-
Medical journals
- Career
Využitie rohovkovej topografie v detskej oftalmológii
Authors: V. Popová 1; D. Tomčíková 1; B. Bušányová 1; F. Kecer 1; A. Gerinec 1; I. Popov 2
Authors‘ workplace: Klinika detskej oftalmológie Národného ústavu detských chorôb a Lekárskej fakulty Univerzity Komenského v Bratislave 1; Klinika oftalmológie Univerzitnej nemocnice Ružinov a Lekárskej fakulty Univerzity Komenského v Bratislave 2
Published in: Čes. a slov. Oftal., 79, 2023, No. 5, p. 258-265
Category: Original Article
doi: https://doi.org/10.31348/2023/30Overview
Aim: To introduce the topic of pediatric keratoconus, highlighting the importance of routine corneal topography and tomography in children and adolescents from predisposed groups. To attempt to ensure the early detection of keratoconus and its subclinical form, enabling early treatment, which brings better expected postoperative results.
Material and methods: Using the corneal tomograph Pentacam AXL we examined children and adolescents with astigmatism equal or greater than
2 diopters (in at least one eye) and patients with at least one risk factor such as eye rubbing in the case of allergic pathologies, positive family history of keratoconus or certain forms of retinal dystrophy. In total, we included 231 eyes (116 patients), of which 54 were girls and 62 were boys.
Results: The Belin-Ambrósio deviation index parameter was evaluated, in which we classified a total of 41 eyes as subclinical keratoconus and 12 eyes as clinical keratoconus. Next, the corneal maps were evaluated individually, in which we included a total of 15 eyes as subclinical keratoconus and 6 eyes as clinical keratoconus. In our group, compared to the control group, subclinical and clinical keratoconus occurred most often in the group of patients with astigmatism and in the group of so-called “eye rubbers”. After individual evaluation, keratoconus occurred more frequently in boys than in girls in our cohort.
Conclusion: Most patients with keratoconus are diagnosed when there is a deterioration of visual acuity and changes on the anterior surface of
the cornea. Corneal topography and tomography allows us to monitor the initial changes on the posterior surface of the cornea, and helps us to detect the subclinical form of keratoconus and the possibility of its early treatment. Therefore, it is important to determine which groups are at risk and groups in which corneal topography and tomography should be performed routinely.Keywords:
keratoconus – topography and tomography – astigmatism – eye-rubbing
INTRODUCTION
Although keratoconus is most often not diagnosed until adulthood, ectatic corneal processes begin already during puberty and adolescence. There are certain groups of patients who are predisposed to the occurrence of this disorder [1]. The largest such group comprises so-called “eye-rubbers”. This group includes patients with disorders that lead to frequent eye rubbing, such as chronic blepharitis, allergic conjunctivitis, vernal keratoconjunctivitis (Fig. 1) and patients with atopic disorders [2]. The pathophysiological basis of the onset of ectatic disease in this case is an imbalance between apoptosis and the proliferation of keratocytes as a consequence of chronic damage to the epithelium. Another cause of eye-rubbing may be for example oculodigital reflex upon a background of Leber congenital amaurosis or other retinal dystrophies, in which it is assumed to be the cause of the onset of keratoconus [3]. Another group consists of “syndrome disorders”. These include Down syndrome (Fig. 2) [4] and diseases of the conjunctival tissue, such as Marfan syndrome and Ehlers-Danlos syndrome [1].
1. Patient with vernal keratoconjunctivitis 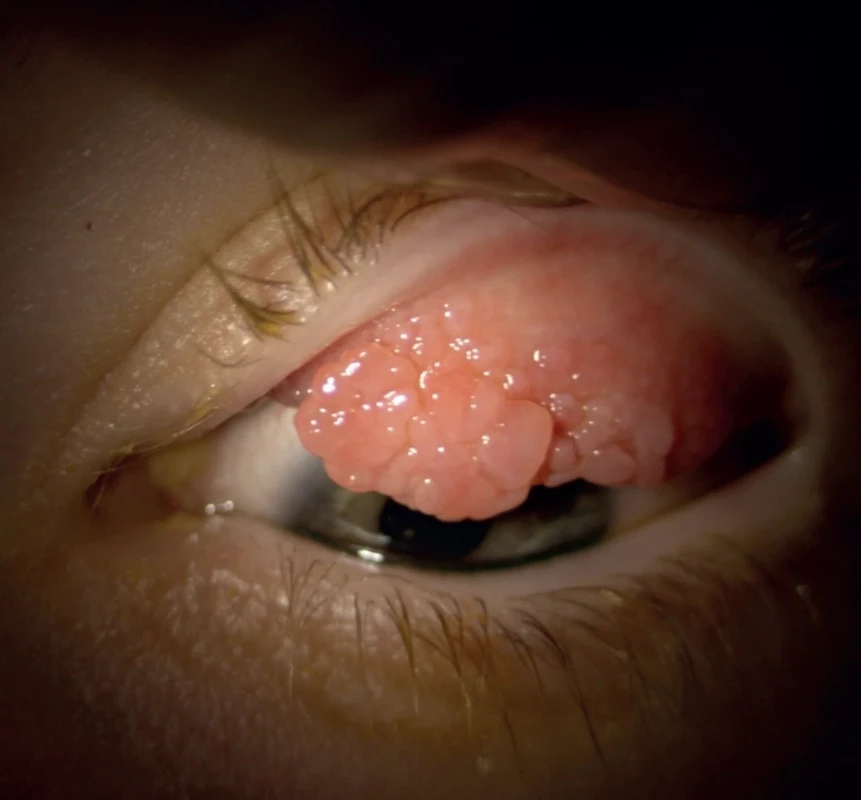
2. Corneal bulging in keratoconus in a patient with Down syndrome 
Over the course of the last 20 years, the management and treatment of keratoconus has changed dramatically. Thanks to the arrival of corneal topography and tomography, our capacity to identify early ectatic changes has improved, and this is now possible far earlier than previously. With the arrival of interventions such as corneal cross-linking, intrastromal corneal ring or a combination thereof, a new form of visual rehabilitation is opening up in doctor-patient cooperation for this disease [5]. Treating patients with a mild stage of keratoconus at a younger age produces better results, and for this reason it is important to ensure early identification of children and adolescents with keratoconus [6].
MATERIAL AND METHOD
Our cross-sectional study was conducted at the Department of Pediatric Ophthalmology of the National Institute of Children’s Diseases and Faculty of Medicine, Comenius University Bratislava, during the period from September 2019 to August 2021. All the patients were examined on corneal tomography with the instrument Pentacam® HR AXL (Oculus company). The patients were selected at random from children who were under observation at our department and came for a routine check-up, or reported to us for an examination for the first time on the basis of a recommendation from the regional ophthalmology clinic. We divided the patients into five groups:
-
- The first group consisted of patients with a value of astigmatism of ≥ 2 Dcyl from an average value of automatic refraction (minimum 3 measurements) in artificial mydriasis in at least one eye (57 eyes). At the same time, however, patients from the following groups were excluded: patients with any form of ocular allergy or other atopic disease, patients with a family history of keratoconus, patients with any confirmed form of retinal dystrophy, genetically confirmed Down’s syndrome and patients with any disorder of the conjunctival tissue. This group shall be referred to in the text, graph and tables below as “astigmatisms”.
- The second group was made up of “eye-rubbers”. It consisted of patients suffering from some form of ocular allergy or atopic disease (chronic seasonal allergic conjunctivitis, vernal keratoconjunctivitis (VKC), blepharitis) and who frequently rubbed their eyes (at least 10 times per day for a minimum of 5 seconds – 80 eyes). This group shall be referred to in the text, graph and tables below as the “eye-rubbers”.
- The third group consisted of patients with a family medical history of keratoconus within second degree relatives. If a patient was also an “eye-rubber”, a note was made of this, but the patient remained classified by priority into this third group. This group shall be referred to in the text, graph and tables below as “FH” – 17 eyes.
The fourth group was made up of patients who had one confirmed form of retinal dystrophy (Retinitis pigmentosa, cone dystrophy, Leber congenital amaurosis). If a patient was also an “eye-rubber”, a note was made of this, but the patient remained classified by priority into this fourth group. This group shall be referred to in the text, graph and tables below as “RD” (group of patients with The exclusion criterion for all groups was previous eye surgery, ocular trauma, prematurity, chronic or overcome acute uveitis, increased intraocular pressure or glaucomatous disorder or suspicion thereof.
We set the lower age limit at 5 years with respect to the fact that performing an examination on Pentacam requires cooperation, in which the patient under examination must not move or blink during the time of the scan, which is 2–3 seconds. The upper age limit was set by the dispensary age limit, which is 19 years at our clinic. The model of examination we selected was a 3D scan with 25 individual images, and only those scans with a quality specification of “OK” were accepted. If the quality field was indicated red or yellow (the scan was not correct), then the scan was repeated.
For an analysis of the condition of the cornea on Pentacam we selected the parameter BAD/D (Belin-Ambrósio deviation index), which is recommended by several studies as the strongest parameter in the detection of subclinical form of keratoconus [7]. The exceptional quality of this parameter consists in its combination of the values of anterior elevation in the thinnest point, posterior elevation in the thinnest point, changes of anterior elevation, changes of posterior elevation, corneal thickness in the thinnest point, location of the thinnest point, pachymetric progression, Ambrósio relational thickness and Kmax. Each parameter is displayed individually with a standard deviation. The final overall assessment is obtained on the basis of regression analyses, with an endeavor to ensure the best possible differentiation of a normal cornea from keratoconus [8]. We selected the cut-off values on the basis of recommendations compiled from an extensive review of studies, and a value for subclinical keratoconus of ≥ 1.31 and ≥ 2.02 for clinical keratoconus [9].
We also assessed each cornea individually. The finding was diagnosed as keratoconus in the case that the topographic and tomographic parameters attested to keratoconus and there was presence of at least one of the following symptoms on examination on a slit lamp: stromal thinning, Vogt’s striae, Fleischer ring, cracks in the Descemet’s membrane or scissor reflex on retinoscopy, with deterioration of best corrected visual acuity. We defined subclinical keratoconus as pertaining to an eye which did not manifest any of the symptoms upon examination on a slit lamp, with no presence of scissor effect on retinoscopy and with best corrected visual acuity of 1.0, whereas paracentral corneal thinning and localized steepening on the posterior surface of the cornea, or numerous changes on the anterior surface of the cornea were present from the topographical and topometric parameters.
We included a total of 231 eyes (116 patients) in our cohort, which comprised 54 girls and 62 boys. The representation of the individual groups was as follows: 57 eyes were represented in the group of astigmatisms, 80 eyes in the group of eye-rubbers, 23 eyes in the group of retinal dystrophies (RD), 17 eyes in the group with positive family history (FH), and 54 eyes in the control group. The numbers and percentage representation in the individual groups are summarized in Table 1.
For the statistical analysis we used the system IBM® SPSS® Statistics.
1. Count and percentage representation of patients in individual groups 
RD – group of patients with retinal dystrophy, FH – group of patients with positive family history 2. Average values of BAD/D by gender 
BAD/D – Belin-Ambrósio deviation index RESULTS
The mean value of BAD/D in the larger groups was 1.11. The lowest BAD/D value was -0.99 and the highest value 11.90. In girls the mean value was slightly lower (1.06) than in boys (1.15). For a comparison see Table 2. The mean values within the individual groups were as follows. We recorded the highest mean BAD/D value in the group of astigmatisms, which was 1.44, followed by the group of eye-rubbers with 1.25, then the group with positive FH with 0.92, while the lowest values were 4. retinal dystrophy) – 23 eyes.
We wished to assemble a further risk group from patients with genetically confirmed Down syndrome and disorder of the conjunctival tissue (Ehler-Danlos syndrome, Marfan syndrome, mitral valve prolapse). However, due to the insufficient number of patients able to undergo an examination on Pentacam we were unable to assemble this group.
The fifth group was formed by a control group of healthy children. This included children with emmetropia or refractive errors of a mild degree, with a value of astigmatism of less than 2 Dcyl. – 54 eyes recorded in the control group and the RD group, both with 0.76. See Table 3 and Graph 1.
In the individual groups we analyzed the data distribution with the aid of tests for normality and also with the aid of visual inspection. With regard to certain deviations from the normal distribution of data, in the further analyses we selected non-parametric tests. We set the alpha value of significance at 0.05. For a comparison of the averages we used a Kruskal-Wallis test with post-hoc correction of significance.
In total the averages between the groups were statistically significantly different (Kruskal-Wallis, P = 0.004). The post-hoc analysis of the differences in averages between the individual groups and the control group are displayed in Table 4. The differences between the individual groups were statistically insignificant (P > 0.05). The only significant difference was between the group of astigmatisms and the control group (P = 0.002).
Within the framework of the stipulated cut-off BAD/D values for subclinical keratoconus of ≥ 1.31 and ≥ 2.02 for clinical keratoconus, we classified a total of 41 eyes as subclinical keratoconus. These included 16 eyes from the group of astigmatisms, 11 eyes from the group of eye-rubbers, 6 eyes from the RD group, and 4 eyes in both the control group and the group with a positive family history.
According to BAD/D we indicated 12 eyes as clinical keratoconus, of which 5 were from the group of astigmatisms and 6 eyes from the group of eye-rubbers, 1 eye from the control group and no keratoconus in the groups with RD and positive family history. See Graphs 2 and 3.
Following an individual evaluation of the topographic and tomographic parameters, we indicated 15 eyes as subclinical keratoconus, of which 5 were in the group of astigmatisms, 6 in the group of eye-rubbers, 1 in the group of RD, 3 in the group of FH and none in the control group. We indicated 6 eyes as clinical keratoconus, of which 3 eyes were in the group of astigmatisms, 2 in the group of eye-rubbers, 1 in the group of FH and none in the control or TRD groups.
Graph 4 illustrates a comparison of the number of cases of clinical and subclinical keratoconus following an individual evaluation and according to BAD/D.
In our cohort we diagnosed keratoconus in total in 5 boys aged 7, 12, 12, 13 and 14 years, and in 1 girl aged 8 years. Of these, 3 patients belonged to the group with astigmatism higher than 2 Dcyl, and other than this they did not manifest any other predisposing factor. Keratoconus was determined in 2 patients from the group of eye-rubbers, and these patients also had astigmatism of ≥ 2 Dcyl in at least one eye. One patient was from the group with a positive family history, and simultaneously was an eye-rubber and had astigmatism of ≥ 2 Dcyl in at least one eye.
3. Average values of BAD/D according to individual groups 
BAD/D – Belin-Ambrósio deviation index, RD – group of patients with retinal dystrophy, FH – group of patients with positive family history 4. Comparison of average BAD/D values of individual groups with the control group 
RD – group of patients with retinal dystrophy, FH – group of patients with positive family history DISCUSSION
The differences is the number of subclinical and clinical cases of keratoconus according to BAD/D and following individual assessment of the topographic and tomographic parameters can be explained by the fact that BAD/D has a higher sensitivity and specificity in the detection of keratoconus, since it was developed and is also recommended as a screening method [10]. Sensitivity and specificity differs depending on the selected cut-off values, though the need to detect all patients with suspected keratoconus, above all candidates for refractive surgery, requires high sensitivity and is associated with a greater number of false positive results [11].
In the study conducted by Henriquez, 6 out of 10 screening indexes from Pentacam were associated with the appearance of false positive results in the diagnosis of subclinical keratoconus. In this study the results in general also showed that the levels of false positive cases were highest in groups with hyperopic astigmatism and mixed astigmatism [12]. This explains the high BAD/D values in the group of astigmatisms in our cohort.
Because certain predisposing risk factors may be associated with the early onset of keratoconus, topography is routinely indicated in such cases. Keratoconus is a frequent complication of VKC. The prevalence of keratoconus may be as high as 26.8% among patients with VKC, whereas abnormal topography of the cornea may appear in up to 71% of such patients. This is more severe and progresses more rapidly, with marked deterioration of vision and with an increased need for keratoplasty. Treatment with CXL and corneal transplantation appears to be equally effective for patients with VKC in comparison with patients without VKC. However, postoperative complications are higher in patients with VKC, and require careful observation and strict monitoring of local inflammation [13]. Totan et al., who studied the incidence of keratoconus in patients with VKC using a quantitative evaluation of videokeratographic maps, determined an incidence of keratoconus in 26.8% of cases [14]. An increased incidence of keratoconus was recorded in male patients, as well as patients with a long duration of the disease, mixed or palpebral form of VKC and advanced corneal lesions. They came to the conclusion that the higher incidence of keratoconus in their study was caused by the timely detection of mild to subclinical forms of keratoconus with the aid of corneal topography, in which they emphasized its importance for patients with VKC. Similarly, an increased incidence of keratoconus resembling maps was determined in the pediatric population with VKC in Nepal. Out of 115 examined subjects it was present in 13 (11.3%) [15]. By contrast, a low incidence in comparison with the other literature was published by Caputo et al., where in children with VKC, topographic signs of keratoconus were detected in only 5 out of 651 patients (0.77%). Two of these were bilateral [16]. The importance of topographic examination for subclinical forms was highlighted also by Umale et al., in which an analysis of topographic corneal maps demonstrated an asymmetric bow tie formation with a lower slant in 17.11% of patients. None of these patients had clinical evidence of keratoconus [17]. Ophthalmologists in India succeeded in assessing the elevation of the posterior cornea with the aid of Sirius tomography in children with VKC, and comparing it with healthy children of a corresponding age and sex, in which they again confirmed a higher incidence of keratoconus in children with VKC with the aid of the condition of the posterior surface of the cornea [18]. Léoni-Mesplié et al. examined epidemiological aspects of keratoconus in 49 children and compared them with 167 adult patients. They determined that allergies, as well as eye-rubbing, were more common in children, and that allergy occurred in 67.3% of children in comparison with 47.3% in adults. Eye-rubbing occurred in 91.8% of children as against 70% of adults [2]. As a consequence, the authors recommend that corneal topography is routinely performed on all young allergic boys with a medical history of eye-rubbing and recently appearing corneal astigmatism. In our study we also recorded a higher incidence of keratoconus in boys than in girls, which was also confirmed by the higher mean BAD/D values in the male sex.
The association of keratoconus with certain types of retinal dystrophies has been described in older studies by several authors [19,20]. According to Morichini’s study of refractive errors in patients with retinitis pigmentosa, a refractive error was demonstrated in as many as 93% of cases. In 79% it was associated with myopia, in 60% with astigmatism, in 10% with hypermetropia and in 4% astigmatism was greater than 2.5 D [21]. With regard to the incidence of refractive errors (especially myopia and astigmatism) in patients with retinitis pigmentosa and deterioration of vision upon the background of the underlying pathology, not only incipient but also the advanced stage of keratoconus may easily be overlooked. A condition has been described in which bilateral keratoconus was diagnosed in two brothers with retinitis pigmentosa, one of whom had bilateral keratoconus in the stage of hydrops. Presentation with keratoconus in such an advanced stage is not common, but in the case of patients with retinitis pigmentosa this may be caused by deteriorated visual function, which prevents the perception of any changes of vision caused by keratoconus [22]. For this reason, patients with retinitis pigmentosa also constitute a suitable group for routine examination by means of corneal topography.
Last but not least, in the interest of identifying the early stages of keratoconus, examinations should be conducted in families in which this disease has occurred. Above all it is necessary to examine the children of patients who have undergone treatment for keratoconus [1].
Although in our study only the group of astigmatisms was determined to be statistically significant in comparison with the control group following the post-hoc correction, there was also a clinically significant difference in the group of eye-rubbers. In the groups with a positive family history and retinal dystrophies we were able to include only a smaller number of patients, which may have led to a distortion of the results.
CONCLUSION
Keratoconus is a progressive disorder of the cornea, which originates during adolescence. The majority of patients with keratoconus are not identified until a deterioration of visual acuity occurs, which is the result of changes on the anterior surface of the cornea. Corneal topography and tomography enable us to observe also initial changes on the posterior surface of the cornea, and thereby assist us in detecting subclinical form of keratoconus. Early diagnosis of patients with a mild stage of keratoconus at a young age brings us greater benefits than waiting until the patient is older and the pathology is in a more advanced and serious stage, hence the importance of early identification of children and adolescents with keratoconus. For this reason, despite our own statistical results, we agree with several published studies that certain groups of children, for example patients with higher astigmatism or a tendency to rub their eyes due to an ocular allergy, primarily VKC, patients with various forms of retinal dystrophies and last but not least children with a family history of keratoconus should be routinely examined with the aid of corneal topography and tomography, at least once per year.
1. Average value of BAD/D in individual groups 
RD – group of patients with retinal dystrophy, FH – group of patients with positive family history,
BAD/D – Belin-Ambrósio deviation index2. Graphic distribution of the number of cases of subclinical keratoconus according to BAD/D 
RD – group of patients with retinal dystrophy, FH – group of patients with positive family history,
BAD/D – Belin-Ambrósio deviation index5. Graphical distribution of the number of cases of clinical keratoconus according to BAD/D 
RD – group of patients with retinal dystrophy, FH – group of patients with positive family history 3. Comparison of the number of clinical and subclinical keratoconus after individual assessment and according to BAD/D 
RD – group of patients with retinal dystrophy, FH – group of patients with positive family history,
BAD/D – Belin-Ambrósio deviation index)
Sources
1. Santodomingo-Rubido J, Carracedo G, Suzaki A, Villa-Collar C, Vincent SJ, Wolffsohn JS. Keratoconus: An updated review. Cont Lens Anterior Eye. 2022 Jun;45(3):101559.
2. Léoni-Mesplié S, Mortemousque B, Mesplié N, et al. Aspects épidémiologiques du kératocône chez l’enfant [Epidemiological aspects of keratoconus in children]. J Fr Ophtalmol. 2012 Dec;35(10):776-785. French.
3. Elder MJ. Leber congenital amaurosis and its association with keratoconus and keratoglobus. J Pediatr Ophthalmol Strabismus. 1994; 31(1): p. 38-40.
4. Kristianslund, O. and L. Drolsum, Prevalence of Keratoconus in Persons With Down Syndrome in a National Registry in Norway. JAMA Netw Open, 2021. 4(3): p. e210814.
5. Strmeňová E. Keratokónus - Diagnostika a liečba theses.cz, “Masaryk University, Faculty of Medicine, Brno”, 2014: p.37-110.
6. Olivo-Payne A, Abdala-Figuerola A, Hernandez-Bogantes E, Pedro-Aguilar L, Chan E, Godefrooij D. Optimal management of pediatric keratoconus: challenges and solutions. Clin Ophthalmol. 2019 Jul 10;13 : 1183-1191.
7. Shetty R, Rao H, Khamar P, et al. Keratoconus Screening Indices and Their Diagnostic Ability to Distinguish Normal From Ectatic Corneas. Am J Ophthalmol. 2017 Sep;181 : 140-148.
8. Belin MW, Ambrósio R. Scheimpflug imaging for keratoconus and ectatic disease. Indian J Ophthalmol. 2013 Aug;61(8):401-406.
9. Motlagh MN, Moshirfar M, Murri MS, et al. Pentacam® Corneal Tomography for Screening of Refractive Surgery Candidates: A Review of the Literature, Part I. Med Hypothesis Discov Innov Ophthalmol. 2019 Fall;8(3):177-203.
10. Hwang ES, Perez-Straziota CE, Kim SW, Santhiago MR, Randleman JB. Distinguishing Highly Asymmetric Keratoconus Eyes Using Combined Scheimpflug and Spectral-Domain OCT Analysis. Ophthalmology. 2018 Dec;125(12):1862-1871.
11. McMonnies CW. Screening for keratoconus suspects among candidates for refractive surgery. Clin Exp Optom. 2014 Nov;97(6):492-498.
12. Henriquez M, Izquierdo L, Maldonado C, Cañola L, Cerrate M, Hadid MG. Diagnostic ability to distinguish high astigmatism from ectatic cornea using 2 different Scheimpflug devices. Investigative Ophthalmology & Visual Science. 2018. 59(9): p. 4761-4761.
13. Wajnsztajn D, Solomon A. Vernal keratoconjunctivitis and keratoconus. Curr Opin Allergy Clin Immunol. 2021 Oct 1;21(5):507-514.
14. Totan Y, Hepşen IF, Cekiç O, Gündüz A, Aydin E. Incidence of keratoconus in subjects with vernal keratoconjunctivitis: a videokeratographic study. Ophthalmology. 2001 Apr;108(4):824-827.
15. Gautam V, Chaudhary M, Sharma AK, Shrestha GS, Rai PG. Topographic corneal changes in children with vernal keratoconjunctivitis: A report from Kathmandu, Nepal. Cont Lens Anterior Eye. 2015 Dec;38(6):461-465.
16. Caputo R, Versaci F, Pucci N, et al. Very Low Prevalence of Keratoconus in a Large Series of Vernal Keratoconjunctivitis Patients. Am J Ophthalmol. 2016 Dec;172 : 64-71.
17. Umale RH, Khan MA, Moulick PS, Gupta S, Shankar S, Sati A. A clinical study to describe the corneal topographic pattern and estimation of the prevalence of keratoconus among diagnosed cases of vernal keratoconjunctivitis. Med J Armed Forces India. 2019 Oct;75(4):424-428.
18. Kavitha V, Heralgi MM, Aafreen S. Comparison of posterior corneal elevation in children with and without vernal keratoconjunctivitis using a new tomographer. Indian J Ophthalmol. 2021 Aug;69(8):2060-2063.
19. Moschos M, Droutsas D, Panagakis E, Tsioulias G, Tsalouki M. Keratoconus and tapetoretinal degeneration. Cornea. 1996 Sep;15(5):473-476.
20. Freedman J, Gombos GM. Bilateral macular coloboma, keratoconus, and retinitis pigmentosa. Ann Ophthalmol. 1971 Jun;3(6):664-665.
21. Sieving PA, Fishman GA. Refractive errors of retinitis pigmentosa patients. Br J Ophthalmol. 1978 Mar;62(3):163-167.
22. Zemba M, Zaharia AC, Dumitrescu OM. Association of retinitis pigmentosa and advanced keratoconus in siblings. Rom J Ophthalmol. 2020 Jul-Sep;64(3):313-321.Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology

2023 Issue 5-
All articles in this issue
- Úvod do Doporučených postupů
- XXXI. Výroční sjezd ČOS. Anonce
- Vzpomínka na doc. Hejcmanovou. Nekrolog
- Doporučené postupy diagnostiky a léčby diabetického makulárního edému
- Doporučené postupy diagnostiky a léčby diabetické retinopatie
- Screening diabetické retinopatie a diabetického makulárního edému
- Využitie rohovkovej topografie v detskej oftalmológii
- Torpédo makulopatia. Kazuistika
- Determination of Corneal Power after Refractive Surgery with Excimer Laser: A Concise Review
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Doporučené postupy diagnostiky a léčby diabetického makulárního edému
- Doporučené postupy diagnostiky a léčby diabetické retinopatie
- Screening diabetické retinopatie a diabetického makulárního edému
- Torpédo makulopatia. Kazuistika
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

