drug and a polymer generally forms the basis of controlled oral drug delivery. They can be prepared by direct compression of the powder blend and by granulation prior to compression®
40). The most common approaches to achieve a controlled release are to embed the drug in a hydrophilic (swellable) matrix tablet based on the swelling of hydrophilic polymers41–44), a lipophilic matrix systems containing fats and waxes as carriers or insoluble matrices34, 45–48).
Eudragits® are attractive matrix forming materials due to their high chemical stability, good compatibility properties, and a large variety of available grades with different physicochemical characteristics. Insoluble Eudragit® copolymers are known to form skeleton (insoluble) diffusion-controlled matrix tablets by participation of individual (meth)acrylic copolymers or their mixture mainly together with different commonly used soluble or insoluble fillers. Moreover, a combination of insoluble Eudragits® with other insoluble polymers (e.g. ethylcellulose – EC) have been described in the literature49). Both types of poly(meth)acrylates can be also an important component of swellable polymeric matrices (mainly hydroxypropyl methylcellulose – HPMC) to optimize drug release performance by adjusting gel layer characteristics in dependence on their physicochemical properties41).
Insoluble Eudragit® polymers
In technology of mono-component matrix tablets based on insoluble Eudragit® polymers, a combination of a freely soluble API/insoluble filler (or no filler)50) and a poorly soluble API/soluble filler is considered advantageous. In the first case, an insoluble filler supports the regular sustained drug release, in the second case, a soluble filler prevents a rapid disintegration of an almost insoluble matrix system. For a very soluble drug, a burst effect is typically associated with such a system. That can be demonstrated on Eudragit® NM-based matrix tablets with microcrystalline cellulose (MCC, Avicel® PH 101) as an insoluble filler for sustained release of a freely soluble drug (diltiazem hydrochloride) or a sparingly soluble drug (caffeine). In the experiment, Eudragit® NM 30 D was used to granulate the drug-filler mixture. It has been concluded that the 12 hours extended release of a freely soluble drug can be achieved with the drug-filler ratio 1 : 1 and the polymer concentration from 11.3% and 13.8%. On the contrary, the same system did not work with API caffeine and a higher tendency for fast disintegration was observed47). As a prevention of rapid disintegration, Apu et al.22) prepared an Eudragit® RS PO/RL PO matrix system with a prolonged release of poorly soluble carbamazepine (water solubility: 17.7 mg/L) in combination with soluble Ludipress® LCE (96.5% lactose, 3.5% povidon 30) in the role of the filler.
Insoluble Eudragit® polymers with both good swelling capacity and permeability can be successfully used for the acceleration of drug release from matrices based on more hydrophobic EC or oppositely an EC addition to Eudragit® formulations prolongs drug release51). In the study of Sánchez-Lafuente et al.49), didanosine, an antiretroviral drug, was incorporated into directly compressed matrices using different ratios of Eudragit® RS and EC (Ethocel® 100). The results showed a progressive increase in the drug dissolution rate with an increasing ratio of Eudragit® RS in the polymeric matrix.
Due to their insolubility, these polymers play a key role in a slowdown of drug dissolution rate and elimination/reduction of the burst effect of very soluble APIs from HPMC and other swellable matrices to obtain zero-order kinetics or near zero-order kinetics. Their insoluble character decreases the penetration of the dissolution medium into the inner matrix structure in a concentration-dependent manner and lowers the diffusion extent. Even a thin polymeric layer inside a matrix can be observed for Eudragit® copolymers with more plastic character (e.g. Eudragit® NE) as demonstrated in Figure 3. A deeper explanation of this effect can be seen in the creation of a significantly larger interface between a highly soluble drug and insoluble Eudragit® polymers due to formation of nano-sized drug particles in HPMC K4M/Neusilin® US2 matrices as demonstrated by ssNMR spectroscopy52).
3. The thin Eudragit® NE layer inside the HPMC K4M matrix structure (image from scanning electron microscopy)52) 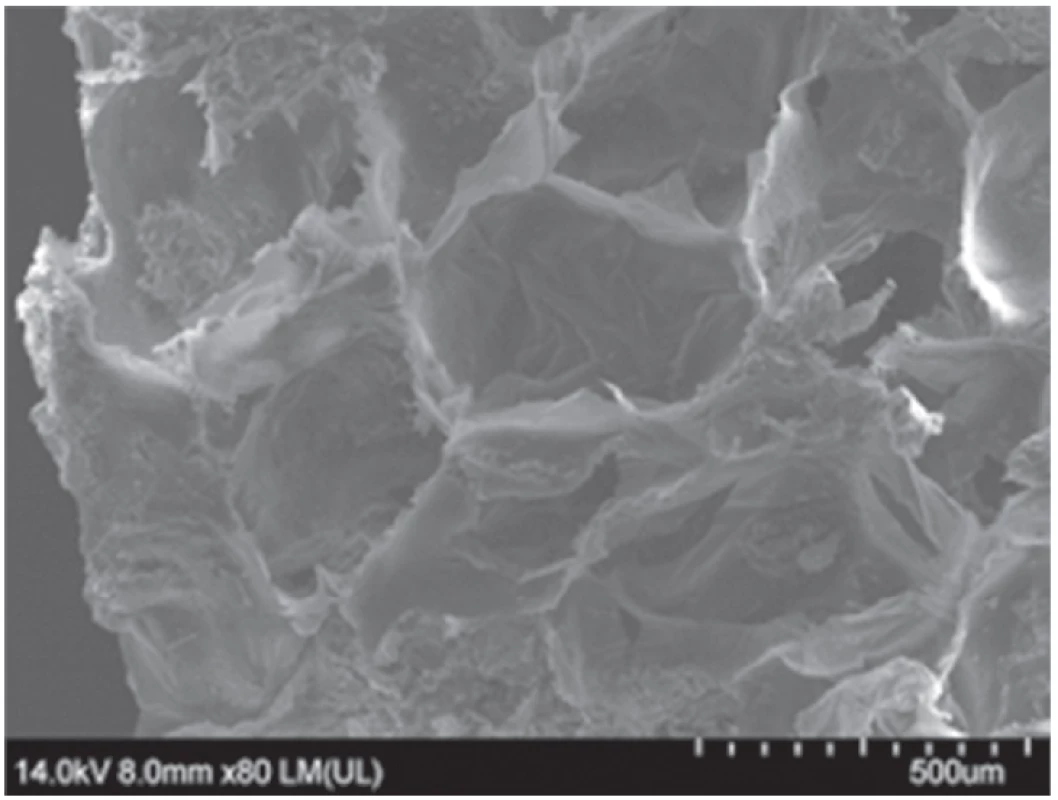
For this reason, a combination of HPMC and insoluble Eudragit® copolymers has been intensively investigated and a lot of literature sources can be found. As an example, Reddy et al.53) published a significant burst release reduction of freely soluble nicorandil by granulation of the HPMC K4M/drug mixture (4 : 1) with ethanolic solutions of Eudragits® RL 100 (10%) and RS 100 (4 and 8%). The initial release during the first 2 hours significantly decreased when Eudragit® RL (10%) was used (19.7 vs. 32.6% in reference); a lower burst effect reduction was achieved for Eudragit® RS (27.4 vs. 30.8% for reference).
In the study published by Tomuta et al.54), the HPMC K100M matrix tablets containing a very soluble drug (metoprolol tartrate) were prepared by fluid bed granulation with Eudragit® NE 40 D as the granulation liquid. The obtained results revealed that increasing amounts of both polymers (HPMC K100M and Eudragit® NE) led to a decreased release of the drug amount, nevertheless without a significant reduction of burst effect as the concentration of Eudragit® NE was rising.
An interesting addition of excipients in relation to granulation process was performed in the experiment published by Roy et al.55). Matrix tablets of the very soluble metformin hydrochloride were prepared by low-shear wet granulation. The formulations of an intragranular mixture included a combination of HPMC K200M with either Eudragit® RS 100 or Eudragit® RL 100 and an addition of HPMC K15M extragranularly. The obtained results revealed that at the 26% concentration HPMC K200M was able to sustain the drug release for 12 hours and the combined effect of HPMC K15M as an extragranular excipient and Eudragit® RS 100 displayed a significant role in drug release. It has been concluded that various grades of HPMC, at suitable concentration, in combination with polyacrylate polymers can be used effectively to modify the release rates in hydrophilic matrix tablets, prepared by wet granulation technique.
Moreover, Eudragit® RS 100 showed a more prolonged drug release compared to the Eudragit® RL 100 based formula. The burst effect reduction of freely soluble metformin was also observed when Eudragit® RS PO was combined with the natural resinous material gum copal and/or damar gum exhibiting a highly hydrophobic structure (MCC PH 101 was used as the filler)56).
Due to the cationic character of Eudragit® RL/RS, drug release of very soluble APIs from HPMC K4M/Eudragit® matrices can be significantly prolonged by an addition of an oppositely charged surfactant. This effect was demonstrated in the experimental study published by Nokhodchi et al.57) investigating the effects of various anionic (lauryl sulphate), cationic (cetyl trimethyl ammonium bromide) and non-ionic (Tween® 65 and Span® 60) surfactant types, its concentrations and the different ratios on the release rate of highly soluble propranolol hydrochloride. The different concentrations of surfactants were incorporated into HPMC K4M-Eudragit® RS matrices prepared by direct compression. The dissolution rate of the drug from the matrices was evaluated at pH 1.2 or 6.8. The results showed that the release rate of propranolol hydrochloride decreased as the concentration of anionic surfactant increased due to complexation with the drug and the polymer. The non-ionic surfactant caused an increase and the cationic surfactant had a little effect on the drug release rate.
Newly, insoluble Eudragit® polymers participate in the formulation of bi-layered tablets delivering drugs that require a loading dose followed by a maintenance dose. In such a system, one layer contains a quantity of drug for conferring immediate release (fast disintegrated part), while the second layer contains a quantity of drug for extended release. Polymers Eudragit® RL and RS can be utilized in the sustained release layers58).
Soluble Eudragit® polymers
Some of anionic polymers can be also used in matrix tablet technology. However, applicability of these polymers in sustained release matrix formulations is limited because they are non-gelling and soluble in intestinal fluids, often necessitating the use of a gelling polymer, such as HPMC59), hydroxyethyl cellulose, hydroxypropyl cellulose60) and xanthan gum61). However, the solubility of these enteric polymers at intestinal pH allows them to form pores in certain matrix systems, where interstitial channels are created due to the dissolution of these polymers, which enables enhanced drug release via diffusion through the channels59). Tatavarti et al.62) explained the effect of the methacrylic acid polymer Eudragit® L 100-55 by a significant decrease in matrix micro-environmental pH, enhancing the release of weakly basic drugs papaverine hydrochloride and verapamil hydrochloride from HPMC K4M hydrophilic matrices. For the former, the release increased with an increase in the levels of the methacrylic polymer used (influence of pH and polymer solubility). For the latter, incorporation of Eudragit® L 100-55 resulted in release retardation due to an interaction between the anionic polymer and the cationic drug and the extent of retardation increased with an increase in the polymer level.
Mixtures of pH-dependent and pH-independent polymers can be employed in matrix tablets formulations with weakly basic drugs. While in the stomach, the polymer mixture ensures low permeability, in the alkaline intestinal environment, the entero-soluble polymer dissolves and acts as a pore former, and increases solubility of the drug2). This approach was followed by Corti et al.63). In their study, pH-dependent Eudragit® L 100-55 with hydrophilic swellable polymers such as chitosan and HPMC K4M was combined. They performed complexation of the very soluble drug metformin with hydrophobic cyclodextrin and dispersed it in the matrix carrier. It was concluded that the method of complexation determined the dissolution profile but the combination of Eudragit® L and chitosan was able to release the drug at the desired profile.
Huang et al. achieved a desirable release profile of diphenhydramine by incorporating Eudragit® L in a carnauba wax matrix system64). Tatavarti and Hoag employed Eudragit® L and malic acid as pH modifiers in the HPMC E4M matrix. Both compounds resulted in pH-independent drug release65). Cha et al. included both Eudragit® L and S (1 : 1 ratio in concentration of 10% and 20%) into polyethylene oxide based matrix tablets to achieve pH-independent minocycline release66).
Incorporation of cationic polymers such as Eudragit® E in the HPMC K4M matrix system has been attempted by Rao et al.67) in order to control the release of a weakly acidic drug. Eudragit® E 100 facilitated a creation of relatively constant microenvironmental pH resulting in pH-independent release of divalproex sodium in dissolution media at different pH conditions (pH 1.0, 4.5, 6.8).
Combination of insoluble and soluble Eudragit® polymers
Various types of (meth)acrylate copolymers are often combined to achieve the desired dissolution profile of the drug from matrices. The controlled drug release kinetics close to zero-order kinetics can be achieved by combining Eudragits® with pH-dependent solubility and insoluble poly(meth)acrylates. Ceballos et al.7) prepared extended-release theophylline matrix tablets by direct compression or by the solvent method [drug and polymer(s) were dissolved in a 95% ethanolic solution and then the solvent was removed in a rotary evaporator at 60 °C; the residue was stored at room temperature in a desiccator for 24 h and sieved (75–150 µm) before use] and different pH-dependent (Eudragit® L 100, L 100-55 and S 100) and pH-independent (Eudragit® RL PO and RS PO) polymer combinations. Combining the solubility and permeability characteristics of the two polymer groups was the key to modulating the release profiles. Dissolution profiles of tablets (pre-prepared by the solvent method) showed a stronger controlled release effect due to a reduction in the particle sizes of the drug, leading to interactions between the polymers and the drug. Nevertheless, the results obtained from direct compression were more advantageous, as matrix tablets gave a total release after 6 hours and resulted in more reproducible release rates. Matrix tablets based on L 100/RL PO and L 100/RS PO mixtures gave the best results, displaying the highest percentage of total theophylline release, and the matrix formulation allowed to obtain more regular release profiles. This was achieved by a combination of the good erodible properties of L 100 with the swelling properties of RL PO and RS PO polymers. Thanks to high flexibility, Eudragits® NE or NM can be incorporated into Eudragit® L based formulations and act as plasticizers2). On the other hand, if Eudragit® L is added in an Eudragit® NM based formulation, drug release is retarded in acidic environment and enhanced in alkaline conditions68).
Floating tablets with Eudragits®
Gastroretentive systems can remain in the gastric region for several hours and hence significantly prolong the gastric residence time of drugs. They are of particular interest for drugs that are locally active in the stomach (e. g. 5-flurouracil, antacids, prostaglandins), have an absorption window in the stomach or in the upper small intestine (e.g. ketoprofen atenolol, levo DOPA, salbutamol, sotalol), are unstable in the intestinal or colonic environment (e.g. captopril), or exhibit low solubility at high pH values (metoprolol, propranolol, verapamil, diazepam)69, 70–72).
Unlike multi-particulate systems73), Eudragit® polymers are not commonly used in the technology of gastroretentive floating tablets, but some examples can be found in the scientific literature. Generally, the tablets exhibiting floating properties containing polymers from Eudragit® group are manufactured by direct compression or hot-melt (HME). HME of Eudragit® RS PO (65 % per tablet) in the presence of sodium bicarbonate (10% per tablet) was used as the unique technique for the preparation of a floating tablet system for acetohydroxamic acid and chlorpheniramine maleate. It was that sodium bicarbonate was decomposed to CO2 gas, sodium carbonate and water during HME. The prepared HME tablets exhibited a more porous structure as CO2 gas was generated in softened acrylic polymers at elevated temperature. They released the drugs in a sustained manner and floated on the surface of the acidic media for 24 hours70). Another approach is a combination of oppositely charged Eudragit® E and Eudragit® L 100-55 as matrix formers for the development of floating tablets for metronidazole. As single polymers, they are not suitable for the development of floating matrices, but their mixture at the optimum 1 : 1 weight ratio ensured superior floating and sustained drug release in 0.1M HCl. This behaviour was explained by the creation of IPEC in the presence of sodium bicarbonate which likely mediated the IPEC by raising the microenvironment pH around the Eudragit® L particles. This allowed a significant ionization of Eudragit® L and its interaction with Eudragit® E74). Similarly, the IPEC complexation between Eudragit® E and oppositely charged hydrophilic polymer carrageenan was used to develop floating matrix tablets for metronidazole. The matrix tablets consisting of the drug, pre-prepared IPEC (complexation weight ratio 0.6, drug : polymer ratio 1 : 2) and effervescent sodium bicarbonate achieved a fast (lag time less than 30 s) and prolonged floating duration with drug release correlating with zero-order kinetics for more than 10 hours75).
Coated tablets with Eudragits®
Film coating is a common step in tablet manufacture that can be used to improve product appearance, organoleptic properties, or to facilitate swallowing. Functional film coats can also be used for product stabilisation and to modify or delay drug release76). Film coating can be performed with a poly(meth)acrylate organic solvent and water dispersion. The type of solvent can have a major influence on the resulting film structure and subsequent release kinetics77). The tablet coating method is an effective tool for targeting the API to the small intestine or colon. Unlike matrix tablets with controlled drug release, the coated tablets with a functional Eudragit® film are commonly used in pharmacotherapy. Table 5 provides an overview of such medicines available in the Czech Republic.
5. Enteric coated tablets currently available in the Czech Republic78) 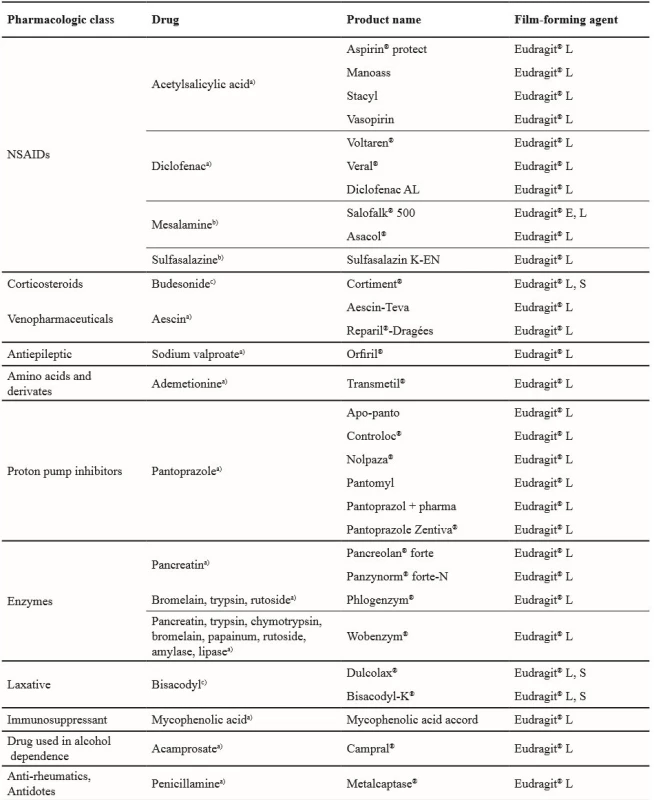
Drug release area: a) upper small intestine, b) terminal ileum and colon, c) colon Small intestine delivery
The application of an enteric coating to a solid dosage form is a well-established approach to prevent drug release in the stomach and to allow release in the small intestine. It is used to prevent the degradation of acid-labile APIs in the gastric environment or to protect the stomach from irritant compounds79). This is usually achieved by applying an outer polymeric film coat with pH-dependent solubility/permeability, or possibly, by using a drug-containing matrix with suitable swelling/solubility characteristics, i.e. delayed release.
If a polymeric film coat is used, the type of polymer and the thickness of the coating will be of importance for the onset of drug release. A threshold pH – for solubility of the polymer – slightly below 6.0 is generally considered to be sufficient to achieve protection from release in the stomach followed by a fast onset of release in the small intestine, e.g. polymers such as hydroxypropyl methylcellulose phthalate and Eudragit® L, which are soluble above pH 5.5, will usually dissolve immediately after the dosage form has been emptied from the stomach80). The double-coated system was developed to accelerate drug release in the upper small intestine. The system comprises an inner coat (partially neutralised Eudragit® L 30 D-55 and organic acid) and an outer coat (standard Eudragit® L 30 D-55). As an example, prednisolone tablets were coated with double layer formulations with inner coats neutralised to pH 5.6 in the presence of 10% citric acid or adipic acid79). Eudragit® L 100-55 can be used for a dry powder coating process. Unlike aqueous coating, powder coating minimizes partitioning of the drug into the film coating during the coating process. Eudragit® L 100-55 was pre-plasticized with triethyl citrate using hot-melt extrusion. Chlorpheniramine maleate and theophylline tablets were powder-coated with pre-plasticized Eudragit® L 100-55. The drug release properties of powder-coated tablets were dependent on the curing time, coating level and plasticizer content. The drug release rate from powder-coated theophylline tablets was controlled with slightly lower coating levels81).
Colon delivery
Targeted drug release to the colon is required for the treatment of local diseases associated with this area (Crohn’s disease, ulcerative colitis or intestinal cancer) but also for the potential to ensure a systemic delivery of proteins (e.g. insulin) and therapeutic peptides82).
To achieve colon site-specific delivery via oral administration, several approaches such as use of pH-dependent, time-dependent, pressure-dependent, microbially activated or newly also combined drug delivery systems have been designed83, 84). Among various polymeric substances, soluble and insoluble Eudragit® copolymers in the form of polymeric coating are essential components of all above-mentioned systems exhibiting very often a multi-layered structure85). Generally, anionic Eudragits® (L, S, FS) are used for their pH-dependent solubility allowing to overcome the acidic stomach compartment with strongly fluctuating pH and residence time and shift the drug release more distally in the human intestine in dependence on their own unique dissolvement pH and a film coat thickness. On the other hand, insoluble Eudragits® (especially RL and RS type) due to their properties play the key role in a system resistance and prolonging drug release86, 87). Soluble cationic type Eudragit® may find the application as an enteroresistant polymer. An example is the CODESTM, a colon targeted delivery system. It has been developed by utilizing a unique mechanism involving lactulose, which acts as a trigger for site specific drug release in the colon. It consists of a traditional tablet core containing lactulose and drug, which is coated with Eudragit® E (acid soluble material), and the outer layer is an enteric material, Eudragit® L. Between Eudragit® E and L layer there is an insulating layer made up of hypromellose. The system passes unchanged through the stomach environment and dissolves in the small intestine, where it is still protected by an inner envelope that is slightly permeable and swells. Once the tablet arrives in the colon, the bacteria enzymatically degrade lactulose into an organic acid which ensures the pH decrease resulting in Eudragit® E dissolving and drug release88, 89).
Among Eudragit® copolymers, Eudragit® FS 30 D (dissolves at pH > 7.0) is a preferable coating material which combines together a side-specific drug release in the colon, aqueous processing and flexible coatings18, 19). Tablets with the 10% coating level of Eudragit® FS could maintain their integrity for 5 hours, approximating colon arrival time and then they released the drug instantaneously or in prolonged manner90). In in vivo conditions, this polymer exhibited consistent intra - and inter-subject performance, with the site of disintegration focused on the ileo-caecal junction and ascending colon91). It is also a part of the composite oral colon-specific drug delivery coated system (together with Eudragit® RL/RS) under the trade name EUDRACOL®, registered by Evonik/Degussa Röhm GmbH92), which has a supreme position in the development of multiparticulate colon drug delivery systems. Another example of the Eudragit® FS (polyanion) importance is a preparation of a film-coated formulation for colon drug delivery based on its combination with Eudragit® RL 30 D (polycation) in two separated layers. An investigation of film behaviour in the conditions mimicking the gastrointestinal environment was performed. Being in the neutral medium, carboxyl groups of Eudragit® FS 30 D are completely ionized interacting with quaternary ammonium groups of Eudragit® RL 30 D chains to form a 3D structure based on IPEC which strongly modified the release profile of the model drug diclofenac sodium93).
Eudragit® S (solubility at pH > 7.0) has been routinely used as a coating material for pH-dependent ileo-colonic drug delivery systems as a part of commercially available products. In contrast to Eudragit® FS, a failure of disintegration followed by slow and incomplete dissolution has been described for Eudragit® S-coated tablets in vivo. The performance inconsistency has been attributed to intra - and inter-individual variability in intestinal pH, transit, volume of colon liquid, etc. In this case, Eudragit® S-coated dosage forms do not work optimally and patients are not effectively treated91). To overcome this problem, the dual mechanism (pH – Eudragit® S/bacterial – resistant starch) coating provides colon specificity and both mechanisms work as a failsafe, ensuring drug targeting94). Another possibility to ensure ileo-colonic targeting is to accelerate dissolution of the Eudragit® S film in the desired GIT area. Similarly as for the upper small intestine drug delivery, Eudragit® S has been used in the development of a double coating system, in which prednisolone tablets were coated with partially neutralized Eudragit® S and a buffer agent (10% KH2PO4, neutralization pH of 8.0), followed by a second coat of standard Eudragit® S. The prepared coated tablets exhibited a significant acceleration of coat dissolution and a subsequent rapid drug release compared to the Eudragit® S single-layer-coated tablets in the medium simulating distal small intestine95). Eudragit® S100 film (6.0% w/v) prevented premature release of 5-flourouracil from matrices consisting of a polymeric blend of Carbopol 71GNF : guar gum (4 : 1) during an in vitro release caecal study96).
Anionic pH-dependent Eudragit® copolymers have also found an application in the Multimatrix® (MMX®) system which is a drug formulation developed to facilitate release of high API concentrations into the colon with a homogeneous distribution along all colonic segments, particularly the most distal ones. The MMX® formulation is characterized by a lipophilic matrix dispersed in a hydrophilic structure, moreover with gastro-resistant, pH-dependent coating (Eudragit® L, S, FS)97). The mechanism of drug release concerns the gastro-resistant coating, which avoids the release of the embedded compound until the tablet is exposed to a pH of 7 or higher, which is normally reached in the terminal ileum. After reaching this site, the activity of the tablet core, which consists of hydrophilic excipients (for driving the tablet to swell into a viscous gel mass to lower the release of the drug) and lipophilic excipients (slowing the penetration of aqueous fluids into the tablet core), results in a homogenous and prolonged exposure of the whole colonic mucosa to the embedded substance98). Several drugs were incorporated to the MMX® systems. In particular, MMX® mesalamine, budesonide and parnaparin formulations have been investigated in patients with ulcerative colitis, and the first two have reached worldwide registration for the treatment of this disease. Moreover, MMX®-rifamycin is being positively tested in the treatment of colonic bacterial infections, including traveller’s diarrhea97). Budesonide MMX (Cortiment®) has been available in the Czech Republic since 2015.
Recently, a technologically demanding drug delivery system based on a doubly coated multiple-unit tablet was developed for bisacodyl to decrease the intestinal irritation and/or the systemic adverse effect of this stimulant laxative. Bisacodyl solubilized in surfactants was adsorbed into the porous carrier and primarily coated with different combinations of pH-sensitive polymers (Eudragit® L and Eudragit® S) and a time-dependent release polymer (Eudragit® RS). Prepared granules were compressed into tablets and coated again with pH-sensitive polymers (Eudragit® L/Eudragit® S = 1 : 1). The granules with a 12.5% coating of Euragit® L/Eudragit® S/Eudragit® RS mixture in the ratio 1 : 5 : 4 and the final tablets with 25% coating of Eudragit® L effectively retarded the drug release in the simulated gastric and small intestinal fluids but drug liberation in the colonic fluid was over 50%83).
Conclusion
Eudragit® polymers are widely used acrylic pharmaceutical excipients. Due to their unique properties, Eudragit® polymers have made significant contributions to many types of formulations. Poly(meth)acrylate copolymers provide controlled release effects for solid dosage forms as multi-unit systems or matrix tablets. Being a synthetic polymer, Eudragits® display good reproducibility and other advantages associated with synthetic polymers. Further, these are regarded as non-biodegradable, non-absorbable, and non-toxic functional excipients, thus circumventing issues accompanying synthetic polymers. The combination of these polymers shows that different release patterns can be achieved based on their interactions, giving the polymers a variety of properties. Combining differently charged (meth)acrylate copolymers appears to be an interesting field of investigation, providing advantages in the processing and modulation of release profiles.
Acknowledgments
This work was supported by the project ITA VFU Brno: FaF/Vetchý/ITA 2019.
Conflict of interest: none.
Received August 25, 2019 / Accepted September 23, 2019
PharmDr. Jakub Vysloužil, Ph.D. (✉)
Department of Pharmaceutics,
Faculty of Pharmacy University of Veterinary and Pharmaceutical Sciences Brno
Palackého 1,
612 42 Brno,
Czech Republic
e-mail: jakub.vyslouzil@gmail.com
Sources
1. Evonik, Healthcare. Healthcare Evonik. Eudragit. [Online] 7. 1. 2019 [Cited: 7. 1. 2019.] https://healthcare.evonik.com/product/health-care/en/products/pharmaceutical-excipients/EUDRAGIT/
2. Skalsky B., Petereit H. Chemistry and application properties of polymethacrylate systems. In: Felton J. W., McGinity L. A. Aqueous polymeric coatings for pharmaceutical dosage forms 3. New York: Informa Healthcare USA 2008.
3. Tu J., Shen Y., Mahalingam R., Jasti B., Li X. Polymers in oral modified release systems. In: Park H., Wen K. Oral controlled release formulation design and dru delivery: Theory to practice l. New Jersey: John Wiley & Sons 2011; 71–88.
4. Nollenberger K., Alberts J. Poly(meth)acrylate-based coatings. Int. J. Pharm. 2013; 457(2), 461–469.
5. Malá R., Jirásková J., Rabišková M. Vodné disperze polymerů v obalech řídících uvolňování léčiv. Chem Listy 2014; 108(11), 1046–1052.
6. Thakral S., Thakral N. K., Majumdar D. K. Eudragit®: a technology evaluation. Expert Opin. Drug Eval. 2013; 10(1), 131–149.
7. Ceballos A., Cirri M., Maestrlli F., Corti G., Mura P. Influence of formulation and process variables on in vitro release of theophylline from directly-compressed Eudragit matrix tablets. Farmaco 2005; 11–12, 913–918.
8. Vasileiou K., Vysloužil J., Pavelková M., Vysloužil J., Kubová K. Velikostně redukované mikročástice na bázi Eudragitu® RS připravené metodou odpaření rozpouštědla – sledování vlivu vybraných proměnných na testované parametry. Čes. slov. Farm. 2017; 6, 274–280.
9. Vysloužil J., Bavolarová J., Kejdušová M., Vetchý D., Dvořáčková K. Cationic Eudragit® Polymers as Excipients for Microparticles Prepared by Solvent Evaporation Method. Čes. slov. Farm. 2013; 6, 249–254.
10. Gallardo D., Skalsky B., Kleinebudde P. Controlled release solid dosage forms using combinations of (meth)acrylate copolymers. Pharm. Dev. Technol. 2008; 13(5), 413–423.
11. Patra C. N., Priya R., Swain S., Jena G. K., Panigrahi K. C., Ghose D. Pharmaceutical significance of Eudragit: A review. Futur. J. Pharm. Sci. 2017; 3(1), 33–45.
12. Qi, S., Gryczke, A., Belton, P., Craig, D. Q. Characterisation of solid dispersions of paracetamol and Eudragit® E prepared by hot-melt extrusion using thermal, microthermal and spectroscopic analysis. Int. J. Pham. 2008; 1–2(354), 158–167.
13. Liu J., Cao F., Zhang C., Ping Q. Use of polymer combinations in the preparation of solid dispersions of a thermally unstable drug by hot-melt extrusion. Acta Pharm. Sin. B 2013; 3(4), 263–272.
14. Yang Z., Nollenberger K., Alberts J., Craig D. Qi S. Microstructure of an immiscible polymer blend and its stabilization effect on amorphous solid dispersions. Mol. Pharm. 2013; 10(1), 2767–2780.
15. Li J., Lee I. W., Shin G. H., Chen X., Park H. J. Curcumin-Eudragit E PO solid dispersion: A simple and potent method to solve the problems of curcumin. Eur. J. Pharm. Biopharm. 2015; 94, 322–332.
16. Hrubý M., Filippov S. K., Felklová V., Štěpánek P. Přírodou inspirované polymery citlivé na vnější podněty pro dopravu léčiv. Chem Listy 2015; 109(7), 482–487.
17. Kadian S. S., Harikumar S. L. Eudragit and its Pharmaceutical Significance. Pharmainfo. [Online] 2016 [Cited: 1. 30. 2019] KADIAN, Satish Singh; Harikumar S. L. http://www. pharmainfo. net/satishsinghkadian/publications/eudragit-and-its-pharmaceutical-significance
18. Huyghebaert N., Vermeire A., Remon J. P. In vitro evaluation of coating polymers for enteric coating and human ileal targeting. Int. J. Pharm. 2005; 298(1), 26–37.
19. Joshi M. Role of Eudragit in targeted drug delivery. Int. J. Curr. Pharm. Res. 2013; 5(2), 58–62.
20. Brady J., Durig T., Lee P. I., Li J. X. Polymer Properties and Characterization. In: Zhang Y., Mantri G. G. Z., Chen R. V., Yu Y., Qui L. Developing Solid Oral Dosage Forms. 2. Cambridge: Academic Pres 2017; 181–223.
21. Apu A. S., Pathan A. H., Kibria G., Jalil R. U. In vitro release kinetic study of theophylline from eudragit RS PO and eudragit RL PO matrix tablets. J. Pharm. Sci. 2009; 8(1), 1–6.
22. Apu A. S., Pathan A. H., Shrestha D., Kibria G., Jalil R. U. Investigation of in vitro release kinetics of carbamazepine from Eudragit® RS PO and RL PO matrix tablets. Trop. J. Pharm. Res. 2009; 8(2), 145–152.
23. Aleksiev A., Kostova B., Rachev D. Development of Eudragit Based Sustained Release Systems of Galantamine Hydrobromide. Int. J. Pharm. Sci. Rev. Res. 2014; 27(1), 135–140.
24. Müller-Albers J., Guha A., Assmus M. Use of an advanced new enteric combination polymer with multiple unit pellet systems and other multiparticulates. Am. Pharm. Rev. 2018; 7.
25. Moustafine R. I., Margulius E. B., Sibgatullina L. F. Kemenova V. A., van den Mooter G. Comparative evaluation of interpolyelectrolyte complexes of chitosan with Eudragit® L100 and Eudragit® L100-55 as potential carriers for oral controlled drug delivery. Eur. J. Pharm. Biopharm. 2008; 70(1), 215–225.
26. Mustafin R. I., Kabanova T. V., Semina I. I., Bukhovets A. V., Garipova V. R., Shilovskaya E. V. Biopharmaceutical assessment of a polycomplex matrix system based on carbomer 940 and Eudragit E PO for colon-specific drug deliery. Pharm. Chem. J. 2011; 45(8), 491–494.
27. Moustafine R. I., Kemenova V. A., van den Mooter G. Characteristics of interpolyelectrolyte complexes of Eudragit E. Int. J. Pharm. 2005; 294.
28. Li L., Wang L., Jiang S, Wang Y., Zhang X. Insights into the mechanisms of chitosan - anionic polymers-based matrix for extended drug release. Int. J. Pharm. 2014; 476, 253–265.
29. Mašková E., Kubová K., Vetchý D. Využití (meth)akrylátových kopolymerů v technologii matricových tablet s řízeným uvolňováním léčiva. Chem Listy 2015; 109, 14–20.
30. Ofokansi K. C., Kenechukwu F. C. Formulation development and evaluation of drug release kinetics from colon-targeted ibuprofen tablets based on Eudragit RL 100-chitosan interpolyelectrolyte complexes. ISRN Pharm. 2013; 1–8.
31. Azarmi S., Ghaffari F., Löbenberg R., Nokhodchi A. Mechanistic evaluation of the effect of thermal-treating on Eudragit RS matrices. Farmaco 2005; 60(11–12), 925–930.
32. Hoag S., Nyamweya N. N. Influence of coloring agents on the properties of polymeric coating systems. In: Felton L. A., McGinity J. W. Aqueous polymeric coatings for pharmaceutical dosage forms. New York: CRC Press 2008; 7, 191–222.
33. Azarmi S., Farid J., Nokhodchi A., Bahari-Savari S. M., Valizadeh H. Thermal treating as a tool for sustained release of indomethacin from Eudragit RS and RL matrices. Int. J. Pharm. 2002; 246(1–2), 171–177.
34. Dave V. S., Fahmy R. M., Bensley D., Hoag S. W. Eudragit® RS PO/RL PO as rate-controlling matrix-formers via roller compaction: Influence of formulation and process variables on functional attributes of granules and tablets. Drug Dev. Ind. Pharm. 2012; 38(10), 1240–1253.
35. Kubová K., Peček D., Hasserová K., Doležel P., Pavelková M., Vysloužil J., Muselík J., Vetchý D. The influence of thermal treatment and type of insoluble poly(meth)acrylates on dissolution behavior of very soluble drug from hypromellose matrix tablets evaluated by multivariate data analysis. Pharm. Dev. Technol. 2017; 22(2), 206–217.
36. Sarisuta N., Lawanprasert P., Puttipipatkhachorn S., Srikummoon K. The influence of drug-excipient and drug-polymer interactions on adhesive strenght of Ranitidine Hydrochloride film-coated tablets. Drug Dev. Ind. Pharm. 2006; 32, 463–471.
37. Stroyer A., McGinity J. W., Leopold C. S. Solid state interactions between the proton pump inhibitor omeprazole and various enteric coating polymers. J. Pharm. Sci. 2006; 95(6), 1342–1353.
38. Pignatello R., Ferro M., Puglisi G. Preparation of solid dispersions of nonsteroidal anti-inflammatory drugs with acrylic polymers and studies on mechanisms of drug-polymer interactions. AAPS Pharm. Sci. Tech. 2002; 3(2), 35–45.
39. Pignatello R., Spadaro D., Vandelli M. A., Forni F., Puglisi G. Characterization of the Mechanism of Interaction in Ibuprofen-EudragitRL100 Coevaporates. Drug Dev. Ind. Pharm. 2004; 30(3), 277–288.
40. Bose A., Wong T. W., Singh N. Formulation development and optimization of sustained release matrix tablet of Itopride HCl by response surface methodology and its evaluation of release kinetics. SPJ 2013; 21(2), 201–213.
41. Quinten T., Gonnissen Y., Adraens E. Development of injection moulded matrix tablets based on mixtures of ethylcellulose and low-substituted hydroxypropylcellulose. Eur. J. Pharm. Sci. 2009; 37(3–4), 207–216.
42. Fu S., Buckner I. S., Block L. H. Inter-grade and inter-batch variability of sodium alginate used in alginate-based matrix tablets. AAPS Pharm. Sci. Tech. 2014; 15(5), 1228–1237.
43. Phaechamud T., Ritthidej G. C. Sustained-release from layered matrix system comprising chitosan and xanthan gum. Drug Dev. Ind. Pharm. 2007; 33(6), 595–605.
44. Dvořáčková K. Principy uvolňování léčiv z perorálních matricových tablet obsahujících hypromelosu. Chem Listy 2009; 103(1), 66–72.
45. Ozyacizi M., Gokce H. E., Ertan G. Release and diffusional modeling of metronidazole lipid matrices. Eur. J. Pharm. Biopharm. 2006; 63(3), 331–339.
46. Deepika B., Sameen S., Nazneen N., Madhavi A., Raju K. N., Rao K. N., Dutt K. R. Matrix drug delivery system: A review. Eur. J. Pharm. Med. Res. 2018; 5(1), 150–154.
47. Dvořáčková K., Kalėdaitė R., Gajdziok J., Rabišková M., Bajerová M., Muselík J., Lažauskas R., Pečiura R., Bernatonienė J. The development of Eudragit® NM-based controlled-release matrix tablets. Medicina 2012; 48(4), 192–202.
48. Tolia G., Li S. K. Study of drug release and tablet characteristics of silicone adhesive matrix tablets. Eur. J. Pharm. Biopharm. 2012; 82(3), 518–525.
49. Sánchez-Lafuente C., Faucci M. T., Férnandez-Arévalo M., Álvarez-Fuentes J., Rabasco A. M., Mura P. Development of sustained release matrix tablets of didanosine containing methacrylic and ethylcellulose polymers. Int. J. Pharm. 2002; 234, 213–221.
50. Tabandeh H., Mortazavi S. A., Guilani T. B. Preparation of sustained-release matrix tablets of aspirin with ethylcellulose, Eudragit RS100 and Eudragit S100 and studying the release profiles and their sensitivity to tablet hardness. Iran J. Pharm. Res. 2010; 2(4), 201–206.
51. Chithaluru K., Tadikonda R. R., Gollapudi R., Kandula K. K. Formulation and invitro evaluation of sustained release matrix tablets of losartan potassium. Asian J. Pharm. Clin. Res. 2011; 4(3), 18–22.
52. Naiserová M., Kubová K., Vysloužil J., Pavloková S., Vetchý D., Urbanová M., Brus J., Vysloužil J., Kulich P. Investigation of dissolution behaviour HPMC/Eudragit®/magnesium aluminometasilicate oral matrices based on NMR solid-state spectroscopy and dynamic characteristics of gel layer. AAPS Pharm. Sci. Tech. 2018; 19(2), 681–692.
53. Reddy K. R., Mutalik S., Reddy S. Once-daily sustained-release matrix tablets of nicorandil: formulation and in vitro evaluation. AAPS Pharm. Sci. Tech. 2003; 4(4), 480–488.
54. Tomuta I., Alecu C., Dudas D., Leucuta S. E. Optimization of metoprolol tartrate modified-release matrix tablet formulation using Eudragit NE as binder for metoprolol fluid bed granulation. Asian J. Pharm. 2014; 6(2), 101–106.
55. Roy H., Brahma C. K., Nandi S., Parida K. R. Formulation and design of sustained release matrix tablets of metformin hydrochloride: Influence of hypromellose and polyacrylate polymers. Int. J. App. Basic Med. Res. 2013; 3(1), 55–63.
56. Wadher K. J., Kakde R. B., Umekr M. J. Formulation and evaluation of a sustained-release tablets of metformin hydrochloride using hydrophilic synthetic and hydrophobic natural polymers. Indian J. Pharm. Sci. 2011; 73(2), 208–215.
57. Nokhodchi A., Norouzi-Sani S., Siahi-Shadbad M. R., Lotfipoor F., Saeedi M. The effect of various surfactants on the release rate of propranolol hydrochloride from hydroxypropylmethylcellulose (HPMC)-Eudragit matrices. Eur. J. Pharm. Biopharm. 2002; 54(3), 349–356.
58. Moodley K., Pillay V., Choonara Y. E., du Toit L. C., Ndesendo V. M., Kumar P., Cooppan S., Bawa P. Oral drug delivery systems comprising altered geometric configurations for controlled drug delivery. Int. J. Mol. Sci. 2012; 13(1), 18–43.
59. Oren P. L., Seidler W. M. K. Sustained release matrix. 4968508 USA, November 6, 1990.
60. Ashgar L. F. A., Chandran S. Design and evaluation of matrix base with sigmoidal release profile for colon-specific delivery using a combination of Eudragit and non-ionic cellulose ether polymers. Drug Deliv. Transl. Re. 2011; 1(2), 132–146.
61. Asghar L. F. A., Chure C. B., Chandran S. Colon specific delivery of indomethacin: effect of incorporating pH sensitive polymers in xanthan gum matrix bases. AAPS Pharm. Sci. Tech. 2009; 10(2), 418–429.
62. Tatavarti A. S., Muller F. X., Hoag S. W. Evaluation of the deformation behavior of binary systems of methacrylic acid copolymers and hydroxypropyl methylcellulose using a compaction simulator. Int. J. Pharm. 2008; 348(1–2), 46–53.
63. Corti G., Cirri M., Maestrelli F., Mennini N., Mura P. Sustained-release matrix tablets of metformin hydrochloride in combination with triacetyl-β-cyclodextrin. Eur. J. Pharm. Biopharm. 2008; 68, 303–309.
64. Huang H. P., Mehta S. C., Radebaugh G. W., Fawzi M. B. Mechanism of drug release from an acrylic polymer-wax matrix tablet. J. Pharm. Sci. 1994; 83(6), 795–797.
65. Tatavarti A. S., Hoag S. W. M2icroenvironmental pH modulation based release enhancement of a weakly basic drug from hydrophilic matrices. J. Pharm. Sci. 2006; 95(7), 1459–1468.
66. Cha K. H., Park J., Cho W., Gu D. G., Jeong K., Hwang S. J. Design of pH-independent extended release matrix tablets of minocycline hydrochloride for the treatment of dementia. Arch. Pharm. Res. 2009; 32(11), 1593–1598.
67. Rao, V. M., Engh, K., Qiu, Y. Design of pH-independent controlled release matrix tablets for acidic drugs. Int. J. Pharm. 2003; 252(1–2), 81–86.
68. Çetin M., Süleyman H., Çadirci E., Demir E. T., Polat B., Hacimüftüoglu A. Preparation and in vivo evaluation of Eudragit® L100/Eudragit® NM 30D enteric granules containing diclofanac sodium: anti-inflammatory and ulcerogenic activity. Turk. J. Pharm. Sci. 2010; 7(3), 237–248.
69. Arora S., Ali J., Ahuja A. Khar R. K., Baboota S. Floating Drug Delivery Systems: A Review. AAPS Pharm. Sci. Tech. 2005; 6(3), 372–390.
70. Fukuda M., Peppas N. A., McGinity J. W. Floating hot-melt extruded tablets for gastroretentive controlled drug release system. J. Control. Release 2006; 115(2), 121–129.
71. Jagdale S. C., Agavekar A. J., Pandya S. V., Kuchekar B. S., Chabukswar A. R. Formulation and evaluation of gastroretentive drug delivery system of propranolol hydrochloride. AAPS Pharm. Sci. Tech. 2009; 10(3), 1071–1079.
72. Oth M., Franz M. Timmermans J., Möes A. The bilayer floating capsule: a stomach-directed drug delivery system for misoprostol. Pharm. Res. 1992; 9(3), 298–302.
73. Kaushik A. Y., Tiwari A. K., Gaur A. Role of excipients and polymeric advancements in preparation of floating drug delivery systems. Int. J. Pharm. Investig. 2015; 5(1), 1–12.
74. Bani-Jaber A. K., Alkawareek M., Al-Gousous J., Abuhelwa A. Y. Floating and sustained-release characteristics of effervescent tablets prepared with a mixed matrix of Eudragit L-100-55 and Eudragit E PO. Chem. Pharm. Bull. 2011; 59(2), 155–160.
75. Bani-Jaber A., Al-Alani L., Alkhatib H., Al-khalidi B. Prolonged intragastric drug delivery mediated by Eudragit® E-carrageenan polyelectrolyte matrix tablets. AAPS Pharm. Sci. Tech. 2011; 12(1), 354–361.
76. Elder D. Design, formulation and manufacture of film-coated drug products. Eur. Pharm. Rev. 2017; 22(5), 37–40.
77. Khatri P., Desai D., Shelke N., Minko T. Role of plasticizer in membrane coated extended release oral drug delivery. J. Drug. Deliv. Sci. Technol. 2018; 44, 231–243.
78. SÚKL – Státní ústav pro kontrolu léčiv. [Online] 21. 4 2019 [Cited: 21. 4. 2019] http://www.sukl.cz/modules/medication/search.php
79. Liu F., Lizio R., Mier C., Petereit H. U., Blakey P., Basit A. W. A novel concept in enteric coating: A double-coating system providing rapid drug. J. Control. Release 2009; 133(2), 119–124.
80. Wikberg M., Ulmius J., Ragnarsson G. Targeted drug delivery in treatment of intestinal diseases. Aliment. Pharm. Ther. 1997; 11, 109–115.
81. Sauer D., Zheng W., Coots L. B., McGinity J. W. Influence of processing parameters and formulation factors on the drug release from tablets powder-coated with Eudragit L 100-55. Eur. J. Pharm. Biopharm. 2007; 67(2), 464–475.
82. Szente V., Zelkó R. Site-specific drug delivery systems. I. Colon targeted delivery. Acta Pharm. Hung. 2007; 77(3), 185–189.
83. Park H. J., Jung H. J., Ho M. J., Lee D. R., Cho H. R., Choi Y. S., Jun J., Son M., Kang M. Colon-targeted delivery of solubilized bisacodyl by doubly enteric-coated multiple-unit tablet. Eur. J. Pharm. Sci. 2017; 102, 172–179.
84. Hadi M. A., Rao N. R., Rao A. S. Formulation and evaluation of ileo-colonic targeted matrix-mini-tablets of Naproxen for chronotherapeutic treatment of rhumatoid arthritis. Saudi Pharm. J. 2016; 24(1), 64–73.
85. Ren Y., Jiang L., Yang S., Gao S., Yu H., Hu J., Mao D., Peng H., Zhou Y. Design and preparation of a novel colon-targeted tablet of hydrocortisone. Braz. J. Pharm. Sci. 2016; 52(2), 239–250.
86. Amidon S., Brown J. E., Dave V. S. Colon-targeted oral drug delivery systems: Design trends and approaches. AAPS Pharm. Sci. Tech. 2015; 16(4), 731–741.
87. Mehta R., Chawla A., Sharma P., Pawar P. Formulation and in vitro evaluation of Eudragit S100 coated naproxen matrix tablets for colontargeted drug delivery system. J. Adv. Pharm. Tech. Res. 2013; 4(1), 31–41.
88. Philip A. K., Philip B. Colon targeted drug delivery systems: A review on primary and novel approaches. Oman Med. J. 2010; 25(2), 79–87.
89. Katsuma M., Watanabe S., Takemura S., Sako K., Sawada T., Masuda Y., Wilding I. R. Scintigraphic evaluation of a novel colon-targeted delivery system (CODES) in healthy volunteers. J. Pharm. Sci. 2004; 93(5), 1287–1299.
90. Kshirsagar S. J., Bhalekar M. R., Umap R. R. Design, development and in vitro-in vivo study of a colon-specific fast disintegrating tablet. Pharm. Dev. Tech. 2011; 16(5), 449–456.
91. Ibekwe V. C., Liu F., Fadda H. M., Khela M. K., Evans D. F., Parsons G. E., Basit A. W. An investigation into the in vivo performance variability of pH responsive polymers for ileo‐colonic drug delivery using gamma scintigraphy in humans. J. Pharm. Sci. 2006; 95(12), 2760–2766.
92. Mustafin R. I., Bodrov A. V., Kemenova V. A., Rombaut P., van den Mooter G. Interpolymer interaction between countercharged types of Eudragit® RL30D and FS30D in binary films as a method of drug release modification in oral delivery systems. Pharm. Chem. J. 2012; 46(1), 45–49.
93. Moustafine R. I., Bodrov A. V., Kemenova V. A., Rombaut P., van der Mooter G. Drug release modification by interpolymer interaction between countercharged. Int. J. Pharm. 2012; 439, 17–21.
94. Ibekwe V. C., Khela M. K., Evans D. F., Basit A. W. A new concept in colonic drug targeting: a combined pH-responsive and bacterially-triggered drug delivery technology. Aliment Pharm. Ther. 2008; 28(7), 911–916.
95. Liu F., Moreno P., Basit A. W. A novel double-coating approach for improved pH-triggered delivery to the ileo-colonic region of the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2010; 74(2), 311–315.
96. Sinha V. R., Kumar R. V., Bhinge J. R. Influence of polymeric blend of carbomer-gum on the targeted delivery of 5-FU. Polymer Plast. Tech. Eng. 2009; 48(12), 1287–1294.
97. Nardelli S., Pisani L. F., Tontini G. E., Vecchi M., Pastorelli L. MMX® technology and its applications in gastrointstinal diseases. Ther. Adv. Gastroenterol. 2017; 10(7), 545–552.
98. Prantera C., Viscido A., Biancone L., Francavilla A., Giglio L., Campieri M. A new oral delivery system for 5-ASA: preliminary clinical findings for MMX. Inflamm. Bowel. Dis. 2005; 11(5), 421–427.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2019 Issue 5-
All articles in this issue
- Pulse check as a tool to raise awareness of atrial fibrillation in pharmacies in the Czech Republic – a pilot project
- Honoring the 80th Anniversary of the World War II outbrake
-
Pokroky ve farmaceutické technologii
Pracovní den sekce technologie léků - X. zjazd Slovenskej farmaceutickej spoločnosti
- Nové knihy
- (Meth)acrylate copolymers of Eudragit® type in oral tablet technology
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- (Meth)acrylate copolymers of Eudragit® type in oral tablet technology
- Pulse check as a tool to raise awareness of atrial fibrillation in pharmacies in the Czech Republic – a pilot project
-
Pokroky ve farmaceutické technologii
Pracovní den sekce technologie léků - Honoring the 80th Anniversary of the World War II outbrake
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

