-
Medical journals
- Career
Effects of combined hormonal deprivation and fungal elicitation on the production of coumarins in cell suspension cultures of Angelica archangelica L.
Authors: T. Siatka; M. Kašparová
Authors‘ workplace: Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmacognosy, Czech Republic
Published in: Čes. slov. Farm., 2009; 58, 168-171
Category: Original Articles
Overview
The effectiveness of elicitation as a tool to enhance the production of secondary metabolites in plant tissue cultures depends on a complex interaction between the elicitor and the plant cell. The paper investigated the influence of removal of plant growth regulators and light conditions on the cell growth and elicitation of coumarins in Angelica archangelica cell suspension cultures. An autoclaved homogenate of Pythium aphanidermatum was used as the elicitor. Cell cultures of Angelica archangelica were cultured in a medium supplemented with 2,4-dichlorophenoxyacetic acid and benzylaminopurine or in a hormone free medium, and in the dark or under continuous light. The culture growth (both fresh and dry biomass) was not affected adversely in the presence of the fungal elicitor as compared with the control cultures. The contents of coumarins in cells were influenced slightly. The content of coumarins in the medium was stimulated by hormonal deprivation and elicitation depending on the light conditions; the best results were achieved in the dark-growing angelica cell suspension cultures cultured in the hormone free medium. Removal of growth regulators from the culture medium brought about 5-fold increase in coumarins, and by combining hormonal deprivation and fungal elicitation, a 15-fold enhancement of coumarins was achieved, in comparison with control cultures cultured in the presence of plant growth regulators and without elicitor treatment.
Key words:
Angelica archangelica L. – cell suspension cultures – growth – coumarins – growth regulators – light – elicitation – Pythium – flow injection analysisIntroduction
Plant secondary metabolites are unique sources for pharmaceuticals, food additives, flavours, and other industrial materials. Plant cell culture technology shows promise for a large-scale production of valuable plant products. However, it still faces many biological and biotechnological limitations. One of the major obstacles is a low yield of plant secondary metabolites in plant cell cultures 1, 3). Many approaches have been developed to overcome this problem 2, 3), the most notable strategy for improving metabolite yields perhaps being elicitation 4). Elicitors are chemicals or biofactors from various sources that can induce an upregulation of genes. Some elicitors target secondary metabolic genes, which are often associated with defence responses to perceived environmental changes 1, 4) Elicitors may be biotic or abiotic. The biotic elicitors are of biological origin, derived from a pathogen (fungi, bacteria, viruses or herbivores) or from the plant itself (e.g., plant cell wall components). Biotic compounds can be of defined composition, when their molecular structures are known, or have a complex composition when they comprise several different molecular classes making it impossible to define a unique chemical identity (e.g., fungal homogenates or culture filtrates). On the other hand, abiotic elicitors are not of a biological origin and are grouped in physical factors (such as thermal and osmotic stress, radiation, and wounding) and chemical compounds (e.g., heavy metal salts) 5). It is well known that treatment with elicitors causes an array of defence reactions, including the accumulation of secondary metabolites in intact plants 6–8) as well as in cell cultures 9–11). The effectiveness of elicitation as a tool to enhance the production of secondary metabolites depends on a complex interaction between the elicitor and the plant cell. The main factors that can affect this interaction and thereby the elicitation response include elicitor specificity, elicitor concentration and elicitation time, and culture conditions (such as the growth stage, medium composition, and light) 5).
We report here the effects of elicitor concentration, removal of plant growth regulators, and light conditions on the cell growth and elicitation of coumarins in Angelica archangelica cell suspension cultures. An autoclaved homogenate of Pythium aphanidermatum was used as the elicitor.
EXPERIMENTAL PART
Chemicals
2,4-dichlorophenoxyacetic acid and 6-benzylaminopurine (Sigma, St. Louis, U.S.A.); scopoletin (Fluka, Buchs, Germany); dibasic sodium phosphate and monobasic potassium phosphate (Lachema, Brno, Czech Republic).
Instruments
A PS 20A autoclave (Chirana, Brno, Czech Republic); a roller (Vyvojove dilny, Academy of Sciences of the Czech Republic, Praha, CR); a LGA 05 lyophilizer (Janetzki, Leipzig, Germany); a 200S analytical scale (Sartorius, Göttingen, Germany); a single-channel plunger LPC 3001 micropump, a PP05 peristaltic pump for sample delivery, and a TZ 4620 chart recorder (Laboratorni pristroje, Praha, CR); a home-made PTFE valve with exchangeable sample loops; and a Schoeffel FS 970 fluorescence detector (McPherson, Chelmsford, U.S.A.).
Cell suspension cultures and elicitor treatment
Cell suspension cultures of Angelica archangelica were established as described previously12) and grown in liquid Murashige and Skoog medium 13) supplemented with 2 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D), 0.4 mg/l benzylaminopurine (BA), and 30 g/l sucrose. They were agitated in 250 ml flasks containing 30 ml of the medium on a roller apparatus at 8 rpm, incubated at 25 °C under continuous light (3,500 lux) or in darkness, and subcultured every 14 days. The culture used in this study was kept in vitro for three years.
The freeze-dried powdered mycelia of Pythium aphanidermatum were suspended in distilled water, autoclaved (121 °C, 15 min), and appropriate amounts (0.5, 1, 5, 10, and 50 mg) in 2 ml per flask were added to angelica cell cultures on day 10 following the transfer to a fresh medium. Control cultures received 2 ml of sterile distilled water. For testing the effects of hormonal deprivation on the elicitation, cell cultures were grown as one subculture (14 days) in a medium without 2,4-D and BA, and then, in the second subculture in the medium free of growth regulators, were elicited on day 10 as mentioned above.
Elicited and control cultures were harvested 48 hours after treatment, and the growth of cultures and production of coumarins were evaluated.
Analytical procedures
Cells were separated from the culture medium by vacuum filtration using a Buchner funnel with filter paper.
Filtered cells were weighed for fresh weight determination, and then freeze-dried to obtain dry weight.
Coumarins in freeze-dried cells and in the culture medium were quantified fluorometrically by flow injection analysis as described in detail previously 14).
All data presented are the mean values of three replicates with standard deviations. Student’s t-test was used for statistical analysis of data, and differences with P < 0.05 were considered as statistically significant.
Results and discussion
In view of the variability in elicitation responses due to different factors, the optimization of medium composition and culture conditions represent an important aspect in elicitation protocols 5). Growth regulators 15–17) as well as light 18–20) influence the metabolism of plant cells, and therefore, we examined the effects of hormonal deprivation and light conditions on the elicitation of coumarins in Angelica archangelica suspension cultures after addition Pythium aphanidermatum homogenate as the elicitor. The cultures were cultured in a medium supplemented with 2,4-dichlorophenoxyacetic acid and benzylaminopurine or in a hormone free medium, and in the dark or under continuous light.
The culture growth (both fresh and dry biomass) was not affected adversely in the presence of the fungal elicitor as compared with the control cultures (Tables 1 and 2), independently of the medium composition or light conditions. Similarly to Angelica archangelica suspension cultures, the fungal elicitor did not influence the growth in Sanguinaria canadensis cell cultures grown in a medium with or without 2,4-dichlorophenoxyacetic acid 17).
1. Effect of Pythium aphanidermatum elicitor on biomass accumulation and production of coumarins in Angelica archangelica cell suspension cultures grown under dark and light conditions in the medium supplemented with growth regulators (2,4-D and BA) (Data represent as the means with standard deviations. Values with asterisks * are significantly higher in comparison with control cultures, P < 0.05) 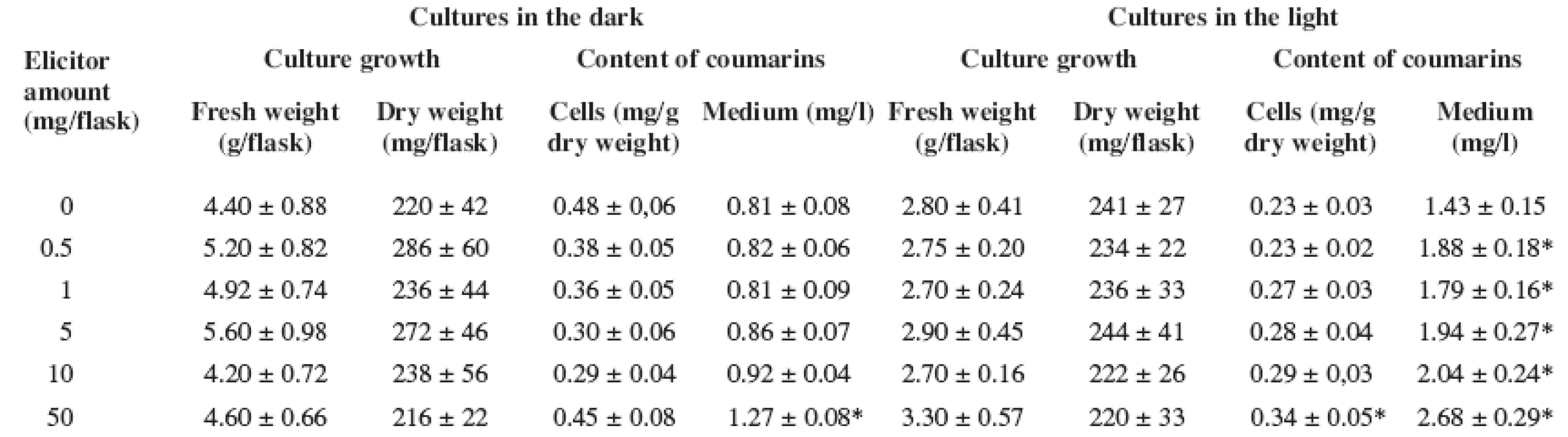
2. Effect of Pythium aphanidermatum elicitor on biomass accumulation and production of coumarins in Angelica archangelica cell suspension cultures grown under dark and light conditions in the hormone free medium (Data represent as the means with standard deviations. Values with asterisks * are significantly higher in comparison with control cultures, P < 0.05) 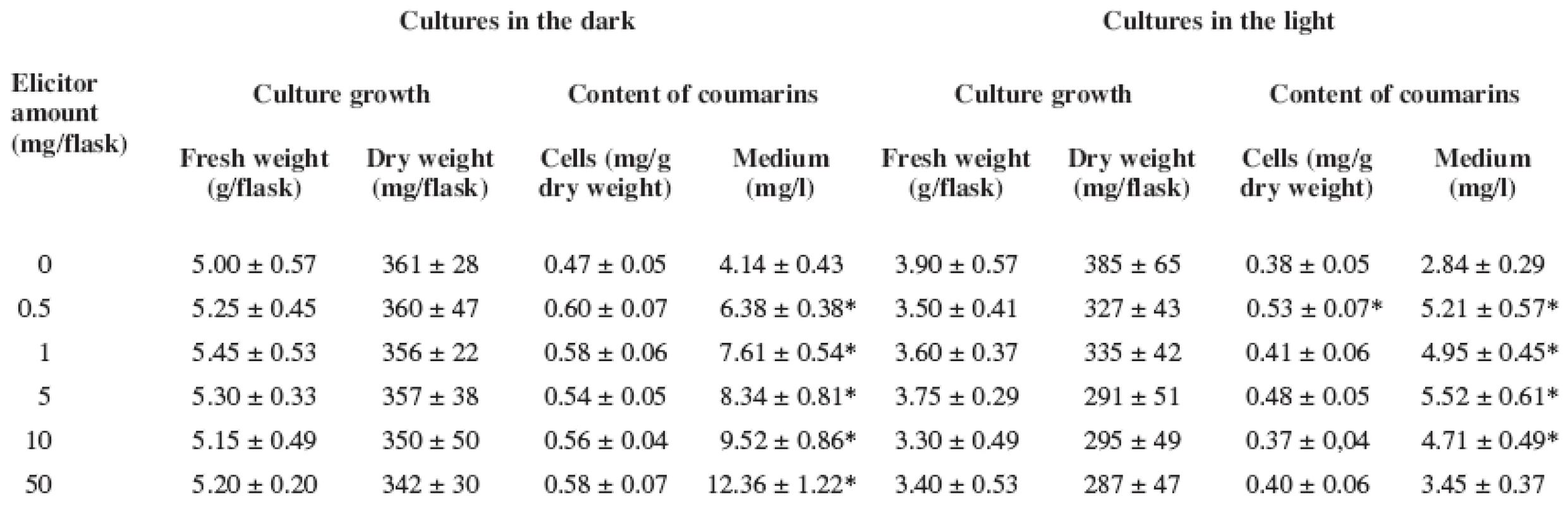
As for the production of coumarins in Angelica archangelica suspension cultures, a better response to the fungal elicitor treatment was achieved in the hormone free medium. In cultures grown in the medium with growth regulators (Table 1), enhanced accumulation of coumarins was found in the culture medium in the light. This was a 1.8-fold increase over the untreated control at the highest elicitor concentration. In cultures grown in the medium without growth regulators (Table 2), the contents of coumarins in cells were influenced only slightly under both light and dark conditions. The highest yields of coumarins were achieved in the culture medium in the dark-growing cultures; the level of coumarins rose with an increasing concentration of the fungal elicitor up to a threefold amount compared with the control cultures. Similar findings regarding the effects of combined hormonal deprivation and fungal elicitation have been reported on alkaloid accumulation in Sanguinaria canadensis cell suspension cultures 17). Our results are in agreement with those observed in Hypericum perforatum cell suspension cultures where the production of hypericin was stimulated with different effectiveness after elicitor treatment depending on light conditions 18).
This work was supported by grant No MSM 0021620822 of the Ministry of Education, Youth and Sports of the Czech Republic.
Address for correspondence:
PharmDr. Tomáš Siatka, CSc.
Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmacognosy, Czech Republic
Heyrovského 1203, 500 05 Hradec Králové
e-mail: siatka@faf.cuni.cz
Received 10 July 2009
Accepted 8 August 2009
Sources
1. Zhao, J, Davis, L. C., Verpoorte, R.: Biotechnol. Adv., 2005; 23, 283.
2. Roberts, S. C.: Nat. Chem. Biol., 2007; 3, 387.
3. Roberts, S. C., Shuler, M. L.: Curr. Opin. Biotechnol., 1997; 8, 154.
4. Kolewe, M. E., Gaurav, V., Roberts, S. C.: Mol. Pharm., 2008; 5, 243.
5. Vasconsuelo, A., Boland, R.: Plant Sci., 2007; 172, 861.
6. Bednarek, P., Osbourn, A.: Science, 2009; 324, 746.
7. Ziaratnia, S. M., Kunert, K. J., Lall, N.: S. Afr. J. Bot., 2009; 75, 97.
8. Mandal, S., Mitra, A.: Physiol. Mol. Plant Pathol., 2007; 71, 201.
9. Ferri, M. et al.: Proteomics, 2009; 9, 610.
10. De Alwis, R. et al.: J. Plant Physiol., 2009; 166, 720.
11. Roat, C. Ramawat, K. G.: Plant Biotechnol. Rep., 2009; 3, 135.
12. Siatka, T., Kašparová, M.: Čes. slov. Farm., 2008; 57, 17.
13. Murashige, T., Skoog, F.: Physiol. Plant., 1962; 15, 473.
14. Siatka, T., Solich, P., Kotyk, R.: Pharmazie, 1998; 53, 273.
15. Santner, A., Calderon-Villalobos, L. I. A., Estelle, M.: Nat. Chem. Biol., 2009; 5, 301.
16. Jeong, G.-T, Woo, J-C., Park, D.-H.: Biotechnol. Bioprocess Eng., 2007; 12, 86.
17. Cline, S. D., McHale, R. J., Coscia, C. J.: J. Nat. Prod., 1993; 56, 1219.
18. Walker, T. S., Bais, H. P., Vivanco, J. M.: Phytochemistry, 2002; 60, 289.
19. Jacques, P. et al.: Acta Bot. Gall., 2007; 154, 21.
20. Ceoldo, S. et al.: Plant Sci., 2009; 176, 553.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2009 Issue 4-
All articles in this issue
- Standard prescriptions for the formulation of medicinal preparations in pharmacies III Some possibilities of using isopropyl alcohol
- Determination of the constituents of propolis of different geographical origin
- Cardioprotective effect of 2’,3,4’--trihydroxychalcone in preclinical experiment
- Determination of the coating thickness of HPMC hard capsules by near-infrared reflectance spectroscopy
- The role of flavonoid osajin in renal ischemia-reperfusion model
- Effects of combined hormonal deprivation and fungal elicitation on the production of coumarins in cell suspension cultures of Angelica archangelica L.
- Studies of the properties of tablets from directly compressible isomalt
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Standard prescriptions for the formulation of medicinal preparations in pharmacies III Some possibilities of using isopropyl alcohol
- Determination of the constituents of propolis of different geographical origin
- Studies of the properties of tablets from directly compressible isomalt
- Determination of the coating thickness of HPMC hard capsules by near-infrared reflectance spectroscopy
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

