-
Medical journals
- Career
Therapeutic potential of phytocannabinoids and synthetic derivatives affecting human endocannabinoid system
Authors: J. Péč 1; E. Riedingerová 2; J. Martin 1; Z. Kršková 1; J. Dušek 1
Authors‘ workplace: Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmacognosy 1; Palacký University Olomouc, Faculty of Medicine 2
Published in: Čes. slov. Farm., 2008; 57, 195-207
Category: Review Articles
Overview
From the pharmaceutical and medicinal point of view Cannabis sativa L. is an interesting and perspective material. It contains biologically active cannabinoids whose isolation and identification in the 1960’s enabled a rapid research that has been bringing interesting results to this day. Especially revealing the human endocannabinoid system and the pharmacological effects of cannabinoids or derived synthetic compounds are the most interesting areas. The research in this field continues also toward pharmaceutical dosage forms with convenient route of administration and pharmacokinetics parameters to be used in clinical practice. Cannabinoids open completely new approaches in the treatment of many relevant human diseases and can become perspective and potential remedies. This review article deals with the effects of cannabinoids on human endocannabinoid system, their use in pharmacotherapy, their adverse effects, their interactions with other drugs and the convenient pharmaceutical dosage forms. Additional information concerning laws valid in the Czech Republic and used analytical forensic methods of cannabinoids are reviewed here as well.
Key words:
Cannabis sativa – cannabinoids – endocannabinoid system – pharmacotherapy – adverse effects – drug interactions – legislationCannabis sativa L., also known as cannabis or hemp, from the family Cannabaceae is an annual, dioecious herb which can grow up to 6 meters in height. Palmate leaves, that consist of 3 to 15 lanceolate folioles with dentate border, are typical for the plant 1). The staminate flowers formed racemose inflorescence and pistillate flowers form cyme panicles. The fruits (the hemp seed) are achenes, ensphered with dry perianths and enclosed in supporting bract 2). Cannabis originated in a large area that extends from the Caspian Sea over the central Russia to the north of India and the Himalayas 3). Cannabis is well known especially for its psychotropic effects which are similar to the ones of alcohol intoxication. Feeling of euphoria that slowly changes into a pleasant feeling of calmness and relaxation is called “high”. Cannabis users can also experience sedation effects, cheerfulness, feeling of hunger, heightened sensitivity to perception of colour and music, lethargy and disrupted sense of time and space. These effects are unwanted in the therapeutic use and researchers endeavour to separate them out from the desired effects 4–6).
Content of bioactive cannabis metabolites depends especially on genetic strains and on the climate in which the plants are grown (Table 1). From the secondary plant metabolites point of view, the cannabis is a unique plant that contains cannabinoids which are terpenophenolic, generally C21 tricyclic, compounds that do not occur in any other plant species. Cannabinoids are optically active, whereas for therapeutic effect their importance consists in the chiral centres 6aR and 10aR, respectively the phenolic and methyl group in positions 1 and 9, and the side aliphatic chain which is, in the case of natural cannabinoids, five or three-carbon (Fig. 1). Cannabinoids are secreted by glandular stalked trichomes in the form of resin and, depending on their morphological location on the pant, material of different quality can be obtained (Table 2). The glandular trichomes are highly specialized secretory tissue abundant on calyx, bracts and leaves 3, 7).The most active and discussed representatives are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) to which the researchers pay the greatest attention. Indeed, around 70 different cannabinoids are known to be present in the plant drug at the same time and, depending on the plant variety cultivated, they could be present in different ratio. Cannabinoids are present in fresh plant material; mainly in the form of carboxylic acid which is considered a primary metabolite. During the plant growth, by subsequent storage or exposure to heat and light, the metabolites are decarboxylated to neutral derivatives. Δ9-Tetrahydrocannabinol-2-carboxylic acid (THCA) is thus transformed into neutral, active THC form 1, 5, 8).
1. Main representatives of the phytocannabinoids, their synthetic derivatives and the endogenous cannabinoids 3, 8) *Several numbering system of the cannabinoids exists. For this article the systematic numbering is used as presented on the THC chemical structure. **U-shape conformation of the depicted endogenous cannabinoids widely corresponds with the THC structure and based on this similarity is explained the same receptor system impact by so structurally different molecules. 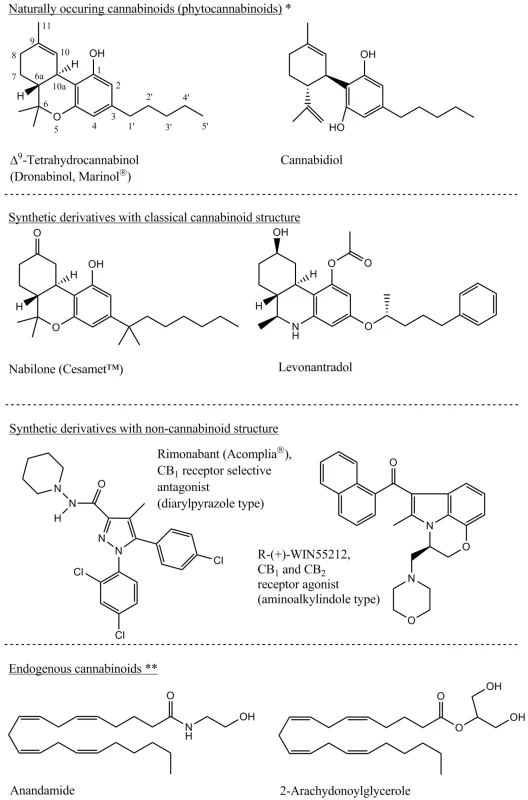
1. Cannabis plants division based on the main cannabinoids production 1, 70, 74, 75) 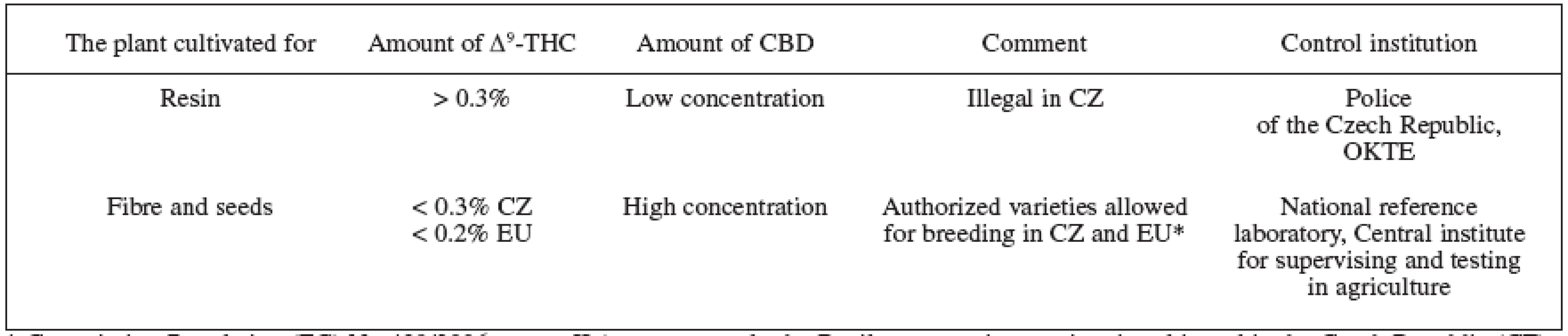
* Commission Regulation (EC) No 489/2006, annex II (at present, only the Beniko monoecious variety is cultivated in the Czech Republic (CZ) on approximately 1500 ha). 2. THC content in different parts of the plant and the names used 7, 9, 28, 68) 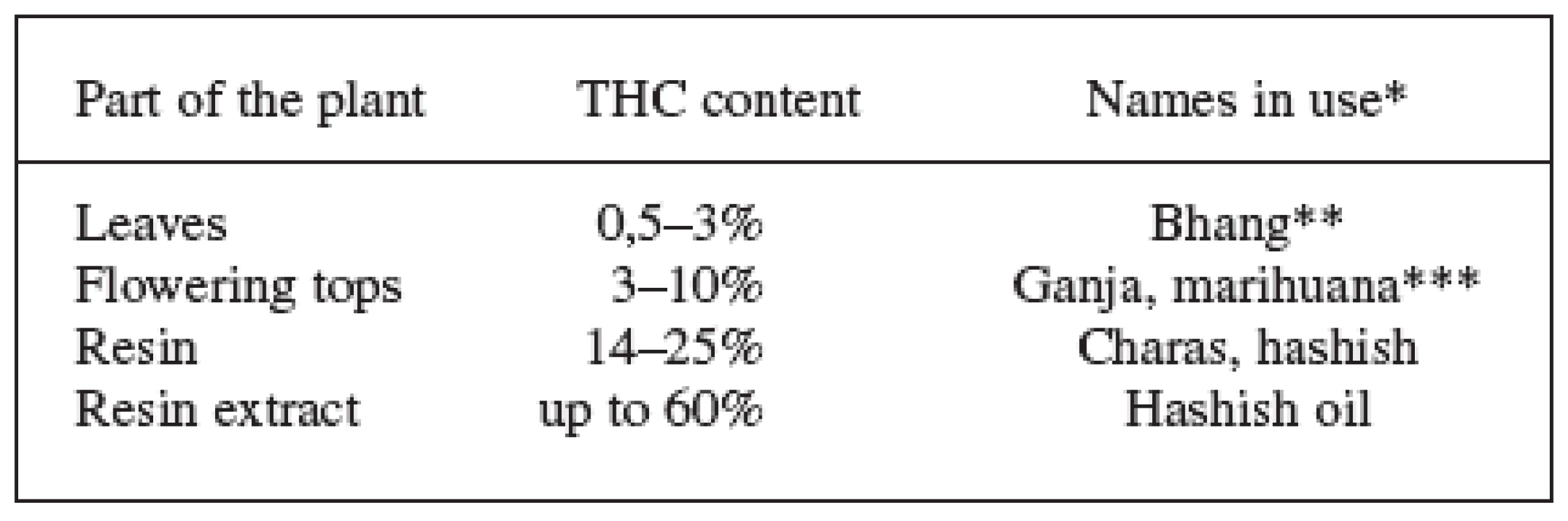
*The terms bhang, ganja and charas originated in India, hashish comes from Arabic and marihuana from Mexico. Other names in use come from Africa: kief and dagga. The Czech slang terms are: tráva, joint, brčko or žilka. **Bhang is low quality material composed of flowers with seeds, leaves and stems. ***Ganja are infertile female flowering tops without seeds and represents high quality material. The word marihuana was originally used to denote cheap tobacco; today, this term is used to indicate cannabis dry leaves and flowering tops. These facts, supplemented with different pharmacological activities of individual cannabinoids and their mutual interactions in the human body, lead to a complicated system that is difficult to evaluate using standard pharmacological and clinical trials. Most of the research is thus focused on the two main cannabinoids, in particular THC and CBD, that also this paper deals with 3). The other cannabinoids also show some degree of activity on the cannabinoid receptors ranging from agonistic action (cannabinol, Δ8-tetrahydrocannabinol) to antagonistic (tetrahydrocannabivarin – cannabinoid with C-3 side chain). Cannabigerol and cannabichromene are another important metabolites with antibacterial, anti-inflammatory and analgesic effects 9, 10). Important source of pharmacological information is also a whole range of chemically changed molecules of plant cannabinoids and information on compounds with completely different structures which interact as agonists or antagonists on the cannabinoid receptors (Fig. 1). Other secondary metabolites of cannabis – like flavonoids, terpenoids, phytosterols, lignans and dihydrostilbenoids – are additional important compounds with effects on human organism. These can also participate on the overall effect of medicinal cannabis by influencing pharmacokinetics and pharmacodynamics of the cannabinoids 3, 10).
Endocannabinoid system
Cannabinoid receptors and endogenous cannabinoids form the endocannabinoid system (ES). Based on the new learnings, this system also includes enzymes of formation and degradation of endocannabinoids and together with a specific membrane transporter form a possible place for interaction with potential drugs 11). Phytocannabinoids present in the plant are not chemically similar to endogenous substances, but share their effects (Fig. 1). Originally the effects of cannabinoids were attributed to the nonspecific mechanism of action and it was believed they interact with phospholipid constituents of biological membranes because of their high lipophility. In the year 1988, a receptor with high affinity to cannabinoids was discovered, it was named CB1, and this one was later followed by the second receptor CB2 in 1993. These important discoveries started research of completely new human signal transduction system. Cannabinoid (CB) receptors are evolutionary very old and they could be found also in another mammals, birds, amphibians or fish 3).CB1 and CB2 are the members of G protein-coupled (Gi/o) family of receptors connected negatively to adenylate cyclase, where the formation of cAMP is lesser. The positively influenced mitogen-activated protein kinase is yet another messenger system. CB1 receptors, via the activation of the mentioned transduction pathways, are also coupled to various types of ion channels (K+, Ca2+), modulation of nitric oxide production, mobilisation of arachidonic acid, and probably also influence by another mechanisms of action that, at present, are not yet completely understood and may not be connected to the known receptor systems 3, 12). Some of the endocannabinoid and phytocannabinoid effects are probably transduced by other types of receptors, like transient potential vanilloid type-1 channel TRPV1, further by peroxisome-proliferator-activated receptor PPARγ, or can serve as an allosteric modulators on the bonding sites, for instance in muscarinic and glutamate receptors 12–14).
Other important finding is the arrangement of ES in the human body. CB1 receptors are the most abundant in the central nervous system (CNS), where their heterogeneous distribution plays important role in the effect of cannabinoids on individual parts of the brain. Based on this knowledge, we can clarify the influence of the presence of CB1 receptors in the cerebral cortex, hippocampus, basal ganglia or cerebellum on cognition, memory or motor functions 12). Relationship to emotion or stress is connected with the distribution of CB1 in amygdala, hippocampus, hypothalamus, prefrontal cortex; hypothalamus, nucleus accumbens, vagus nerve and nodose ganglion are linked to the feeding behaviour. Another important location of CB1 receptors are neurons of algesia perception in the brain itself (periaqueductal gray, rostroventral medulla, thalamus, hypothalamus, brain cortex, limbic areas, amygdala) and also in the brain’s periphery (dorsal horns of the spinal cord, afferent nerve fibres) 13, 15–19). CB1 receptors are also present in the gastrointestinal and reproductive tissues, in some immune cells, sympathetic ganglia, heart, vascular endothelium, lung, urinary bladder, adrenal glands and hypophysis. The location of the ES in various nerve terminals provides the connection of cannabinoid activity with excitatory and inhibitory neurotransmitters like acetycholine, noradrenaline, γ-aminobutyric acid (GABA), dopamine, glutamate or 5-hydroxytryptamine, whose releasing is ordinarily inhibited by CB receptors action. The effect on the excitatory neurons is called depolarization-induced suppression of excitation; for inhibitory neurons, it is depolarization-induced suppression of inhibition. This is a type of short-term synaptic plasticity and, together withlong-term synaptic plasticity, it can ES influence structural and functional character of neurons and synaptic connections that can persist hours to weeks and have important implications on various forms of memory, learning and other functions of CNS 3, 12, 13, 16). Regulation of CB receptors is retrograde, which means that endocannabinoids are secreted from the postsynaptic membrane and then influence the presynapticaly located cannabinoid receptors. ES is an important part of nervous system that guarantees the functional and structural homeostasis and it is attributed the role of “stress-recovery” regulator 20–22).
CB2 receptors and their functions in the organism are much less studied and explored than the case of CB1. The activity of CB2, just like in case of CB1, is negatively coupled with adenylate cyclase but without affecting the ion channels 23). CB2 are mostly located in the immune system, especially in the B lymphocytes and natural killer cells, also in macrophages and T lymphocytes.Spleen, thymus, pancreas and tonsils are more places with high abundance of CB2 receptors. The production of cytokines is regulated here and this influences a wide spectrum of immune functions, for instance, the production of antibodies and proliferation and migration of leukocytes 3, 12, 24). Newer studies notice, that CB2 receptors are also located in the CNS, especially in microglial cells and astrocytes, where they probably play important functions in the inflammatory processes 13, 25, 26).
To-this-day discovered human endogenous cannabinoids are the derivatives of arachidonic acid (Fig. 1). The most important ones include N-arachydonoylethanolamide (anandamide), 2-arachidonoylglycerole (2-AG) and 2-arachidonoylglycerylether (noladin ether). Anandamide acts as a partial agonist of both CB receptors and can be compared with THC. 2-AG is also agonist of both receptors, but with higher affinity than anandamide. Noladin ether has got significantly higher affinity to CB1. These mediators are synthesized de novo from the phospholipids of cell membranes and no reserves are stored 20, 21, 27). The triggering impulse is the depolarization of the postsynaptic membrane accompanied by an increased concentration of Ca2+, or the activation of specific G protein-coupled type (Gq/11) receptors accompanied by the liberation of intracellular storages of Ca2+. The combination of both is also possible in which the relation to interconnection of several neurotransmitter systems can be observed 11, 20). Released endocannabinoids are incorporated into the presynaptic plasma membrane where they interact with CB receptors. During short time, they are transported from the extracellular space by passive or facilitated diffusion, endocytosis or by specific transport carrier back to the cells and enzymatically hydrolysed, however transport mechanism remains controversial subject. Anandamide is cleaved mainly by fatty acid amide hydrolase (FAAH) located postsynapticaly on the membranes of endoplasmic reticulum, Golgi apparatus or mitochondria, while 2-AG by monoacylglycerol lipase located presynapticaly in the cytosol. Different substrate selectivity and heterogeneous tissue location of the hydrolysing enzymes (others have also been identified) can be correlated with the regulation of level and effect duration of diverse endocannabinoids or, possibly, with the regulation of different transduction pathways by one endocannabinoid 11, 15, 28, 29). Cyclooxygenase-2 (COX-2) also takes part in the degradation processes and could influence the tone of ES. Specific metabolites of prostaglandin type are formed (PGE2-G, PGI2-G) from COX-2 metabolism with biological activity on immune, neurological or vascular levels. Interesting association could be also found with COX inhibitors used as analgesic drugs 30).
The psychoactive phytocannabinoid THC is responsible for effects of cannabis with its agonistic activity on CB1 and CB2 receptors. However in some conditions has been found to behave as antagonist, in particular influenced by the density and coupling efficiencies of the receptors, what could have implications in some diseases. Because of the agonistic activity, it became the main model compound for the development of synthetic molecules. On the contrary, the nonpsychoactive CBD acts as CB1 antagonist and is interesting because of its modulation of the THC activity, especially its reduction capability of adverse effects like the sedation, tachycardia, or anxiogenic effects 27, 31, 32). CBD also acts as an inverse agonist of CB2 receptors and, together with other mechanisms (inhibition of cyclooxygenase and lipoxygenase, influence on the prostaglandin and leucotriene production), we can explain its immunosuppressive and anti-inflammatory character. In vivo experiments on rats show that CBD is a pharmacologically interesting active molecule; however, it’s often neglected in the shade of THC. Other interesting activities are the antioxidant and neuroprotective ones that can be important in the treatment of the Parkinson and Alzheimer diseases. Positive results were observed with type-1 diabetes mellitus, cerebral ischemia, rheumatoid arthritis and progression of cancer. Anticonvulsant, anxiolytic, sleep promoting and antiemetic effects were also observed 25, 27, 33).
Therapeutic potential of cannabinoids and drugs in practice
Antiemetic effect of cannabinoids was one of the first indications that began the period of their use in a disease therapy. For the reduction of nausea and vomiting after the chemotherapeutic treatment of cancer patients two preparations are used – synthetically prepared THC (dronabinol) registered as Marinol® (2,5 mg, 5 mg, 10 mg) and synthetic cannabinoid nabilone named Cesamet™ (1 mg). Another indication of Marinol® is prophylaxis and treatment of postoperative nausea and vomiting. Marinol® is registered as a medicinal preparation in the USA and Canada, Cesamet™ extra in the United Kingdom. Recommended dosage of Marinol® for adults in the main indication is 5 mg/m2, 1 to 3 hours before the application of chemotherapy; and every 2 to 4 hours with the maximum of 4 to 6 doses per day afterwards. In the case of postoperative nausea and vomiting the recommended dosage is 5–15 mg/m2 every 3 to 6 hours. Cesamet™ is administered twice a day, 1 or 2 mg. The maximum dose is 6 mg and is divided into three smaller doses per day 34). Another substance – levonantradol, with its intramuscular administration is limited by adverse effects on CNS 28). The control of emesis with cannabinoids is based on the presence of CB1 receptors in brainstem. The motility inhibition of digestive tract is caused by the CB1 receptors’ location in cholinergic nerve terminals of the myenteric plexus with inhibition of acetylcholine release 19, 35). The interest in the use of cannabinoids for this indication is gradually decreasing due to the introduction of modern treatment by antagonists of serotonin (5-HT3) and neurokinin (NK 1) receptors that do not bring about any psychotropic adverse effects. Nevertheless, for patients whose nausea and vomiting cannot be controlled with conventional therapy, cannabinoids are a possible solution 28, 34).
Appetite stimulation is a potential treatment to improve the health of patients suffering from cachexia that most of the time occurs in advanced stages of cancer or AIDS patients. For this purpose, the agonistic effect of cannabinoids on CB1 receptors is used for the stimulation ofanabolic metabolism. Marinol® is also registered for the treatment of AIDS related anorexia. The recommended dosage is 2,5 mg before meal with maximum daily dose 20 mg 28, 34). The established immunosuppressive effects of cannabinoids, e.g. the decrease in the level of CD4+ and CD8+ lymphocytes, don’t play a negative role in this indication, although to confirm this, it is still necessary to carry out long term supplementary studies. Other positive aspects, attributed to cannabis use with HIV infected patients, consist in a better control of nausea and stomach sickness, improvement of anxiety and poor quality of sleep associated with the disease itself and with the antiretroviral therapy 28, 36). The discovery of cannabinoid effects on the appetite led to further research in the field of metabolic processes control and ES was revealed as important physiological regulator of energetic metabolism with impact on food intake and caloric balance. This regulation involves CNS regions, introduced in previous section, and also periphery tissues that include white adipose tissue, liver, pancreatic islet, gastrointestinal tract and skeletal muscles. With the discovery of cannabinoid receptors antagonists, obesity and metabolic syndrome became the next therapeutic targets. The mechanism of action does not only consist in simple reduction of food intake, but mainly also in affecting the complex regulation mechanisms of central neuropeptides (e.g. proopiomelanocortin, neuropeptide Y, corticotropin-releasing and thyrotropin-releasing hormone, prepro-orexin, melanin-concentrating hormone) and the periphery hormones (leptin, insulin, ghrelin, glucocorticoids, malonyl-coenzyme A, cholecystokinin). The antagonists of CB1 receptors in periphery organs decrease in weight by the increased maturation of adipocytes without storing lipids in fatty tissues, by the enhanced lipolysis and by the regulation of glucose homeostasis. Another positive effect on the progress of metabolic syndrome is the periphery regulation of dyslipidemia by decreasing plasma levels of triglycerides, free fatty acids and LDL lipoproteins. Positive effects are also observed with insulin resistance in patients suffering from type-2 diabetes mellitus 16, 37, 38). FAAH polymorphism is also associated with genetic predisposition to obesity 25). Rimonabant (Fig. 1) is so far the most studied selective antagonist of CB1 receptors and is contained in medicinal preparation Acomplia®, also registered in the Czech Republic. Acomplia® is used as a supplement for diet and exercise of the obesity treatment and it must be prescribed, usually with recommended dosage 20 mg/day 39, 40). The principle adverse effect of this drug is the increased occurrence of depressions, a fact that was also pointed out by the State Institute for Drug Control of the Czech Republic (SUKL) in their July 19, 2007 report. The basic contraindication is therefore the treatment of patients with psychic disorders 16, 34, 41, 42). Owing to the positive results of the obesity treatment and to other positive accompanying effects, we can assume the CB1 antagonists to take important place in clinical practice, while other compounds are in the phase I. and II. of clinical trials 17).
Analgesia mediated by ES is another interesting field in which the so far most effective opioid system is not affected. Vide variety of pain types are influenced by the CB1 agonists starting with the inflammatory to acute to neuropathic pains; whereas the physiological role of ES is expected on the pain sensitivity modulation. On the contrary, the CB1 antagonists abolish the analgesics effect. In the antinociceptive transduction pathway affected by ES, the CNS regions are represented (please, see above). The most important of them seem to be namely amygdala, periaqueductal gray and rostroventral medulla that are represented in regulatory processes of descending pain pathway. The antinociceptive modulation on the level of spinal cord and periphery nerves is also expected. The so-far discovered mediators involved in the transduction pain pathway affected by ES are neurotransmitters GABA and glutamate that participate in the antinociceptive effect after the activation of CB1 receptors. There is also hypothesis of analgesic potential of not very explored CB2 receptors. Activation of both opioid and endocannabinoid systems leads to the synergistic analgesic effect 18, 23, 43). The peroral THC (10, 15 and 20 mg) and benzopyranoperidine (synthetic analogue of THC, 4 mg) were tested on oncologic patients and the analgesic effects were positive.Ajulemic acid had positive effect on chronic neuropathic pain; the intramuscularly applied levonantradol was then successfully used to ease the post surgery pain.Sativex® (a sublingual spray) was successfully tested on the rheumatoid arthritis pain, the neuropathic pain that accompanies multiple sclerosis (please, see next section) and on the cancerous pain 28, 31, 44). There were other types of pain where positive results were achieved – to mention a few, they were the trigeminal neuralgia, migraine, and the muscle and neuropathic pains of AIDS patients. Nevertheless, the results of clinical trials of cannabinoids analgesic effects are often not clear, primarily in association with effects on pain with different pathophysiological background 25, 28, 45).
Multiple sclerosis is an autoimmune neurodegenerative disease accompanied by demyelinating lesions and the cannabinoids are used here with partial success. Marihuana, hashish, peroral THC, nabilone and cannabis extracts with major content of THC, CBD administered perorally or sublingually, and their mixtures were tested in clinical trials. Using often inconclusive results, the main symptoms associated with the disease (where the improvement was observed) were assigned. Among them was the spasticity, pain, tremor and quality of sleep. Sativex® seems to be a promising drug; it is registered in Canada for the symptomatic relief from neuropathic pain caused by multiple sclerosis. In addition to the mentioned positive effects, the Sativex® clinical studies revealed other positive effects like the improvement of urinary bladder dysfunction and patients’ mobility 19, 25, 28, 46). The mechanism of action concerning pain and spasticity probably consists in influencing the motor neurones tone and in the lack of their inhibition by the descending pathways of higher nerve centres because of their demyelinization. Some additional functions can be attributed to the CB2 receptors and their anti-inflammatory potential with respect to the autoimmune character of multiple sclerosis 24, 26, 47). Like in the case of pain modulation, based on the complexity of ES, possible different effects of cannabinoids on diverse parts of the nervous system can be expected. This can bring non-unequivocal results in clinical trials. The positive effects in cases of multiple sclerosis are generally accepted, at least with some groups of patients, even though it is necessary to evaluate possible adverse effects associated with long term use 45, 48, 49).
The effects of cannabinoids and the functions of ES were also investigated in cases of other neurodegenerative diseases; Parkinson’s (PD) and Alzheimer’s disease (AD), amiotrophic lateral sclerosis (ALS) and Huntington’s chorea (HC) 25, 26, 50, 51). In all of the mentioned ones, the pathophysiology of neurodegeneration is significantly connected with the progress of inflammatory process, primarily in the astrocytes and microglial cells.These cells are part of the CNS immune system with protective character. Unfortunately, the toxic effect and increased destruction of neurons occurs during their excessive activation. Several possible mechanisms of neuroprotection connected to ES can be attributed – e.g. the protection against glutamatergic excytotoxicity or ischemia, the Ca2+ influx reduction followed by inhibition of subsequent noxious cascades, the impact on other metabolic pathways by kinases phosphorylation, the suppression of production of TNF-α, the induction of expression of transcription factors and neurotrophins, or the antioxidant activity mediated by phenol groups of the cannabinoids 19, 50, 52). In the case of the mentioned neurodegenerative diseases, both known cannabinoid receptors are involved, where the CB2 effects on the modulation of inflammatory processes and their location in the microglial cells are important discoveries. Concerning AD, the amyloid peptide plagues removal by CB2 receptor specific agonists can present a new therapeutic intervention. CB1 receptors are also closely connected with the neuroprotective effects and they support new functional synapses formation; but on the other hand, this could lead to the deterioration of AD symptoms because the acetylcholine production in hippocampus decreases. This effect is also known in marihuana consumption by healthy people where the short memory impairment appears and it is the proof of connection between cognition processes, memory and ES. Some positive effects in AD were achieved in one trial with 6 patients who were administered dronabinol (2,5 mg) 25, 26, 50, 51). In the case of PD, the unambiguous results after nabilone or rimonabant treatment were not observed; the activation of CB1 receptors can rather lead to the apoptosis of dopamine neurones. However, the involvement of ES in the disease development and its symptoms is important with possible therapeutic potential 19, 22, 25, 28, 50). The CB2 anti-inflammatory effects delaying the ALS progression but, on the contrary, the activation of CB1 receptors probably negatively influences the survival of motor neurons. Only beneficial effects on spasticity, appetite stimulation and sleep disorders were observed in clinical trials using THC onALS. In the HC case mentioned last, a low concentration of endocannabinoids in CNS was observed and the activation of ES could be helpful for motor problems control. Nevertheless, no positive results were achieved during several human clinical trials. The inhibitors of endocannabinoid degrading enzymes, or the inhibitors of their reuptake, appear to be promising therapeutics for CNS. Positive effects in clinical trials are often achieved for both, the agonists and the antagonists of CB receptors; therefore the increase and the decrease the endocannabinoid activity in the organism could bring positive results depending on dosing and the stage of the disease. The influence of ES on the neurodegenerative diseases is indisputable and very promising for new drugs development; however, the research in this field and recognition of all regulation mechanisms are in the beginnings 22, 25, 50, 51). The question that remains is if it is possible to prevent the progress of neurodegeneration during the beginning stages with the ES implication and its neuroprotective effects, or if it is possible to use the affected CB receptors “only” for the symptom suppression.
The immunosuppressive and anti-inflammatory effects caused by CB2 receptors are characterized by decreased production of pro-inflammatory (IL-1, IL-2, IL-6, IL-12, TNF and IFN-γ) and by increased production of anti-inflammatory cytokines (IL-4, IL-10). By revealing theseeffects, the potential of chronic inflammatory diseases treatment opens. In this respect, the ajulemic acid was developed and it is a promising active substance which has also inhibitory effect on lipoxygenase and COX-2 19, 24, 26, 33, 53).The anti-inflammatory potential was already mentioned in relation with the neurodegenerative diseases (please, see the above mentioned). The ajulemic acid, CBD, and Sativex® were tested with partial positive effects in rheumatoid arthritis cases. Especially the attenuation in joint disease progression and the suppression of the immune reactivity are the main positive results that were achieved 24).
For completeness’ sake, the following paragraph lists other potential indications with respect to the ES influence. In most cases, the in vitro data are available, sometimes obtained from the utilization of animal models; however, the number of clinical trials conducted on humans is limited.
- A possibility opens in the therapies of Gilles de la Touarette’s syndrome (perorally administered THC reduced the motor and vocal tics and obsessive-compulsive behavioural disorders), of the spinal cord injuries, epilepsy (possible anticonvulsive effects – the most promising is CBD), of anorexia, stroke, osteoporosis, insomnia, respiratory system (asthma bronchiale, bronchodilation and anti-inflammatory effect – on the contrary, higher doses of THC caused bronchoconstriction by irritation), of the cardiovascular (e.g. atherosclerosis prevention, hypertension and metabolic syndrome influence – please, see also the above) and the gastrointestinal disorders (reduction of emesis, gastric secretion and intestinal motility, therapeutic potential for the treatment of chronic inflammatory bowel disease) 19, 24, 25, 28, 35). Attenuating fibrosis and reversing steatosis by CB1 antagonists was also revealed 54, 55). Equally interesting area is the effect on the reproductive system where the relations of ES have been explained on uterus, on pregnancy progress and on sperm maturation (by lowering their production, viability and motility) 15, 25, 56).
- Reduction of intraocular pressure connected to glaucoma was observed after application of marihuana or THC (eye drops in concentration 0,05 and 0,1%). Disadvantages are the short period of duration, central adverse effects after the systemic administration and the possibility to utilize other, more effective and less toxic drugs 25, 28).
- The possibility of cancer therapy with cannabinoids utilization is based on the observation of heightened expression of CB receptors on some cancer cells. By stimulation of receptors, we can achieve the selective influence of tumour with the possibility of direct growth inhibition,cell death and the migration inhibition or, indirectly, by the mechanisms angiogenesis inhibition and by affecting the immune system. An important role can also be played by cannabinoids’ degrading metabolites, for example, the ethanolamine. On the other hand, in cases when cancer cells don’t express CB receptors, the applied cannabinoids can cause the inhibition of the immune system which can lead to the suppression of antitumor immune response. Positive results caused by the CB receptors activation were observed for thyroid, breast, colon and prostate cancer, in myeloid malignancies, melanoma, hepatocellular carcinoma and some types of brain tumours. In the case of lung cancer and bladder carcinoma, squamous cell carcinoma, glioblastoma, astrocytoma and kidney carcinoma, cannabinoids can, on the contrary, stimulate the tumour growth. The cells differentiation state may also be an importantfactor. Only further studies will reveal if the targeting of ES will become one of the existing cancer treatment procedures 14, 25). Sativex® is used in Canada for the relief of pain caused by advanced cancer stages when high doses of opiates are insufficient 46).
- The ES studies also focus on mood disorders. Influencing emotions and the anxiolytic and antidepressant effects are interesting pharmacological goals. It is important to point out here the opposite effects (please, see also the part that deals with adverse effects) when the cannabinoids could provoke or exacerbate depressions and possibly cause anxiety; this undesirable fact is being explained mainly by different dosing of cannabinoids or by the predisposition of an individual person 13, 22). Cognitive processes or the nervous system development are other prospective areas for the ES investigation 13, 57).
- The interconnection between ES and the opioid receptors, dopaminergic system, and the possibility to regulate the brain reward processes together with the reinforcement of craving effects, are the impulses for the development of new drugs to be used in the alcohol, opiates or nicotine addiction therapies25,58,59). Possible implications on the modulation of mesolimbic dopaminergic pathways are also involved in the reward processes and motivation aspects of feeding, with importance in the case of obesity treatment 38, 58).
Degrading enzymes inhibitors (please, see the endocannabinoid system) are currently other perspective target for drug development. The main advantages consist in the absence of the CNS adverse effects which accompany the agonists application that affects the CB receptors directly. The higher specific action, only in places where endocannabinoids are produced and degraded, is also explained. Possible application areas correspond with the above-mentioned indications 15, 29, 59, 60).
Adverse effects, safety and interactions with other drugs
Because of the extensive effects of cannabinoids, it is possible to observe a lot of adverse effects associated with their application, especially on the cardiovascular, respiratory and nervous systems. The use of marihuana can lead to psychological disorders and to addiction. The adverse effects associated with the cardiovascular system are tachycardia, hypertension, palpitation and orthostatic hypotension. The toleration to these and also some other THC mediated effects develops within several days to weeks. This phenomenon is explained by reduction of CB1 receptor density and coupling efficiency. The adverse effects associated with nervous system are dry mouth, nausea, drowsiness, numbness, dizziness and nightmares. Marihuana has also been reported to cause visual disturbances, blurred vision, dry eyes, reddening and burning eyes, mydriasis, photophobia and muscle weakness. Acute toxic psychosis is linked to hallucinations, delusions, depersonalization, fear of dying, paranoia, anxiety and depression. Long term marihuana use can result in psychological dysfunction, affecting person’s ability to concentrate and recall events. Concerning the impairment of psychomotor functions, it is important to note here the person’s unfitness to drive motor vehicles or operate heavy machinery while she/he is under the cannabinoids influence 3, 4, 27, 34, 53).
Considering the above-mentioned adverse effects, the variability in effects on different patients, the lack of clinical data, and, all this linked with current unavailability of standardized plant material, the treatment-experimentations with cannabis are generally not recommended.Patients suffering from cardiovascular diseases are at a much higher risk of stroke and acute myocardial infarction. Other risks to mention include the high risk of the development or the impairment of psychological disorders like schizophrenia, psychosis, bipolar disorders, depression,eating disorders, or panic and anxiety disorders. Marihuana can also cause undesirable weight gain in patients with diabetes and obese patients and its daily consumption is an independent predictor of steatosis in patients with chronic hepatitis C. People suffering from chronic obstructive pulmonary disease, asthma, tuberculosis, cancer, or patients after transplantations, should also avoid non-professional cannabis applications. Pregnant and breastfeeding women should not use marihuana because of its negative impact on structural and neurobehavioral defects in the foetus. Moreover, prenatal exposure to marihuana may increase the risk of childhood leukaemia.
Marihuana and cannabinoids interact with whole range of drugs. The potentiating effect of opioids, barbiturates, benzodiazepines, muscle relaxants and alcohol appear to lead to the excessive CNS depression. Decrease of effectiveness is observed in the protease inhibitors and the theophylline; the fluoxetine can provoke manic episodes. Sildenafil was observed to present a higher risk of myocardial infarction. The combination with tricyclic antidepressants, anticholinergic agents and α-agonists presents an increased risk of tachycardia and hypertension exacerbation. Other compounds that are dangerous when combined with cannabis are naltrexone, disulfiram, neuroleptic antipsychotics and anaesthetic agents. When administered in combination with systemic corticosteroids, an increased risk of immunosuppression develops 4, 34, 53, 55).
Because of the high cannabinoids metabolism by hepatic cytochrome system, it is possible that the pharmacokinetic interaction could occur with other drugs. The increased biological availability of cannabinoids could be caused by the enzyme inhibitors like macrolide antibiotics (claritromycin, erythromycin), antimycotics (itraconazole, fluconazole, ketoconazole and miconazole), calcium antagonists (diltiazem, verapamil), HIV protease inhibitors (ritonavir), amiodarone and isoniazid. On the contrary, rifampicin, carbamazepine, phenobarbital, phenytoin, primidone, troglitazone and Saint John’s Wort preparations induce enzymatic processes and cause quicker liver metabolism of cannabinoids. Other interactions can be expected with drugs which are strongly bound to plasma proteins 4, 34).
Pharmaceutical dosage forms and pharmacokinetics
Most of the traditional application forms of the plant material include the preparation step – heating of the sample (smoking, vaporization, baking) to achieve decarboxylation of cannabinoid acid form (e.g. THCA) onto neutral analogue (e.g. THC) 1, 4). The principal route of cannabis administration is smoke inhalation which provides almost immediate onset of effects with maximum plasmatic concentrations that occur after about 9 minutes and a minimal liver first-pass metabolism. The variability of biological availability 2–56% is caused especially by differences in smoking dynamics and the quality of the plant material. A related, positive effect is the feasibility to titrate the desired degree of effects; the negative one is then the uncertainty in the dose delivery 61, 62). However, smoking is (in the modern medicine) an inappropriate administration form mainly because of the carcinogenic compounds production and the established social standards. When compared with regular cigarettes, marihuana smoke contains higher amounts of carcinogenic compounds which could trigger lung carcinoma formation. Marihuana smoking is also connected with higher occurrence rates of head and neck tumours, with the development of pharyngitis, rhinitis and chronic obstructive pulmonary diseases 53, 63, 64). Modern method of inhalation of cannabinoids from plant material consists in the use of vaporizer that uses temperature regulated hot air to extract the plant material’s drug (or its liquid extract) and the forming fine smoke is accumulated in a small bag from which it is subsequently inhaled. This administration route is more convenient from the application point of view; good bioavailability of cannabinoids (54% of extracted cannabinoids, while the mean absorption by lungs from whole dose is 30–40%), and the carcinogenic compounds production reduction 65, 66). During smoking and optimized heating in the vaporizer, THCA completely converses into THC but, when smoking the material, partial destruction takes place as well 61, 67).
Soft gelatine capsules of the Marinol® medicinal preparation represent a peroral dosage form in which the cannabinoids are dissolved in oil since their lipophilic character allows to do that. The most suitable vehicles known are the sesame oil and the glycocholate that increase the bioavailability of cannabinoids. Cesamet™ is also prescribed for peroral application. This route of administration is however considered less convenient due to its pharmacokinetic parameters, especially because of its slow absorption, degradation in the stomach and a significant liver first-pass metabolism of cannabinoids. Bioavailability after peroral administration ranges from 4 to 20% with the plasma concentrations peak after 4 to 6 hours 34, 68).
Oral spray Sativex® applied onto the mouth mucosa generates in one dose (100 μl) 2.7 mg of THC and, simultaneously, 2.5 mg of CBD which represents 70% of the total extract. Other phytocannabinoids (5%), flavonoids, terpenoids and phytosterols are also present in minor amounts if plant extract is used. The effect onset appears after 15 to 40 minutes which allows patients an effective dose titrating and minimizes the adverse effects. An electronic dosage instrument was developed by the GW Pharmaceuticals, a company that is involved in the cannabis-based medicine research – including Sativex®.This instrument could be used to dose Sativex® and there would be advantages, for instance, the possibility of data storage, the compliance check by physician or pharmacist, the potential to control the dosage, the overdose or abuse prevention 3, 31, 46, 61). Sativex® utilizes the information that plant extract has, in many respects, more favourable effects than single, isolated compound. In case like this, the standardized content of simultaneously administered THC and CBD is important. This conception is well known also in other medicinal plants used in modern phytotherapy and it is interpreted by the synergistic and antagonistic action of the sum compounds in the whole extract. The Tetranabinex® and Nabidiolex® extracts with high content of THC and CBD, respectively, are also available 31, 68).
THC-hemisuccinate in the form of rectal suppositories has, in comparison with perorally-applied dosage forms, advantages owing to higher absorption and lower firs-pass metabolism and this fact approximately doubles the bioavailability. Another advantageous delivery route for cannabinoids from the pharmacokinetic point of view can be the transdermal patches with gradual release 61). To make the list complete, we should mention the cannabis decoction as a traditional dosage form which has been used, for example, in ethnomedicine in Jamaica and the preparation spread also to Europe. However, neutral cannabinoids’ dissolvability in water is limited. THC dissolvability is only about 17%, whereas its acid analogue THCA stands at 63%, where the conversion of THCA to THC in boiling water is minimal.It is assumed that the effects of the cannabis decoction or its infusion could be triggered by the acid forms of cannabinoids. Not only THCA, but also the combination of other acid forms (e.g. cannabigerolic acid, tetrahydrocannabivarinic acid), or possibly some other secondary metabolites like flavonoids, could represent an interesting therapeutic source 69).
Owing to the cannabinoid lipophility, the plasmatic concentration of absorbed THC rapidly decreases by the distribution into highly perfused organs (lung, heart, brain and liver) and by the liver metabolism. Important cannabinoids storage compartment is fatty tissue. The distribution volume of THC is large 10 l/kg and it is bound in plasma lipoproteins in the volume ranging between 95 and 99%. THC rapidly crosses the placenta and the concentration is 3 to 6 times lower in the cord blood than it is in the maternal blood.On the contrary, the concentration in breast milk could be several times higher. The main metabolism of THC takes place in liver, but other organs like brain, intestine and lung are also metabolically active. Principal hepatic enzyme systems responsible for THC oxidation are CYP 450, 2C9, 2C19 and 3A4 and more than 100 THC metabolites have been identified. During the THC oxidation, 11 hydroxytetrahydrocannabinol (11-OH-THC) is formed as a predominant compound which has a psychoactive effect too. Another metabolic step is the oxidation onto carboxylic acid with formation of pharmacologically not active 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-9-COOH). THC-9-COOH and its glucuronide conjugates are main end products of human THC metabolism.Cytochrome systems may also oxidize other parts of the cannabinoid molecule, primarily position 8 or the side aliphatic chain. CBD metabolism is analogous to THC with oxidation of methyl group and formation of carboxylic acid as final product. Based on the pharmacokinetic observations, CBD does not significantly influence the THC metabolism. The THC elimination is carried out from 65% by faeces (mainly 11-OH-THC), 20% by urine (mainly THC-9-COOH glucuronide conjugates) and 80–90% is excreted within 5 days. The half-life of the terminal elimination of cannabinoids is, of course, variable because of their gradual release from lipid-storage compartments and their enterohepatic circulation. It can take several weeks before the metabolites are eliminated after a long-term use of marihuana or pure cannabinoids. Cannabinoids pharmacokinetics or the profiles of the discovered body metabolites are important parameters for rational pharmacotherapy and for the detection of exposure to addictive compounds in the forensic sciences 34, 61).
Forensic analysis and cannabis-related legal provisions in force in the Czech Republic
From the breeder’s point of view, the cannabis plant can be divided into two groups. One group includes plants that are grown to produce fiber and seeds, the other one consists of plants with high content of psychoactive THC, or of plants with a specific profile of other cannabinoids 3, 70).
The Czech legislation deals with the hemp farming in the Addictive Substances Act (No. 167/1998, of the Register of Laws and Regulations) which prohibits growing hemp cultivars (Cannabis genus) that may contain amounts greater than 0.3% of the substances in the tetrahydrocannabinols group. These, together with resin, are prohibited to obtain. The hemp, for the purpose of the law, is understood as the above-ground part of the plant that may have blooming or seed-bearing cyme. Plants with high concentration of THC, or with other desirable cannabinoids, are used in research and for medicinal purposes. For instance, Bedrocan, Bedrobinol and Bediol are cultivars that yield dried drug (Cannabis flos), available upon prescription in Dutch pharmacies, and vary in the relative content of THC and CBD 4, 71). At present, there is no registered medicament containing cannabis extract, tincture or dronabinol (nor any other cannabis-based medicament) on the Czech market (information by Jitka Židlická, Press and Information Centre of SUKL, March 11, 2008). However, pharmacy is an important health care institution permitted to handle addictive substances and preparations and it has the potential to issue cannabis and cannabinoid-based medicaments accompanied by the required information (Table 3).
3. Cannabis products listed per the Addictive Substances Act No.167/1998 of the Register of Laws and Regulations 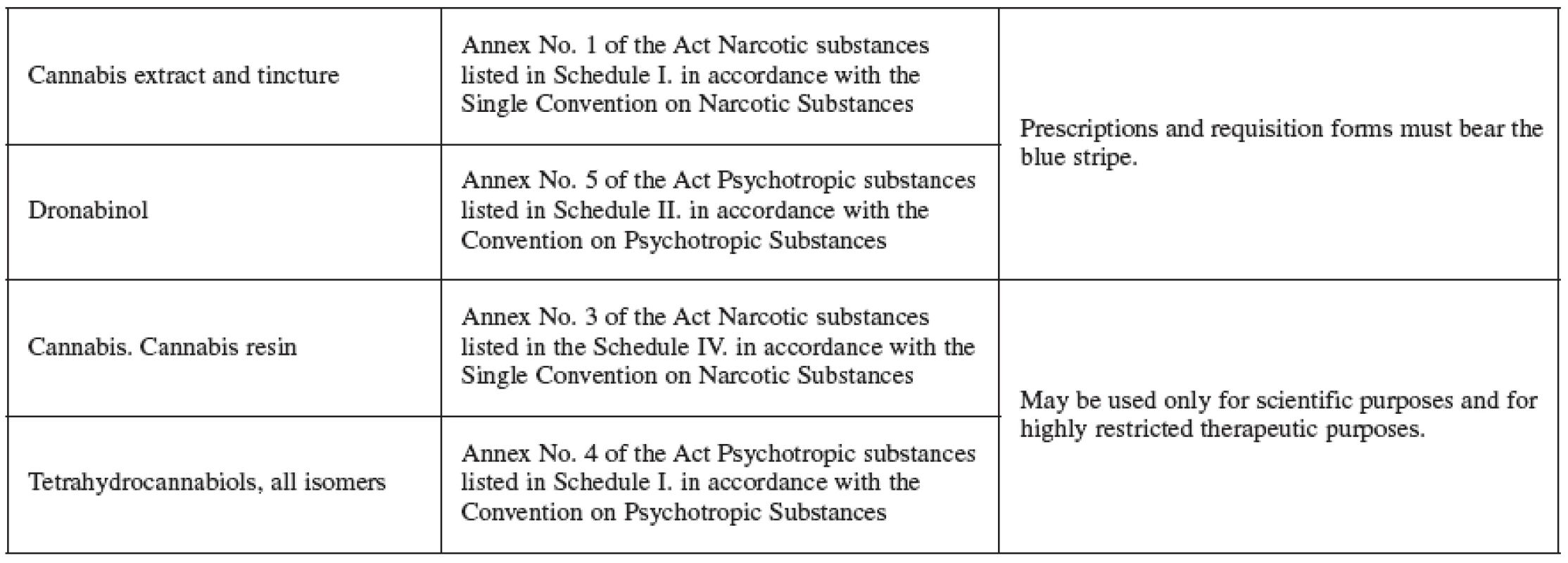
Some of the more important names of THC-rich plant cultivars sold on the Dutch, Canadian, or Swiss markets and known to recreational users are: Skunk (the name derives from its distinct smell), Durban Poison, Northern Lights, Blueberry or White Widow 3). It is necessary to point out right here that the unauthorized production, import, export, sale and possession of such cultivars (with high content of THC) is illegal in the Czech Republic and is punishable by law (No. 140/1961, of the Register of Laws and Regulations). The legal sanctions imposed depend on the gravity of the offence and can range from financial penalties from 1 to 15 years of imprisonment. A new bill modifies the wording of the penal law, lists cannabis as a soft drug, sanctions its possession with 1-year imprisonment and, in cases of growing cannabis in “quantity larger than small”, the sanction may be a 6-month imprisonment. This quantity is also newly defined and is, concurrently with the bill to modify the wording of the penal law, published in the Statutory Orders by the Cabinet that are based on the binding guidelines by the Police President (No. 39/1998), on the guidelines by Supreme Public’s Prosecutor (No. 6/2000) and on expert opinions. In case of THC, the quantity larger than small has been set to 1.0 g (approximately 20 doses, 50 mg each) and up; the number of plants grown was set to 3 and up. The possession and cultivation of quantity smaller than small is then judged in accordance with the Misdemeanour Act (No. 200/1990, of the Register of Laws and Regulations) and financial penalties may range up to 15 000 CZK 72, 73). The Czech Republic is one of the countries with low tolerance for growing and using hemp with high content of psychoactive cannabinoids, even if the hemp is grown for personal use only or used in own empiric therapy of diseases. The current trend, as we can tell from the wording of the new penal law bill, is trying to find a compromise between a more liberal approach and the radical concept of rejecting all drugs and newly divides them according to the extent of their abuse and the hazard they present. The bill doesn’t fully liberate the cannabis plant’s cultivation for personal purposes and the plant’s private use. The decisions on individual violations will be made by state attorneys and courts 73).
When testing seized plant samples for the presence of THC, the Criminal Laboratories of Police, Department of Criminalistic-technical Expert Opinions (OKTE) use gas chromatography – FID detection. An alternative method consists in the utilization of the high-performance liquid chromatography with UV/VIS detection that can detect, due to the exclusion of heat, the initial concentrations of THC and THCA (personal information by Ing. Ivo Vykydal, OKTE, Hradec Králové). The chemical analysis should utilize the method that can detect both THC and THCA and, by adding up the concentrations, it should quantify the total amount of psychoactive THC that develops when the drug is applied after being heated up. Another alternative consists in the quantitative transformation of THCA to THC before the analysis itself. The listed sources indicate that the THCA transformation to THC during gas chromatography analysis does not progress quantitatively 67).
The analysis of biological materials, namely blood and urine, is based on the detection of cannabinoids themselves and their body metabolites of which the most important ones are 11-OH-THC and THC-9--COOH. The testing is done by authorized toxicology experts and by laboratories specialized in toxicology, for instance, by forensic medicine institutes or by institutes for clinical biochemistry and diagnostics at university hospitals. The preliminary screening utilizes immunochemical methods that are later followed by a chemical analysis that can identify and quantify a whole line of cannabinoids and their metabolites, even in their trace concentrations. Also, the thin-layer chromatography systems (Toxi Lab® THC II by Varian Inc.) are available, as well as the gas and liquid chromatography methods that use the mass spectrometry for detection. Mass spectrometry is under the given conditions highly selective (minimal interference with structurally similar compounds) and the most sensitive analytic detector today (the detection limit depends on the metabolite tested, most of the time 1 ng/ml for THC-COOH). Each citizen, possibly an employer too, have an option to monitor the use of marihuana by commercially available detection systems. These screening methods are based on the tested metabolites immunochemical reactions (most commonly THC-9-COOH in urine, THC in perspiration and saliva) with specific antibodies and are interpreted by the presence or absence of set colours on the detection strip (Dynex Test®, OralStat® Mavand, DrugWipe, Cozart®, Envitec SmartClip®, Bio-Rad Tox/See™, Triage®, Syva® Rapid Test etc.). These tests are just preliminary tests whose positive results must be confirmed with subsequent analytic method. Urine, perspiration and saliva are used in testing most often, but smears from objects such are keyboards, cell phones and other personal objects can be used as well. The detection limits are given by the manufacturer and correspond with the expected concentrations of tested metabolites in the individual biological samples. The Police of the Czech Republic and sporadically also Metropolitan Police use these detection systems primarily to identify drivers impaired by narcotics and psychotropic drugs. The use of hairs as a biological material has more criminalistic importance in special cases and the analysis requires high-quality laboratory equipment with mass spectrometry and protected laboratory space against contamination of analyzed compounds (personal information by PharmDr. Viktor Voříšek, Institute of Clinical Biochemistry and Diagnostics, Hradec Králové University Hospital) 61).
Low THC content (less than 0.3%) cultivars are grown in the Czech Republic and throughout the European Union (EU) to produce fibre and seed. Check samples are taken at the places of cultivation and any cultivated areas larger than 100 m2 are subject to registration. The EU further regulates the farming of the hemp fibre cultivars with its farm subsidy policy and such cultivars must comply with the requirement that THC content is lower than 0.2% (tab. 1) 3, 70).
Conclusion
”From ethnopharmacology to modern pharmaceutics”. These fitting words can be used in the conclusion to sum up the cannabis and its chemical substances. The ethnopharmacology is the science that re-discovers the long-forgotten natural remedies of our forefathers, primarily their medicinal plants, examines the plant’s original effects, compares them with modern scientific approaches and utilizes the millenniums of human coexistence with nature and its gifts. In spite of the fact that the cannabis has never been completely forgotten, it has been reprobated and restricted by different legislations for many years, due to its potential of being abused, which had a negative impact on cannabis research. There is no doubt that cannabis is a drug that affects the human central nervous system and is thus the subject to legal provisions and different countries’ legal approach ranges from a benevolent to strict prohibition of use. The permission to use cannabis for medicinal purposes with ailing people whom the drug’s content substances bring relief still remains very questionable; not only in the Czech legislation. These issues are not only a complicated and waste topic for the lawmakers, policies on drugs, and the possible development of dependency and of other health problems, but also for the perception and understanding of the general public. In the event that self-treatment or treatment prescribed by doctors was allowed, the health-care staffs would have to be educated so that they could inform patients and legally distribute the cannabis material for medical purposes. The pharmacy should, in this respect, become an unsubstitutable, health-care facility – the centre of knowledge. The research results justify the legitimacy of the use of cannabinoids, of their synthetic derivatives, and of substances affecting the biosynthesis and the degradation of endogenous cannabinoids in the therapy of many serious diseases. It is important to separate these medical achievements from attempts to abuse the cannabis in the form of an illicit drug. Many empirical observations describe the benefits and the risks of the cannabis use and this knowledge can be utilized by research. A long road leads to obtaining modern and safe medicaments – a road that consists of extensive chemical, pharmacological and clinical research. There is a good possibility that medicaments based on the research of cannabis will assist in fighting diseases also in the Czech Republic (please, see Acomplia®) the same way they have already been helping in several other countries.
This work was supported by the Research Project MSM 0021620822.
The author thanks to Col. JUDr. Zdeněk Riedinger (Police of the Czech Republic, Prostějov District Office) for his information on legislation, Ing. Ivo Vykydal (Police of the Czech Republic, OKTE Hradec Králové) for his information on chemical analyses of cannabis material and PharmDr. Viktor Voříšek (Institute of Clinical Biochemistry and Diagnostics, Hradec Králové University Hospital) for his information on toxicological analysis of biologic material.
Received 7 July 2008 / Accepted 17 July 2008
Adresses for correspondence:
Mgr. Jaroslav Peč
Charles University in Prague, Faculty of Pharmacy in Hradec Králové
Department of Pharmacognosy
Heyrovského 1203, 500 05 Hradec Králové
e-mail: jaroslav.pec@faf.cuni.cz
Sources
1. Bruneton, J.: Pharmacognosy, Phytochemistry, Medicinal plants. 2nd ed. Paris, Lavoisier Publishing Inc., 1999, 445–459.
2. Jahodář, L.: Farmakobotanika, semenné rostliny. Praha, Karolinum, 2006, s. 87.
3. Guy, G. W., Whittle, B. A., Robson, P. J. eds.: The Medicinal Uses of Cannabis and Cannabinoids. London, Pharmaceutical Press, 2004, s. 71–79, 103–129, 141–197.
4. http://www.cannabisoffice.nl/eng/index.html, Information for physicians and pharmacists (20. 5. 2008).
5. Hrdina, V., Hrdina, R., Jahodář, L. et al.: Přírodní toxiny a jedy. Praha, Galén a Karolinum, 2004, s. 87.
6. Brown, D. T. eds.: Cannabis: the Genus Cannabis. (Medicinal and aromatic plants: industrial profiles; v. 4). Amsterdam, Harwood Academic Publishers, 1998, s. 55–67, 253–272.
7. Dewick, P. M.: Medicinal Natural Products, A Biosynthetic Approach. 2nd ed. Chichester, J. Wiley & Sons Ltd., 2001, s. 85–89.
8. ElSohly, M. A., Slade, D.: Life Sci., 2005; 78, 539–548.
9. Russo, E. B.: Chem. Biodiv., 2007; 4, 1614–1648.
10. McPartland, J. M., Russo, E. B.: J. Cannabis. Ther., 2001; 1, 103–132.
11. Bisogno, T.: J. Neuroendocrinol., 2008; 20 (Suppl. 1), 1–9.
12. Howlett, A. C., Barth, F., Bonner, T. I. et al.: Pharmacol. Rev., 2002; 54, 161–202.
13. Viveros, M. P., Marco E. M., Llorente R. et al.: Neural Plast., 2007; 2007, 1–12.
14. Sander, B., Flygare, J.: Semin. Cancer Biol., 2008; 18, s. 176–189.
15. Labar, G., Micheaux, C.: Chem. Biodiv., 2007; 4, 1882–1902.
16. Wang, J., Ueda, N.: Curr. Opin. Nephrol. Hypertens., 2008; 17, 1–10.
17. Kunos, G.: Am. J. Med., 2007; 120 (9A), S18–S24.
18. Rea, K., Roche, M., Finn, D. M.: Br. J. Pharmacol., 2007; 152, 633–648.
19. Costa, B.: Chem. Biodiv., 2007; 4, 1664–1677.
20. Hashimotodani, Y., Ohno-Shosaku, T., Kano, M.: Curr. Opin. Neurobiol., 2007; 17, 360–365.
21. Fišar, Z.: Chem. Listy, 2006; 100, s. 314–322.
22. Bisogno, T., Di Marzo, V.: Pharmacol. Res., 2007; 56, 428–442.
23. Hohman, A. G., Suplita, R. L.: AAPS J., 2006; 8 (4), E693–E708.
24. Klein, T., W., Newton, C., T.: Therapeutic Potential of Cannabinoid-Based Drugs. In: Shurin, M., R., Smolkin, Y., S. eds. Immune Mediated Dissease vol. 601. New York, Springer, 2007, s. 395–413.
25. Pacher, P., Sándor, B., Kunos, G.: Pharmacol. Rev., 2006; 58, 389–462.
26. Benito, C., Tolón, R. M., Pazos, M. R. et al.: Br. J. Pharmacol., 2008; 153, 277–285.
27. Partwee, R. G.: Br. J. Pharmacol., 2008; 153, 199–215.
28. Ben Amar, M.: J. Ethnopharmacol., 2006; 105, 1–25.
29. Basavarajappa, B. S.: Protein. Pept. Lett., 2007; 14, 237–246.
30. Rouzer, C. A., Lawrence, J. M.: J. Biol. Chem., 2008; 283, 8065–8069.
31. Russo, E. B., Guy, G. F., Robson, P. J.: Chem. Biodiv., 2007; 4, 1729–1743.
32. Hayakawa, K., Mishima, K., Hazekawa, M. et al.: Brain Res., 2008; 1188, 157–164.
33. Mechoulam, R., Peters, M., Murillo-Rodriguez, E. et al.: Chem. Biodiv., 2007; 4, 1678–1692.
34. Micromedex® Healthcare Series, Thomson Micromedex, http://www.thomsonhc.com (20. 5. 2008).
35. Wright, K. L., Duncan, M., Sharkey, K. A.: Br. J. Pharmacol., 2008; 153, 263–270.
36. Haney, M., Gunderson, E. W., Rabkin, J. et al.: J. Acquir. Immune. Defic. Syndr., 2007; 45, 545–554.
37. Hradec, J.: Remedia, 2005; 2, 163–168.
38. Bellocchio, L., Vicennati, V., Cervino, C. et al.: Am. J. Cardiol., 2007; 100 (Suppl.), s. 7P–17P.
39. http://www.sukl.cz/modules/medication/search.php (20. 5. 2008, Acomplia).
40. Mikro-verze AISLP – ČR, 2008.1 pro MS Windows.
41. Mitchel, P., B., Morris, M., J.: Lancet, 2007; 370, s. 1671–1672.
42. http://www.sukl.cz/sukl-upozornuje-na-kontraindikace-u-pripravku-acomplia (20. 5. 2008).
43. Pan, H. L., Wu, Z. Z., Zhou, H. Y. et al.: Pharmacol. Ther., 2008; 117, 141–161.
44. Nurmikko, T. J., Serpell, M. G., Hoggart, B. et al.: Pain, 2007; 133, 210–220.
45. Lienau, F. S., Füllgraf, H., Moser, A. et al.: Eur. J. Neurol., 2007; 14, 1162–1169.
46. http://www.gwpharm.com/sativex.asp (20. 5. 2008).
47. Docagne, F., Mestre, L., Loría, F. et al.: Expert Opin. Ther. Targets, 2008; 12, 185–189.
48. Smith, P. F.: Expert. Rev. Neurother., 2007; 7, 1157–1163.
49. Papathanasopoulos, P., Messinis, L., Lyros, E. et al.: J. Neuropsychiatry Clin. Neurosci., 2008; 20, 36–51.
50. Centonze, D., Finazzi-Agró, A., Bernardi, G. et al.: Trends Pharmacol. Sci., 2007; 28, 180–187.
51. Micale, V., Mazzola, C., Drago, F.: Pharmacol. Res., 2007; 56, 382–392.
52. Gilbert, G. L., Kim, H. J., Waataja, J. J. et al.: Brain Res., 2007; 1128, 61–69.
53. Seamon, M. J., Fass, J. A., Maniscalco-Feichtl, M. et al.: Am. J. Health-Syst. Pharm., 2007; 64, 1037–1044.
54. Kunos, G., Gao, B.: Gastroenterology, 2008; 134, 622–625.
55. Hézode, C., Zafrani, E. S., Roudot-Thoraval, F. et al.: Gastroenterology, 2008; 134, 432–439.
56. Ricci, G., Cacciola, G., Altucci, L. et al.: Gen. Comp. Endocrinol., 2007; 153, 320–322.
57. Harkany, T., Guzmán, M., Galve-Roperh, I. et al.: Trends. Pharmacol. Sci., 2006; 28, 83–92.
58. Solinas, M., Yasar, S., Goldberg, S. R.: Pharmacol. Res., 2007; 56, 393–405.
59. Fattore, L., Fadda, P., Fratta, W.: Pharmacol. Res., 2007; 56, 418–427.
60. Saario, S. M., Laitinen, J. T.: Bas. Clin. Pharma. & Tox., 2007; 101, 287–293.
61. Huestis, M., A.: Chem. Biodiv., 2007; 4, 1770–1804.
62. Kalant, H.: Clin. Pharmacol. Ther., 2008; 83, 517–519.
63. Aldington, S., Harwood, M., Cox, B. et al.: Eur. Respir. J., 2008; 31, 280–286.
64. Aldington, S., Harwood, M., Cox, B. et al.: Otolaryngol. Head Neck Surg., 2008; 138, 374–380.
65. Hazekamp, A., Ruhaak, R., Zuurman, L. et al.: J. Pharm. Sci., 2006; 95, 1308–1317.
66. http://www.storz-bickel.com/vaporizer/volcano-vaporization-system.html# (20. 5. 2008).
67. Dussy, F. E., Hamberg, C., Luginbühl, M. et al.: Forensic. Sci. Int., 2005; 149, 3–10.
68. Evans, W. C. (eds.): Trease and Evans Pharmacognosy. 15th ed. Edinburg, W. B. Saunders, 2002, s. 51, 53–54, 501–503.
69. Hazekamp, A., Bastola, K., Rashidi, H. et al.: J. Ethnopharmacol., 2007; 113, 85–90.
70. http://www.mze.cz/, Situační a výhledová zpráva len a konopí, červen 2007 (20. 5. 2008).
71. Hazekamp, A.: Cannabinoids, 2006; 1, 1–9.
72. http://www.psp.cz/sqw/text/tiskt.sqw?O=5&CT=410& CT1=0 (20. 5. 2008).
73. http://www.drogy–info.cz/index.php/info/drogy_a_zakon (20. 5. 2008).
74. Kabátová, N.: Bulletin Národní referenční laboratoře; 2005, IX(3), s. 33–58.
75. Zákon o návykových látkách č. 167/1998 Sb.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2008 Issue 5-
All articles in this issue
- History and the present state of hypolipidaemic therapy
- Effect of montelukast on the treatment of asthma bronchiale in pediatric patients with perennial allergic rhinitis
- Impact of demographic factors on consumption of pharmaceuticals in the Czech Republic
- Medicinal preparations in Prague pharmacies at the end of the 16th century
- Therapeutic potential of phytocannabinoids and synthetic derivatives affecting human endocannabinoid system
- Characterization of celluloses by means of viscoelastic parameters
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- History and the present state of hypolipidaemic therapy
- Effect of montelukast on the treatment of asthma bronchiale in pediatric patients with perennial allergic rhinitis
- Medicinal preparations in Prague pharmacies at the end of the 16th century
- Impact of demographic factors on consumption of pharmaceuticals in the Czech Republic
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

