-
Medical journals
- Career
Effects of zinc and cadmium ions on cell growth and production of coumarins in cell suspension cultures of Angelica archangelica L.
Authors: Tomáš Siatka; Marie Kašparová; Jiřina Spilková
Authors‘ workplace: Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmacognosy
Published in: Čes. slov. Farm., 2012; 61, 261-266
Category: Original Articles
Overview
The plant cell may respond to the excess of heavy metals in its environment by various mechanisms, including enhanced biosynthesis of secondary metabolites. In this study, zinc (0 to 1500 μM) and cadmium ions (0 to 100 μM) were tested as potential elicitors of the production of coumarins in angelica cell suspension cultures. In addition, the toxicity of both metals was assessed by evaluating their effect on cell growth (characterized by fresh and dry biomass at the end of a two-week subculture). It has been found that fresh biomass was not influenced up to zinc concentrations of 150 and 300 μM in the dark-grown and light-grown cultures, resp. Then it declined with an increasing zinc level. Zinc at 1500 μM diminished it by 54% and 24% in the dark-grown and light-grown cultures, resp. Dry biomass was influenced in a similar way. Zinc at 1500 μM reduced dry cell weight by 30% and 20% in cultures in the dark and in the light, resp. Cadmium ions did not affect fresh and dry weights of cells up to concentrations of 10 μM and 50 μM in cultures in the dark and in the light, resp. Toxic concentrations of cadmium are by an order of magnitude lower than those of zinc. Cadmium at 50 μM reduced fresh and dry cell weights by 66% and 59%, resp., in the dark-grown cultures. Cadmium at 100 μM caused a decrease in fresh and dry biomass by 40% and 44%, resp., in the light-grown cultures. Neither zinc nor cadmium improved production of coumarins.
Keywords:
Angelica archangelica L. • cell suspension cultures • growth • coumarins • zinc • cadmium • elicitation • light conditions • sequential injection analysisIntroduction
Plant secondary metabolites are economically important as drugs, flavours and fragrances, pigments, pesticides, and food additives1, 2). In recent years the evolving commercial importance of secondary metabolites has resulted in a great interest in the possibility of altering the production of bioactive compounds by means of plant tissue culture technology3). Some examples of successful commercial processes for supply of pharmaceutically valuable substances are shown in Table 14). However, the in vitro production is still facing many biological and biotechnological limitations. One of the obstacles is a low yield of metabolites in plant cell cultures. Since the major roles of plant secondary metabolites are to protect plants from attack by insect, herbivores and pathogens, or to survive other biotic and abiotic stresses, some strategies based on this principle have been developed to improve the yield of such plant secondary metabolites in vitro5). Plants as well as plant cell cultures show physiological and morphological responses to biological, physical, or chemical stress factors which are known as elicitors3, 6). The elicitors include, e.g., components of microbial cells, heavy metal ions, hyperosmotic stress, and ultraviolet radiation, as well as the signalling compounds in plant defence responses such as salicylic acid and methyl jasmonate7–9). The modes of elicitor action are complex. Moreover, since little is known about the biosynthetic pathways of most secondary metabolites, the effect of an elicitor on a plant cell culture cannot easily be predicted10). Therefore the majority of elicitation approaches are empirical and the optimum conditions have to be determined experimentally for each system in particular.
1. Examples of plant cell cultures used for the commercial production of high-value secondary metabolites <sup>4)</sup>. 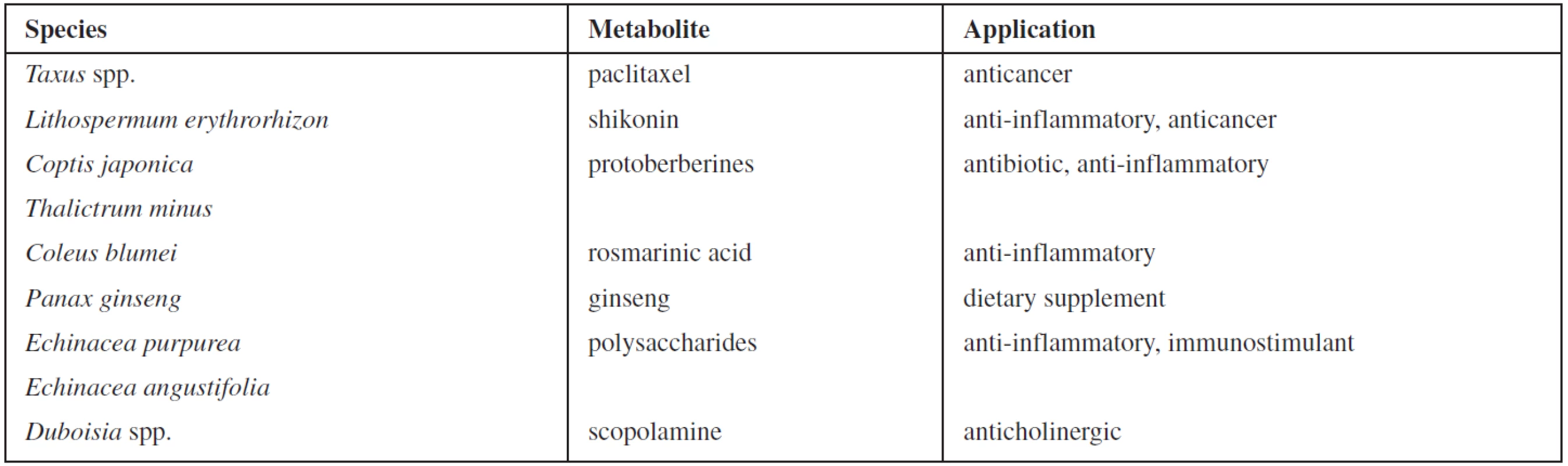
Production of secondary metabolites has been investigated in cell cultures derived from many plant species in the family Apiaceae (Umbelliferae), e.g., Petroselinum crispum (coumarins)11), Anethum graveolens (flavonoids)12), Pimpinella anisum (coumarins)13), Daucus carota (anthocyanins)14), Ammi majus (coumarins)15), Anthriscus sylvestris (lignans)16), and Glehnia littoralis (anthocyanins and coumarins)17).
We report here the effects of zinc and cadmium sulphates as potential elicitors on cell growth and production of coumarins in Angelica archangelica cell suspension cultures.
Experimental part
Chemicals
2,4-dichlorophenoxyacetic acid, 6-benzylaminopurine, and agar (plant cell culture tested, Sigma, Praha, Czech Republic); scopoletin (analytical standard, Fluka, Praha, Czech Republic); zinc sulphate, cadmium sulphate, sodium phosphate dibasic, and potassium phosphate monobasic (p.a., Lachema, Brno, Czech Republic).
Instruments
A PS 20A autoclave (Chirana, Brno, Czech Republic); a roller (Vyvojové dílny, Academy of Sciences of the Czech Republic, Praha, Czech Republic); a 200S analytical scale (Sartorius, Göttingen, Germany); a laboratory centrifuge MPW 342 (MPW Med. instruments, Warsaw, Poland); a laboratory shaker KS 501 (IKA Labortechnik, Staufen, Germany); a peristaltic pump (Alitea Instruments, Seattle, U.S.A); an eight position selection valve (Vici Valco Instruments, Brockville, Canada); and a FS 970 fluorescence detector (Schoeffel Instrument Corp., Westwood, U.S.A.).
Cell suspension cultures and culture conditions
Tissue cultures of Angelica archangelica were derived from a bud of an in spring sprouting one-year old plant grown in the Botanical Garden of Faculty of Pharmacy in Hradec Králové. Callus cultures were established from the bud meristem and maintained by subculturing every five weeks on Murashige and Skoog medium18) supplemented with 2 mg l–1 2,4-dichlorophenoxyacetic acid, 0.4 mg l–1 benzylaminopurine, 30 g l–1 sucrose, and 8 g l–1 agar. The pH of all media was adjusted to 5.7 before autoclaving at 121 °C for 15 min. Cell suspension cultures were initiated from friable calluses in the same medium devoid of agar. They were agitated in 250 ml flasks containing 30 ml of the medium on a roller apparatus at 8 rpm, incubated at 25 ± 1 °C under a 16/8 light/dark photoperiod or in the dark, and subcultured every two weeks.
For testing the effects of metal ions, the cultures were cultured in Murashige and Skoog media supplemented with an appropriate concentration of zinc sulphate (0, 30, 60, 150, 300, 600, and 1500 μM) or cadmium sulphate (0, 0.1, 0.5, 1, 2, 5, 10, 50, and 100 μM). After 14 days, the cultures were harvested, and the cell growth and production of coumarins were evaluated. All experiments were carried out in triplicate and repeated three times.
Analytical procedures
Cells were separated from the culture medium by vacuum filtration using a Buchner funnel with filter paper. For evaluation of the culture growth, filtered cells were washed with distilled water, weighed for fresh weight determination, and then dried at 60 °C to obtain dry weight.
Coumarins in cells and in the culture medium were quantified fluorometrically by sequential injection analysis as described in detail previously19). In brief, the powdered dry cells were extracted three times (always 15 min) by a mixture of equal volumes of methanol and 0.066 M phosphate buffer (pH 6) by shaking at 150 rpm on an orbital shaker at laboratory temperature. The extracts were pooled, adjusted to 25 ml with the extraction mixture, centrifuged at 3.000 rpm for 10 min, and analysed. The culture media were analysed direct. The conditions of the sequential injection analysis were as follows – a carrier stream: water; flow rate: 3 ml/min-1; sample volume: 40 μl; volume of 0.066 M phosphate buffer (pH 6): 100 μl; a 1.5 ml mixing coil; excitation wavelength: 345 nm; and emission wavelength: a cut-off emission filter transparent at ≥ 390 nm. The contents of coumarins were expressed as scopoletin (mg l–1 in the medium and mg g–1 dry weight in the cells).
Experimental data were statistically analysed using a one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test. Differences at p < 0.05 were considered as statistically significant.
Results and discussion
Zinc belongs to the essential trace elements, whereas cadmium has no known biological function in plants20, 21). Zinc participates in several metabolic processes such as protein synthesis, enzyme activation, and metabolism of carbohydrates, lipids or nucleic acids; and it is an integral part of transcription factors controlling cell proliferation and differentiation22). Zinc is suggested to have a stabilizing and protective effect against reactive oxygen species mediated oxidative and peroxidative damage in cells22). Zinc and cadmium can be toxic to plants. The toxic dose depends on the ion concentration and plant species20). There are even plants thriving in metal-enriched environments. Certain plant species, called hyperaccumulators, accumulate and tolerate unusually large amounts of metals compared to other plants and the ambient metals concentration, without symptoms of toxicity20, 23). Zinc and cadmium hyperaccumulators are, for instance, Pistia stratiotes23), Thlaspi caerulescens24), Arabidopsis halleri25), and Sedum alfredii26).
As mentioned above, heavy metal salts may be employed to stimulate production of secondary metabolites in plant tissue cultures27, 28). We have tested zinc and cadmium ions in a wide range of concentrations (zinc up to 1500 μM, cadmium up to 100 μM, based on preliminary experiments; higher concentrations were lethal for the culture) as potential elicitors of production of coumarins in angelica cell suspension cultures. In addition, the toxicity of zinc and cadmium for the culture was assessed by evaluating their effects on cell growth, which was characterized by the fresh and dry weights of cells (the most widely growth parameters used) at the end of a two-week subculture. The cultures were cultured in the dark or in the light because light conditions are an important environmental factor involved not only in the regulation of plant growth and organogenesis, but also in the biosynthesis of primary and secondary metabolites29, 30).
As shown in Figs. 1 and 2, cell cultures of Angelica archangelica grew as a homogeneous suspension consisting of cell clumps with the diameter of about 150 μm. There were no morphological differences between cultures cultured under heterotrophic and photomixotrophic conditions. No cell differentiation or organogenesis was observed.
Fig. 1. Angelica archangelica cell suspension cultures cultured under heterotrophic conditions. Photomicrograph of cell clusters at a magnification of 200x and 400x (the scale is 50 and 20 μm, resp.). 
Fig. 2. Angelica archangelica cell suspension cultures cultured under photomixotrophic conditions. Photomicrograph of cell clusters at a magnification of 200x and 400x (the scale is 50 and 20 μm, resp.). 
Effects of zinc ions on angelica cell suspension cultures are presented in Table 2. Zinc sulphate is a component of the basal Murashige and Skoog medium (MS medium) at a concentration of 30 μM. Fresh biomass was not significantly influenced in a range of zinc concentrations from 0 (MS medium without zinc sulphate) to 150 and 300 μM in the dark-grown and light-grown cultures, respectively. Then it declined with an increasing zinc level. Zinc ions at 1500 μM diminished it by 54% and 24% in the dark-grown and light-grown cultures, respectively, in comparison to control cultures in the standard MS medium. Dry biomass was influenced in a similar way. Zinc ions at 1500 μM reduced dry cell weight by 30% and 20% in cultures cultured in the dark and in the light, respectively. As for production of coumarins, elimination of zinc from the medium as well as its concentrations up to 300 μM did not markedly affect the levels of coumarins in cells and medium in the dark-grown and light-grown cultures. Higher zinc concentrations decreased the contents of coumarins in cells under both light conditions; in the medium, coumarins declined correspondingly in the cultures cultured in the light, but rose in those in the dark, which could be due to damages in the cell membrane or to cell lysis, as a consequence of zinc toxicity. In the same way as in angelica cell cultures, fresh and, similarly to a lesser extent, dry weights of cultured tomato cells decreased with increasing zinc concentrations from 500 to 5000 μM, markedly when zinc concentration was higher than 1000 μM29). Toxicity of zinc was investigated and compared in cell suspension cultures of Arabidopsis halleri, a zinc hyperaccumulator, and Mesembryanthemum crystallinum, a plant shown to be sensitive to zinc: the dry cell weight did not change statistically with increasing zinc concentration in cultures of the former exposed to zinc concentrations up to 1000 μM, whereas cultures of the latter showed inhibition of growth in the presence of increasing concentrations of zinc, considerably from the concentration of 500 μM 25). Zinc ions at concentrations from 300 to 900 μM could cause any significant increase in the overall productivity of the betalains in hairy root cultures of Beta vulgaris32). A slightly positive effect on the production of betalains and no influence on the growth had zinc in cell suspension cultures of Beta vulgaris33). Increased concentration of zinc did not influence cell growth and inhibited camptothecin biosynthesis in Camptotheca acuminata suspension cultures27). Zinc ions decreased biosynthesis of α-tocopherol and improved pigment production in Carthamus tinctorius cell suspension cultures28). Zinc did not significantly affect the growth of Papaver bracteatum cell cultures or alkaloid yields35). Zinc seems to be not too suitable for elicitation of secondary metabolites in plant tissue cultures.
2. Effects of zinc ions on cell growth and production of coumarins in Angelica archangelica cell suspension cultures. Values are means ± standard deviations (n = 3). Asterisks denote significant differences between control (30 μM Zn<sup>2+</sup> as in standard Murashige and Skoog medium) and Zn-treated cultures, P < 0.05. 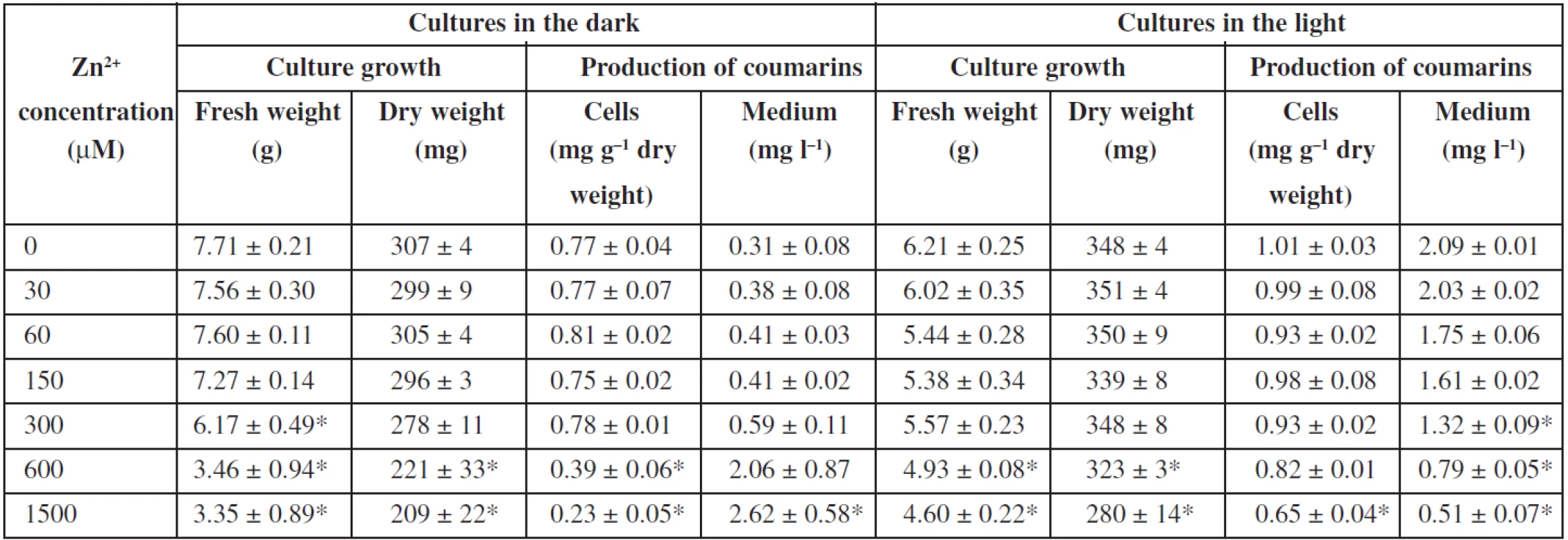
The influence of cadmium ions on angelica cell suspension cultures is shown in Table 3. The presence of cadmium ions did not affect fresh and dry weights of cells up to concentrations of 10 μM and 50 μM in cultures in the dark and in the light, respectively. Toxic concentrations of cadmium are by an order of magnitude lower than those of zinc. Cadmium ions at 50 μM reduced fresh and dry cell weights by 66% and 59%, respectively, in cultures cultured in the dark. Cadmium at 100 μM caused a decrease in fresh and dry biomass by 40% and 44%, respectively, in the light-grown cultures. With regard to biosynthesis of coumarins, addition of cadmium ions to the culture medium did not improve or decrease the production of coumarins in Angelica archangelica cell suspension cultures. The findings are in agreement with those in the whole plant cultures of Centella asiatica, where the asiaticoside production declined in the presence of cadmium ions36). Cadmium from 1 to 500 μM caused growth depression depending on its concentration in whole plant cultures of Dionaea muscipula; on the other hand, an increasing cadmium level led to enhanced anthocyanin biosynthesis37). An increase in growth and, on the contrary, an inhibition of growth occurred in Nicotiana tabacum cell suspension cultures grown at 100 and 200 μM cadmium, respectively38). The dry weight accumulation in the suspension culture of Catharanthus roseus treated with 50 μM cadmium was not different from that of the control culture; cadmium concentrations up to 400 μM lowered culture dry weight, and concentrations higher than 600 μM were lethal for the culture39). Cadmium ions at a concentration of 25 μM reduced the dry biomass yield by about 50% and increased production of tanshinones about 11-fold in cell suspension cultures of Salvia miltiorrhiza8). Production of coumarins in angelica cell cultures was not improved by cadmium, whereas that of gymnemic acid, ajmalicine, and sanguinarine in suspension cultures of Gymnema sylvestre28), Catharanthus roseus39), and Papaver somniferum40), respectively, was stimulated by cadmium treatment.
3. Effects of cadmium ions on cell growth and production of coumarins in Angelica archangelica cell suspension cultures. Values are means ± standard deviations (n = 3). Asterisks denote significant differences between control (without Cd<sup>2+</sup>) and Cd-treated cultures, P < 0.05. 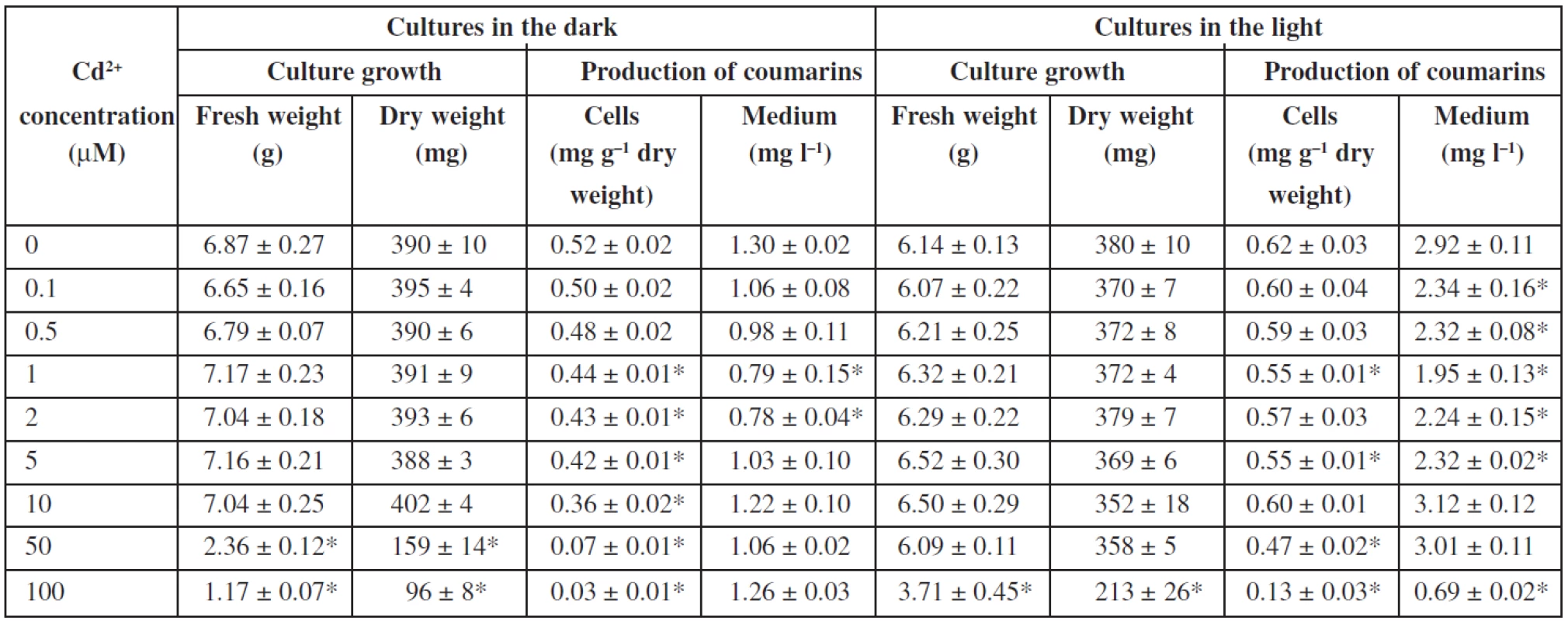
There are many reports concerning the influence of zinc and cadmium on plants41–47). Various mechanisms of how a plant cell reacts to an excess of essential heavy metal ions, e. g. zinc, or the toxic ones that do not play a role in metabolism, e. g. cadmium, were described. They include an increase in the activities of antioxidant enzymes38) and enhanced biosynthesis of amino acids (such as histidine48) and cysteine37)), glutathione21), phytochelatins49), metallothioneins48), organic acids (such as citrate and malate)50), and secondary metabolites33, 39). The latter mentioned way of plant cell reaction attracts research attention because of its possible use for stimulation of secondary metabolism in plant tissue cultures. However, particular mechanisms occur to a different extent depending on the plant species and the kind of heavy metal. On the one hand, several of them, at various intensities, may run together – side by side or one after another. On the other hand, some of them can be involved weakly or not at all. Based on our results, increased biosynthesis of secondary metabolites does not participate in response of angelica cultures to zinc and cadmium treatment. It can be concluded that zinc and cadmium ions are not suitable as elicitors enhancing the production of coumarins in Angelica archangelica cell suspension cultures.
This work was financially supported by the grant of Charles University in Prague SVV 265 004.
Conflicts of interest: none.
Received 5 October 2012 / Accepted 14 November 2012
Tomáš Siatka, Marie Kašparová, Jiřina Spilková
Address:
PharmDr. Tomáš Siatka, CSc.
Charles University in Prague, Faculty of Pharmacy in Hradec Králové,
Department of Pharmacognosy
Heyrovského 1203, 500 05 Hradec Králové
e-mail: siatka@faf.cuni.cz
Sources
1. Jenkins T., Bovi A., Edwards R. Plants: biofactories for a sustainable future? Phil. Trans. R. Soc. A 2011; 369, 1826–1839.
2. Dvořáková M., Valterová I., Vaněk T. Monoterpeny v rostlinách. Chem. Listy 2011; 105, 839–845.
3. Hussain M. S., Fareed S., Ansari S., Rahman M. A., Ahmad I. Z., Saeed M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied. Sci. 2012; 4, 10–20.
4. Kolewe M. E., Gaurav V., Roberts S. C. Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol. Pharmaceut. 2008; 5, 243–256.
5. Zhao J, Davis L. C., Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005; 23, 283–333.
6. Mithöfer A., Schulze B., Boland W. Biotic and heavy metal stress response in plants: evidence for common signals. FEBS Lett. 2004; 566, 1–5.
7. Kašparová M., Dušek J. Effect of biotic elicitation on the production of anthraglycosides by the tissue culture of Rheum palmatum L. Čes. slov. Farm. 1999; 48, 132–135.
8. Zhao J. L., Zhou L. G., Wu J. Y. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol. 2010; 87, 137–144.
9. Yendo A. C. A., de Costa F., Gosmann G., Fett-Neto A. G. Production of plant bioactive triterpenoid saponins: elicitation strategies and target genes to improve yields. Mol. Biotechnol. 2010; 46, 94–104.
10. Bhagwath S. G., Hjortsø M. A. Statistical analysis of elicitation strategies for thiarubrine A production in hairy root cultures of Ambrosia artemisiifolia. J. Biotechnol. 2000; 80, 159–167.
11. Kombrink E., Hahlbrock K. Dependence of the level of phytoalexin and enzyme-induction by fungal elicitor on the growth stage of Petroselinum crispum cell cultures. Plant Cell Rep. 1985; 4, 277–280.
12. Möhle B., Heller W., Wellmann E.: UV-induced biosynthesis of quercetin 3-O-ββ-D-glucuronide in dill cell cultures. Phytochemistry 1985; 24, 465–467.
13. Reichling J., Merkel B. Elicitor-induced formation coumarin derivatives in suspension cultures of Pimpinella anisum. Planta Med. 1993; 59, 187–188.
14. Abe Y., Sawada A., Momose T., Sasaki N., Kawahara N., Kamakura H., Goda Y., Ozeki Y. Structure of an anthocyanin-anthocyanin dimer molecule in anthocyanin-producing cells of a carrot suspension culture. Tetrahedron Lett. 2008; 49, 7330–7333.
15. Staniszewska I., Królicka A., Maliński E., Łojkowska E., Szafranek J. Elicitation of secondary metabolites in in vitro cultures of Ammi majus L. Enzyme Microb. Tech. 2003; 33, 565–568.
16. Koulman A., Kubbinga M. E., Batterman S., Woerdenbag H. J., Pras N., Woolley J. G., Quax W. J. A phytochemical study of lignans in whole plants and cell suspension cultures of Anthriscus sylvestris. Planta Med. 2003; 69, 733–738.
17. Ishikawa A., Kitamura Y., Ozeki Y., Itoh Y., Yamada A., Watanabe M. Post-stress metabolism involves umbelliferone production in anthocyanin-producing and non-producing cells of Glehnia littoralis suspension cultures. J. Plant Physiol. 2005; 162, 703–710.
18. Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962; 15, 473–497.
19. Siatka T., Sklenářová H., Kašparová M., Solich P. Effects of mercury(II) chloride on production of coumarins in Angelica archangelica L. cell suspension cultures. Chem. Listy 2011; 105, 367–370.
20. Memon A. R., Schröder P. Implications of metal accumulation mechanisms to phytoremediation. Environ. Sci. Pollut. Res. 2009; 16, 162–175.
21. Yadav S. K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010; 76, 167–179.
22. Źróbek-Sokolnik A., Asard H., Górska-Koplińska K., Górecki R. J. Cadmium and zinc-mediated oxidative burst in tobacco BY-2 cell suspension cultures. Acta Physiol. Plant. 2009; 31, 43–49.
23. Padmavathiamma P. K, Li L. Y. Phytoremediation technology: hyper-accumulation metals in plants. Water Air Soil Pollut. 2007; 184, 105–126.
24. Hu Y. T., Ming F., Chen W. W., Yan J. Y., Xu Z. Y., Li G. X., Xu C. Y., Yang J. L., Zheng S. J. TcOPT3, a member of oligopeptide transporters from the hyperaccumulator Thlaspi caerulescens, is a novel Fe/Zn/Cd/Cu transporter. PLOS one 2012; 7, e38535.
25. Vera-Estrella R., Miranda-Vergara M. C., Barkla B. J. Zinc tolerance and accumulation in stable cell suspension cultures and in vitro regenerated plants of the emerging model plant Arabidopsis halleri (Brassicaceae). Planta 2009; 229, 977–986.
26. Li T., Xu Z., Han X., Yang X., Sparks D. L. Characterization of dissolved organic matter in the rhizosphere of hyperaccumulator Sedum alfredii and its effect on the mobility of zinc. Chemosphere 2012; 88, 570–576.
27. Pan X. W., Shi Y. Y., Liu X., Gao X., Lu Y. T. Influence of inorganic microelements on the production of camptothecin with suspension cultures of Camptotheca acuminata. Plant Growth Regul. 2004; 44, 59–63.
28. Ch B., Rao K., Gandi S., Giri A. Abiotic elicitation of gymnemic acid in the suspension cultures of Gymnema sylvestre. World J. Microbiol. Biotechnol. 2012; 28, 741–747.
29. Yousefzadi M., Sharifi M., Behmanesh M., Ghasempour A., Moyano E., Palazon J. The effect of light on gene expression and podophyllotoxin biosynthesis in Linum album cell culture. Plant Physiol. Biochem, 2012; 56, 41–46.
30. Chan L. K., Koay S. S., Boey P. L., Bhatt A. Effects of abiotic stress on biomass and anthocyanin production in cell cultures of Melastoma malabathricum. Biol. Res. 2010; 43, 127–135.
31. Muschitz A., Faugeron C., Morvan H. Response of cultured tomato cells subjected to excess zinc: role of cell wall in zinc compartmentation. Acta Physiol. Plant. 2009; 31, 1197–1204.
32. Savitha B. C., Thimmaraju R., Bhagyalakshmi N., Ravishankar G. A. Different biotic and abiotic elicitors influence betalain production in hairy root cultures of Beta vulgaris in shake-flask and bioreactor. Process Biochem. 2006; 41, 50–60.
33. Trejo-Tapia G., Jimenez-Aparicio A., Rodriguez-Monroy M., De Jesus-Sanchez A., Gutierrez-Lopez G. Influence of cobalt and other microelements on the production of betalains and the growth of suspension cultures of Beta vulgaris. Plant Cell Tiss. Org. Cult. 2001; 67, 19–23.
34. Chavan S. P., Lokhande V. H., Nitnaware K. M., Nikam T. D. Influence of growth regulators and elicitors on cell growth and α-tocopherol and pigment productions in cell cultures of Carthamus tinctorius L. Appl. Microbiol. Biotechnol. 2011; 89, 1701–1707.
35. Lecky R., Hook I., Sheridan H. Enhancement of dihydrosanguinarine production in suspension cultures of Papaver somniferum, I. Medium modifications. J. Nat. Prod. 1992; 55, 1513–1517.
36. Kim O. T., Kim M. Y., Hong M. H., Ahn J. C, Hwang B. Stimulation of asiaticoside accumulation in the whole plant cultures of Centella asiatica (L.) Urban by elicitors. Plant Cell Rep. 2004; 23, 339–344.
37. Babula P., Ryant P., Adam V., Zehnalek J., Havel L., Kizek R. The role of sulphur in cadmium(II) ions detoxification demonstrated in in vitro model: Dionaea muscipula Ell. Environ. Chem. Lett. 2009; 7, 353–361.
38. Gratão P. L., Pompeu G. B., Capaldi F. R., Vitorello V. A., Lea P. J., Azevedo R. A. Antioxidant response of Nicotiana tabacum cv. Bright Yellow 2 cells to cadmium and nickel stress. Plant Cell Tiss. Organ Cult. 2008; 94, 73–83.
39. Zheng Z., Wu M. Cadmium treatment enhances the production of alkaloid secondary metabolites in Catharanthus roseus. Plant Sci. 2004; 166, 507–514.
40. Balážová A., Blanáriková V., Bilka, F., Bilková A., Kiňová Sepová H. The effect of three different elicitors on sanguinarine production in suspension cultures of a low-morphine variety of the opium poppy (Papaver somniferum L.). Čes. slov. Farm. 2011; 60, 237–340.
41. Li D. D., Zhou D. M. Acclimation of wheat to low-level cadmium or zinc generates its resistance to cadmium toxicity. Ecotox. Environ. Safe. 2012; 79, 264–271.
42. Sanaeiostovar A. Khoshgoftarmanesh A. H. Shariatmadari H., Afyuni, M., Schulin R. Combined effect of zinc and cadmium levels on root antioxidative responses in three different zinc-efficient wheat genotypes J. Agron. Crop Sci. 2012; 198, 276–285.
43. Hao X. Z., Zhou D. M., Li D. D., Jiang P. Growth, cadmium and zinc accumulation of ornamental sunflower (Helianthus annuus L.) in contaminated soil with different amendments. Pedoshere 2012; 22, 631–639.
44. Martin S. R., Llugany M., Barcelo J., Poschenrieder C. Cadmium exclusion a key factor in differential Cd-resistance in Thlaspi arvense ecotypes. Biol. Plant. 2012; 56, 729–734.
45. Dhankhar R., Sainger P. A., Sainger M. Phytoextraction of zinc: physiological and molecular mechanism. Soil Sediment Contam. 2012; 21, 115–133.
46. Najmanova J., Neumannova E., Leonhardt T., Zitka O., Kizek R., Macek T., Mackova, M., Kotrba P. Cadmium-induced production of phytochelatins and speciation of intracellular cadmium in organs of Linum usitatissimum seedlings. Ind. Crop Prod. 2012; 36, 536–542.
47. Remans T., Opdenakker K., Guisez Y., Carleer R., Schat H., Vangronsveld J., Cuypers A. Exposure of Arabidopsis thaliana to excess Zn reveals a Zn-specific oxidative stress signature. Environ. Exp. Bot. 2012; 84, 61–71.
48. Verbruggen N., Hermans C., Schat H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009; 181, 759–776.
49. Zenk M. H. Heavy metal detoxification in higher plants – a review. Gene 1996; 179, 21–30.
50. Rascioa N., Navari-Izzo F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011; 180, 169–181.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2012 Issue 6-
All articles in this issue
- Comparison of sanguinarine production in suspension cultures of the Papaveraceae plants
- Evaluation of content uniformity of tablets with a low content of the active ingredient with a narrow therapeutic index
- Analysis of pharmaceutical care in dispensing of over-the-counter orlistat
-
Study of local anaesthetics – Part 200
Choice of the optimal type of chitosan for the formulation of local anaesthetics of the carbamate type into hydrogels -
TEACHING COMMUNICATION SKILLS IN PHARMACY PRACTISE
TO KNOW AND TO DO ARE TWO DIFFERENT THINGS -
PATIENT COUNSELING IN PHARMACY PRACTICE
OPTIMIZATION OF DRUG THERAPY - The Czech Pharmaceutical Society 1871 - 2012
- INSTRUCTIONS FOR THE AUTHORS SUBMITTING PAPERS TO THE JOURNAL CZECH AND SLOVAK PHARMACY
- Halloysite – interesting nanotubular carrier for drugs
- Effects of zinc and cadmium ions on cell growth and production of coumarins in cell suspension cultures of Angelica archangelica L.
- Phenyl salicylates – a new group of potential antituberculotics
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Evaluation of content uniformity of tablets with a low content of the active ingredient with a narrow therapeutic index
- Analysis of pharmaceutical care in dispensing of over-the-counter orlistat
- Halloysite – interesting nanotubular carrier for drugs
- Comparison of sanguinarine production in suspension cultures of the Papaveraceae plants
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career



