-
Medical journals
- Career
ENDOPHTHALMITIS IN OPHTHALMOLOGICAL REFERRAL CENTRE IN COLOMBIA: AETIOLOGY AND MICROBIAL RESISTANCE
Authors: Rangel, CM. 1,2,3,4; Parra, MM. 1,2; Corrales, MI. 1,2; D. Garcia 1,2; R. Sánchezávila 5; Varón, CL. 1,3; E. Villareal 1,2; D. Villarreal 6; A. Tello 1,2,4; V. Galvis 1,2,4
Authors‘ workplace: Ophthalmology Foundation of Santander - Carlos Ardila Lulle, (FOSCAL), Floridablanca, Colombia. 1; Autonomous University of Bucaramanga (UNAB), Bucaramanga, Colombia. 2; Industrial University of Santander (UIS), Bucaramanga, Colombia. 3; Virgilio Galvis Eye Centre, Floridablanca, Colombia. 4; Fernández-Vega University Institute, Ophthalmological Research, Foundation, Oviedo University, Oviedo, Spain. 5; OCULAB-Higuera Escalante Clinical Laboratory 6
Published in: Čes. a slov. Oftal., 78, 2022, No. 4, p. 160-173
Category: Original Article
doi: https://doi.org/10.31348/2022/19Overview
Aims: To describe the aetiology and microbial susceptibility profile of endophthalmitis cases treated at an ophthalmological referral centre in Colombia.
Material and Methods: A retrospective descriptive study was carried out with all endophthalmitis cases referred to the Fundación Oftalmológica de Santander FOSCAL (Floridablanca, Colombia) from 1 January 2012 to 31 December 2015.
Results: 121 eyes of 121 patients were evaluated. 77.7% of them were male and the mean age was 42.9 years. Five of them (4.1%) corresponded to endogenous endophthalmitis, and 116 (95.9%) to exogenous endophthalmitis. Of the latter, 66.9% were associated with trauma (almost one-half of them associated with intraocular foreign body), and 29.5% with intraocular surgery. The most common isolated microorganisms in the exogenous endophthalmitis group corresponded to methicillin-resistant and methicillin-sensitive strains of Staphylococcus epidermidis and Staphylococcus aureus, which were mostly susceptible to imipenem, vancomycin and moxifloxacin and resistant to ceftazidime.
Conclusion: Endophthalmitis is a potentially sight-threatening condition, especially in cases of inadequate treatment. Therefore, antimicrobial therapy should be guided by vitreous humour culture to assure that the causative microorganism is susceptible to the selected agent. The results of our study lead us to propose vancomycin, moxifloxacin or imipenem as first-line antimicrobial options.
Keywords:
Phacoemulsification – endophthalmitis – antimicrobial susceptibility – intraocular foreign body – posterior vitrectomy
INTRODUCTION
Endophthalmitis is a serious intraocular inflammatory reaction that could be secondary to an endogenous or exogenous aetiology. Left untreated, it could lead to significant and permanent vision loss. For this reason, it is important to initiate an effective antibiotic treatment promptly, guided by culture and antibiogram which evidence the aetiological microorganism and its susceptibility profile [1-4]. The latter has motivated the publication of a number of studies worldwide. These studies reflect that, for endophthalmitis in general, the most common isolated bacteria is Coagulase-negative staphylococcus, while fungal infection by Candida albicans is frequent in cases of endogenous endophthalmitis. The susceptibility profile varies geographically, depending on the coexistence with associated trauma or immunosuppression, the asepsis techniques, and the antimicrobial prophylaxis regimen of the place where the study was developed [2,5-13].
The chosen treatment could differ, depending on the isolated microorganism, susceptibility profile, severity of the infection and associated risk factors. To our knowledge, this is the first study describing the aetiological profile of endophthalmitis, the associated risk factors and treatment response in the Colombian population.
MATERIAL AND METHODS
A cross-sectional descriptive study was carried out with vitreous humour samples of all endophthalmitis cases referred to the Fundación Oftalmológica de Santander – FOSCAL (Floridablanca, Colombia) from 1 January 2012 to 31 December 2015. Through the analysis of their clinical records, culture and antibiogram results (interpreted separately as susceptible, intermediately susceptible or resistant), the cases were classified as endogenous or exogenous; the latter were divided into posttraumatic and postsurgical presentation.
The posttraumatic cases were distributed according to the place of occurrence of the accident (home, workplace, urban or rural context), trauma mechanism (perforating, penetrating), presence of intraocular foreign body, globe rupture, and associated findings (cataract or retinal detachment). For postsurgical endophthalmitis, the surgery type and postoperative time of clinical presentation were taken into account. All vitreous humour samples were obtained by posterior vitrectomy in operating theatres with the Constellation® system (Alcon, Fort Worth, Texas, USA).
For vitreous humour samples, our protocol includes obtaining a significant sample of undiluted vitreous humour (2 cc). To avoid hypotony during admission, a simultaneous injection of air is performed. In addition, another sample of approximately 3 cc of diluted vitreous humour is taken. These samples are immediately transported to the laboratory, where they are inoculated in duplicate, in three different types of culture broths (BHI, thioglycolate and potato dextrose), and divided into two groups to be kept under aerobic and anaerobic conditions. In addition, upon receiving the sample material, it is also inoculated into solid media: chocolate agar, which is kept under aerobic conditions, and blood agar, which is kept under anaerobic conditions. The growth of microorganisms in the broths is carefully monitored, gram staining and fluorescence microscopy studies are performed. Based on the results, re-inoculations are performed in the appropriate solid media. All inoculation processes are carried out under strict aseptic measures in a laminar flow chamber.
All variables were registered in a database, using 2010 Microsoft Excel software (Redmond, Washington, United States of America). The analysis included the culture and antibiogram results, whereby the isolations were classified by type of microorganism, and whether they were susceptible, showed intermediate susceptibility or were resistant to the determined antimicrobial. The analysis also contemplated the changes in corrected distance visual acuity (CDVA) pre - and post-treatment, measured in Snellen and converted to LogMar for the statistical analysis, and what cases ended up in enucleation, evisceration or phthisis bulbi.
Continuous variables were studied with the Kolmogorov - Smirnov normality test, and any potential difference between the base and the final value for non-parametric variables was evaluated with the Wilcoxon statistical test. The significance level was established in p<0.05, using the statistical software for Windows SPSS v19.0 (SPSS, Inc., Chicago, IL) for every analysis.
RESULTS
One hundred and twenty-one eyes of 121 patients were evaluated, the mean age was 42.9 years, and 77.7% of the patients were male. Five eyes (4.1%) corresponded to endogenous endophthalmitis, and 116 (95.9%) to exogenous endophthalmitis. Of the latter, 81 cases (66.9%) were secondary to trauma (49.4% of them associated with an intraocular foreign body) and 35 cases (28.9%) to ocular surgery: 18 to phacoemulsification (6 early and 12 late onset), 6 to trabeculectomy, 4 to posterior vitrectomy, 4 to intravitreal anti-angiogenic injections, 2 to glaucoma drainage devices, and 1 case associated with scleral suture.
For the exogenous endophthalmitis cases, the initial clinical findings that predicted greater endophthalmitis severity were cataract, intraocular foreign body, rhegmatogenous retinal detachment, and wounds perforating or penetrating the ocular globe. The surgical procedure most commonly associated with endophthalmitis was cataract surgery by phacoemulsification (51.4% of the cases), with a predominant late onset of 6 weeks since the surgery date (34.3%). Instead, those cases related to posterior vitrectomy or intravitreal injections mostly had an early onset. In the whole group of exogenous endophthalmitis, in 48 cases Staphylococcus epidermidis was isolated, and 58.3% of these showed resistance to methicillin. Out of 24 cases where Staphylococcus aureus was isolated, 50% showed resistance to methicillin. The specific characteristics of exogenous endophthalmitis cases are shown in Table 1. The sensitivity analysis to antibiotics, according to the endophthalmitis group (exogenous or endogenous), is shown in Table 2.
1. Characterization of exogenous endophthalmitis 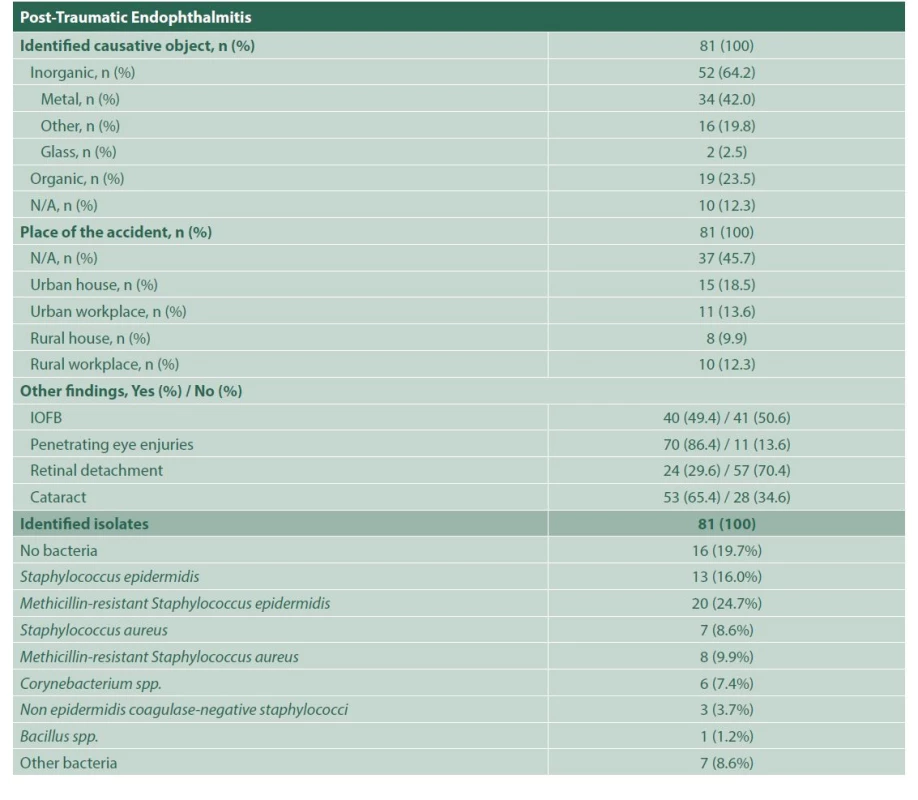
Tab1. continuation 
N/A: information not available, ND – No data, IOFB – Intraocular foreign body, spp – species pluralis, Latin abbreviation for multiple species 2. Bacterial sensitivity profile for each antibiotic in endophthalmitis 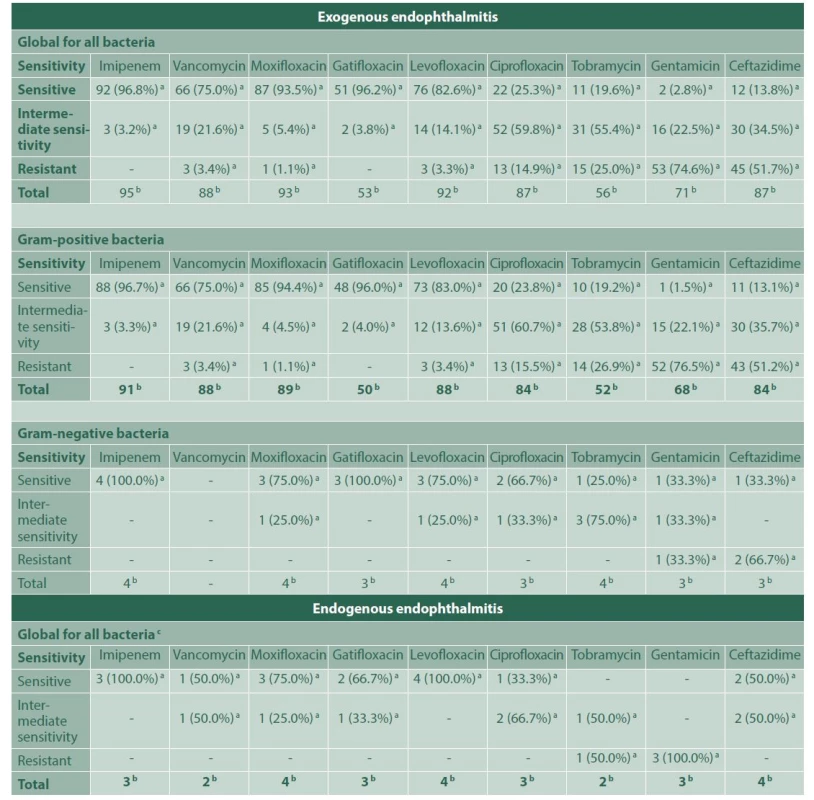
a – Number and percentage of positive cultures for bacteria, within each indicated group; sensitive, with intermediate sensitivity or resistant to that antibiotic, b – Total positive cultures for bacteria using a sensidisk with that particular antibiotic, in each group of bacteria indicated, c – All bacteria were gram-positive In 65 cases (80.2%) of posttraumatic endophthalmitis, a microorganism was isolated, methicillin-resistant Staphylococcus epidermidis being the most common aetiological agent (30.76%), which showed high susceptibility to imipenem (100%) and moxifloxacin (95%), but strikingly lower susceptibility to vancomycin (57.9%), and resistance to ceftazidime (55.6%). These results are shown in Table 3. Sixty-one of the posttraumatic cases received intravenous antibiotic therapy, either with vancomycin plus ceftriaxone (61.7%), or vancomycin plus moxifloxacin (13.5%). In the remaining 20 cases, intravenous treatment could not be obtained from the clinical records. Approximately 85% of the posttraumatic endophthalmitis cases underwent surgery: posterior vitrectomy alone (19.8%), posterior vitrectomy with phacoemulsification (44.4%), and both procedures with intraocular lens (IOL) implant in the same surgery (21%). In addition, 97.5% of these cases received intravitreal vancomycin, either combined with moxifloxacin (89.8%) or ceftazidime (10%). Most of the patients remained stable in time (90.1%); 1 case required evisceration and 7 cases (8.6%) ended up in phthisis bulbi.
3. Bacterial sensitivity to each antibiotic, for the group of endophthalmitis due to ocular trauma 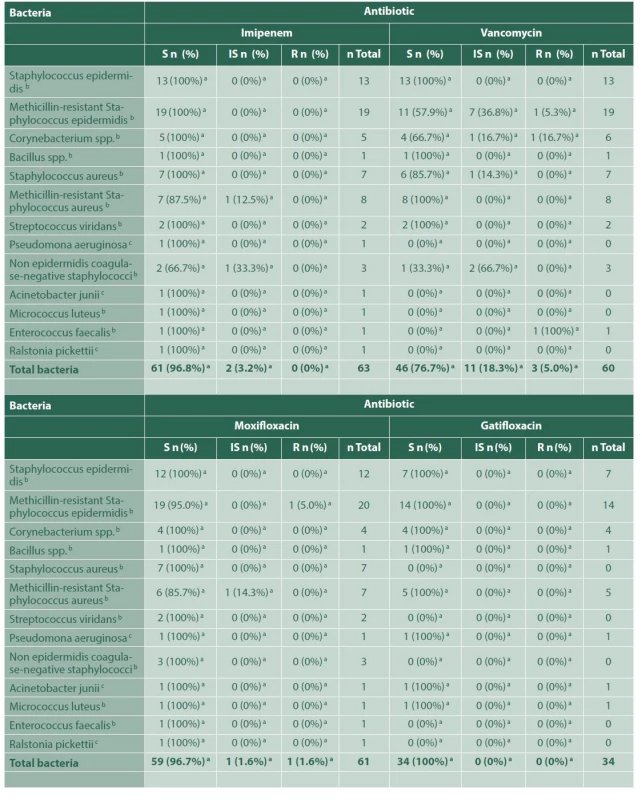
Tab 3. continuation 
a – Number and percentage of bacteria, within each indicated group, sensitive, with intermediate sensitivity or resistant to that certain antibiotic,
b – Gram-positive bacteria,
c – Gram-negative bacteria,
S – Susceptible,
IS – Intermediately susceptible,
R – ResistantRegarding the 35 cases of postsurgical endophthalmitis, a microorganism was isolated in 32 of them (91.4%). Methicillin-resistant Staphylococcus epidermidis was the most frequently found agent (25%), with an adequate susceptibility to imipenem (100%), vancomycin (75%) and moxifloxacin (87.5%), and resistance to ceftazidime in 57.1% of the samples. There was an additional case of co-infection with Candida albicans. None of the cases was associated with Acanthamoeba spp (Table 4). Twenty - three postsurgical cases received intravenous antibiotic therapy, 82.6% of them treated with vancomycin and ceftriaxone, and 17.4% with vancomycin and moxifloxacin. Thirty-three cases of postsurgical endophthalmitis underwent surgery: posterior vitrectomy alone (77.1%), posterior vitrectomy with phacoemulsification (11.4%), and both procedures with IOL (5.7%). All cases received additional intravitreal antibiotic therapy, the most common combination being vancomycin and moxifloxacin (94.3%). Although 2 cases of this group ended up in phthisis bulbi (5.7%), the majority of the cases remained stable.
4. Bacterial sensitivity to each antibiotic, for the group of endophthalmitis due to ophthalmological surgery 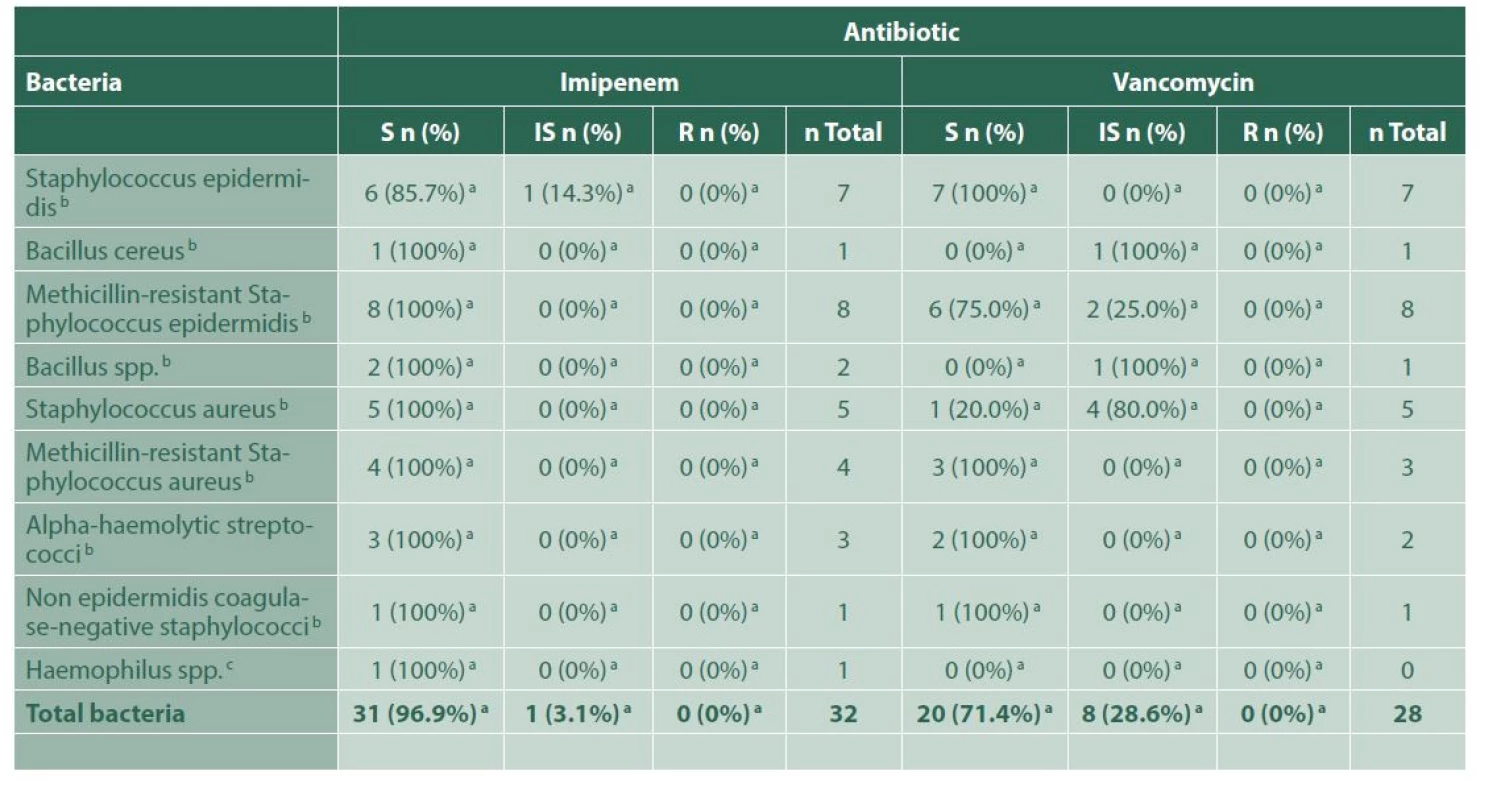
Tab 4. continuation 
a – Number and percentage of bacteria, within each indicated group, sensitive, with intermediate sensitivity or resistant to that certain antibiotic, b – Gram-positive bacteria, c – Gram-negative bacteria, S – Susceptible, IS – Intermediately susceptible, R – Resistant For the 5 cases of endogenous endophthalmitis, these were related to pulmonary tuberculosis, recent sepsis secondary to a hysterectomy procedure, Serratia marcescens sepsis and catheter-related bacteraemia; in one case the source could not be found. The microorganisms found in the 4 positive isolations are shown in Table 5. Three of those cases received intravenous antibiotics, and all of them received intravitreal microbial treatment in different combinations, depending on each sensitivity profile, including vancomycin, ceftriaxone, moxifloxacin and amphotericin B.
5. Bacterial sensitivity to each antibiotic, for the group of endogenous endophthalmitis 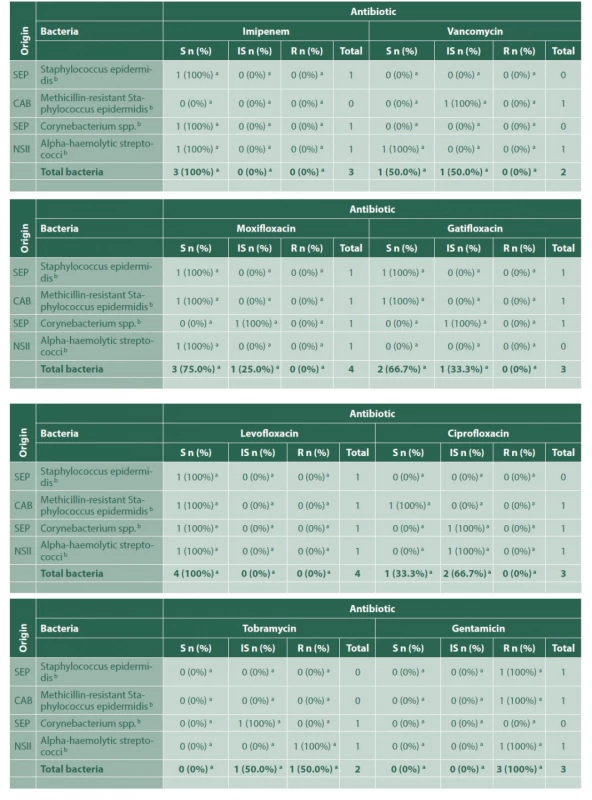
Tab 5. continuation 
CAB – Catheter-associated bacteremia, SEP– Septicemia, NSII – No source of infection identified, a – Number and percentage of bacteria, within each indicated group, sensitive, with intermediate sensitivity or resistant to that certain antibiotic, b – Gram-positive bacteria, S – Susceptible, IS – Intermediately susceptible, R – Resistant Only 1 of the total study cases required evisceration (in the post-trauma group); none of them required enucleation; 9 cases ended up in phthisis bulbi (7.43%).
Post-treatment CDVA, all groups experienced statistically significant improvement, except the endogenous endophthalmitis group. The mean CDVA pre - versus post - treatment was for endogenous endophthalmitis LogMar 2.33 (Snellen 20/4275, i.e. hand motions @ 3 m) vs. 1.35 (Snellen 20/447) (p 0.655); for posttraumatic exogenous endophthalmitis LogMar 1.87 (Snellen 20/1482, i.e. counting fingers @ 1 m) vs. 1,19 (Snellen 20/310) (p 0.002), and for post-intraocular surgery exogenous endophthalmitis LogMar 1.29 (Snellen 20/390) vs. 0.53 (Snellen 20/68) (p 0.007).
DISCUSSION
Endophthalmitis is an ophthalmological emergency, most commonly caused by bacteria or fungi, which enter the ocular globe endogenously or exogenously [10]. Its prognosis varies, depending mostly on the causal mechanism and the time elapsed from the infection time until the initiation of adequate antibiotic treatment for the specific isolated microorganism [1]. Intracameral antibiotics have been shown to diminish the risk of acute postoperative endophthalmitis, but nevertheless, some cases still occur [14,15]. A recent study suggested a seasonal variation of exogenous post-cataract surgery endophthalmitis incidence, but a confusion bias in the analysis has been suggested [16,17].
In a 10-year retrospective study published by Shimel et al., they described the characteristics, isolated microorganisms and susceptibility profile of endophthalmitis cases in the Bascom Palmer Eye Institute from 2002 to 2011. The most frequently isolated agents were Staphylococcus epidermidis in 30.1% of the cases, followed by Streptococcus viridians (10.9%), Staphylococcus aureus (7.8%) and Candida albicans (5.8%). In the present study, Staphylococcus epidermidis was also the most commonly isolated microorganism. Shimel et al. did not study gram-positive bacteria susceptibility for imipenem or ceftazidime, but they reported it for vancomycin (100%) and moxifloxacin (47%). In the present study for methicillin-resistant Staphylococcus epidermidis (gram-positive bacteria), the results were quite different: a susceptibility of 57.9% for vancomycin and 95% for moxifloxacin were found. This finding highlights the fact that results in this matter vary geographically [6].
In a previous report from our Institution in Colombia, methicillin-resistant coagulase - negative staphylococci were the most commonly isolated microorganisms in samples from intraocular fluids taken between June 2011 and January 2012 [18]. For samples taken between January 2013 and June 2016, Staphylococcus coagulase-negative non-epidermidis was the most commonly isolated agent from intraocular fluids, followed by Staphylococcus epidermidis, in samples from intraocular fluids [19].
While the global probability of obtaining a positive vitreous humour culture in cases of posttraumatic endophthalmitis varies between 48.4% and 64.8%, in the present study the value reached 80.2% [5,18,19]. This high positivity of cultures may be associated to several factors, including some related to the patient (contamination at the time of trauma, delay in seeking ophthalmological care). However, on the other hand, it may be influenced by the fact that we follow a very rigorous protocol for sample collection and processing, and have a microbiological unit dedicated to eye infections. We have also had very high culture positivity rates for corneal infections [18,19].
A review of the existent literature on the topic shows significant variability in aetiological agents of infectious endophthalmitis. Coagulase-negative Staphylococcus epidermidis is often the most common agent found, causing endophthalmitis subsequent to trauma, cataract surgery, intravitreal injections and posterior vitrectomy [5-18,20]. Streptococcus spp. and Haemophilus have also been isolated in cases secondary to glaucoma filtration surgery [21-24].
The present study showed similar results as those already previously published, differing slightly in methicillin-resistant Staphylococcus epidermidis being the most common isolation, also in post-glaucoma filtration surgery endophthalmitis.
In the present study, the number of cases with endogenous endophthalmitis was low (5 patients), so further statistical evaluation was not possible. Having said this, the most common isolated microorganisms in cases of endogenous endophthalmitis were Serratia spp. and Staphylococcus epidermidis, while other studies published worldwide report fungi as the first aetiological agent in these cases [8]. This possibly occurred because, in our study cases, sepsis was the most frequently associated pathology in this context.
Ramakrishnan et al. analysed 955 endophthalmitis cases during a period of 10 years, finding, as in the present study, that the most common aetiology of endogenous endophthalmitis were bacteria. However, unlike our findings, they evidenced a high susceptibility for ceftazidime in which methicillin-resistant Staphylococcus epidermidis was resistant to the antimicrobial in 57.1% of the cases. Hence, we do not recommend the intravitreal or intravenous indication of ceftazidime in our population [25].
In a review published by Gokce et al., they stated that only 88% (not all) of posttraumatic endophthalmitis cases require posterior pars plana vitrectomy [26]. However, although we agree with Gokce et al. in that there is not yet a definitive protocol supported by a well-designed, prospective multicentre clinical study for traumatic endophthalmitis, it is very likely that, due to its complexity and cost, this study will not be carried out for a long time. Given the recent advances in vitreoretinal microsurgery, which make it very safe, and the good results of our study, including the fact that none of the patients required enucleation or evisceration, we recommend performing posterior vitrectomy with vitreous humour sampling and intravitreal antibiotic application in all cases of endophthalmitis, regardless of their origin. Although the overall visual results showed improvement, many patients did not reach 20/200, as is also shown in other published case series [5,27,28]. Moreover, there were remarkable anatomical results in our study, compared to other studies that have reported enucleation in 17%, evisceration in 11% and phthisis bulbi in 35% of the cases [26]. However, in an extremely severe case of a patient with endogenous endophthalmitis, who consulted at our Institution at a later date and is not included in this series, bilateral enucleation was required [29].
In our study, all of the endophthalmitis cases post-cataract surgery by phacoemulsification (33 patients) received intravitreal antibiotic application, and 94.2% of them underwent posterior vitrectomy. Also, in the postsurgical period, depending on the case, intravenous antibiotics were indicated in 65.7% of the cases. Otherwise, publications by Kelkar’s group and Gower’s group report rates of posterior vitrectomy in post-cataract surgery endophthalmitis of 46.6% and 45%, respectively. In this subgroup of endophthalmitis, our visual and anatomical results were similar to those reported in other studies [30,31].
The results of the present study show that the majority of endophthalmitis cases in our context have an exogenous origin, trauma being the leading cause. This situation may be explained by the fact that our Institution is a referral centre for rural area patients, and also for patients whose jobs imply a considerable risk of ocular accidents. It draws our attention to the fact that the most common isolated microorganism is part of the skin flora, presumably because the trauma mechanism inoculates those agents more easily than those from the external environment.
Regarding endogenous endophthalmitis, in the present study, there were only 5 cases, 4.1% of our whole sample, which is within the range of the proportion reported in other studies, with respect to endophthalmitis of any cause [32].
Yannuzzi et al., in a study performed in Miami (Fla, USA), found that in 53.1% of the endophthalmitis cases caused by Staphylococcus epidermidis, methicillin resistance was found [33]. The rate of methicillin resistance among S. epidermidis isolates in the present study was slightly higher (58%). On the other hand, while 1 out of 7 (14.3%) of the cases of S. epidermidis endophthalmitis following trauma in the series reported by Yannuzzi et al. was caused by methicillin-sensitive microorganisms, this rate reached 62.5% (20 out of 32 cases) in the present study. Methicillin resistance has been reported in a wide range among studies on endophthalmitis from different countries. Chiquet et al. in France reported that methicillin resistance was found in 45% of patients with post - cataract endophthalmitis in the coagulase-negative Staphylococcus species form [34]. In a study with a large sample size (almost 1 000 consecutive culture-positive endophthalmitis isolates) carried out in New York (NY, USA), including cases collected from 1987 to 2011, researchers found that there was a statistically significant increase in the percentage of isolates that were resistant to methicillin, reaching a percentage of 55% for both S. aureus and S. epidermidis for the period 2007-2011 [35]. Specifically referring to cases of post-traumatic endophthalmitis, in another recent study carried out in China, also with a large sample, more than 1 000 cases of positive culture, Liu et al. found that 60.6% of gram-positive microorganisms isolated were resistant to methicillin. This percentage is similar to that found in the present study. This suggests that methicillin resistance within gram-positive organisms has increased in some areas of the world and is no longer restricted to cases of hospital-acquired infections.
A major limitation of our study is its retrospective nature, and that it includes the analysis of multiple aetiologies with variable clinical courses. Although these make it difficult to draw conclusions, our results will undoubtedly assist in the evidence-based management of endophthalmitis. The present study contributes to the knowledge of local microbiological predominance in endophthalmitis cases and their susceptibility profiles, which lead us to suggest vancomycin, moxifloxacin and imipenem as first-line antimicrobials for infectious endophthalmitis, either its intravitreal, systemic or combined application.
CONCLUSION
Endophthalmitis is a potential sight-threatening condition, especially in cases of inadequate treatment. For this reason, antimicrobial therapy should be guided by vitreous humour culture, to assure that the causative microorganism is susceptible to the selected agent. The results of our study lead us to propose vancomycin, moxifloxacin or imipenem as first-line antimicrobial options.
ACKNOWLEDGMENTS
We thank the Fundación Oftalmológica de Santander FOSCAL, the Higuera Escalante-OCULAB Clinical Laboratory and Universidad Autónoma de Bucaramanga UNAB.
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceutical company. They also declare that this article has not been submitted to another journal and is not printed elsewhere.
Received: 17 January 2022
Accepted: 20 April 2022
First author:
Carlos Mario Rangel, MD
Corresponding author:
Diana García, MD
Centro Oftalmológico Virgilio
Galvis.
Centro Médico Ardila Lulle
Tower A, 3rd floor, module 7
Urbanización El Bosque
loridablanca, Colombia.
E-mail: dianagarcias02@outlook.com
Sources
1. Vaziri K, Schwartz SG, Kishor K, Flynn HW Jr. Endophthalmitis: state of the art. Clin Ophthalmol. 2015; doi: 10.2147/OPTH.S76406
2. Rahmani S, Eliott D. Postoperative Endophthalmitis: A Review of Risk Factors, Prophylaxis, Incidence, Microbiology, Treatment, and Outcomes. Semin Ophthalmol. 2018;33(1):95‐101. doi:10.1080/08 820538.2017.1353826
3. Relhan N, Forster RK, Flynn HW Jr. Endophthalmitis: Then and Now. Am J Ophthalmol. 2018;187:xx‐xxvii. doi: 10.1016/j.ajo.2017.11.021
4. Brockhaus L, Goldblum D, Eggenschwiler L, Zimmerli S, Marzolini C. Revisiting systemic treatment of bacterial endophthalmitis: a review of intravitreal penetration of systemic antibiotics. Clin Microbiol Infect. 2019;25(11):1364‐1369. doi: 10.1016/j.cmi.2019.01.017
5. Bhagat N, Nagori S, Zarbin M. Post-traumatic Infectious Endophthalmitis. Surv Ophthalmol. 2011; doi: 10.1016/j.survophthal. 2010.09.002
6. Schimel A, Miller D, Flynn HW Jr. Endophthalmitis Isolates and Antibiotic Susceptibilities: A 10-Year Review of Culture-Proven Cases. Am J Ophthalmol. 2013; doi: 10.1016/j.ajo.2013.01.027
7. Smith SR, Kroll AJ, Lou PL, Ryan EA. Endogenous bacterial and fungal endophthalmitis. Int Ophthalmol Clin. 2007; doi: 10.1097/ IIO.0b013e31803778f7
8. Sadiq MA, Hassan M, Agarwal A, et al. Endogenous endophthalmitis: diagnosis, management, and prognosis. J Ophthalmic Inflamm Infect. 2015; doi: 10.1186/s12348-015-0063-y
9. Lingappan A, Wykoff CC, Albini TA, et al. Endogenous Fungal Endophthalmitis: Causative Organisms, Management Strategies, and Visual Acuity Outcomes. Am J Ophthalmol. 2012; doi: 10.1016/j. ajo.2011.06.020
10. Durand ML. Endophthalmitis. Clin Microbiol Infect. 2013; doi: 10.1111/1469-0691.12118
11. Sachdeva MM, Moshiri A, Leder HA, Scott AW. Endophthalmitis following intravitreal injection of anti-VEGF agents: long-term outcomes and the identification of unusual microorganisms. J Ophthalmic Inflamm Infect. 2016; doi: 10.1186/s12348-015-0069-5
12. Dossarps D, Bron AM, Koehrer P, Aho-Glélé LS, Creuzot-Garcher C, FRCR net (FRenCh Retina specialist net). Endophthalmitis after intravitreal injections: Incidence, presentation, management, and visual outcome. Am J Ophthalmol. 2015; doi: 10.1016/j. ajo.2015.04.013
13. Solá-Del Valle DA, Modjtahedi BS, Eliott D, Shen LQ. Treatment of Blebitis and Bleb-related Endophthalmitis. Int Ophthalmol Clin. 2015; doi: 10.1097/IIO.0000000000000081
14. Galvis V, Tello A, Camacho PA, Rey JJ. Comment on: Antibiotic prophylaxis in cataract surgery - An evidence-based approach. Indian J Ophthalmol. 2018;66(4):603. doi: 10.4103/ijo.IJO_1309_17
15. Galvis V, Tello A, Sánchez MA, Camacho PA. Cohort study of intracameral moxifloxacin in postoperative endophthalmitis prophylaxis. Ophthalmol Eye Dis. 2014;6 : 1‐4. Published 2014 Jan 16. doi:10.4137/OED.S13102.
16. Kim SH, Yu MH, Lee JH, Yoon JS, Rah SH, Choi M. Seasonal variation in acute post-cataract surgery endophthalmitis incidences in South Korea. J Cataract Refract Surg. 2019;45(12):1711‐1716. doi: 10.1016/j.jcrs.2019.07.022
17. Rey JJ, Camacho PA, Serrano SE, Ochoa ME, Galvis V, Tello A. Confusion bias and seasonal variation of exogenous endophthalmitis incidence. J Cataract Refract Surg. 2020;46(3):491‐492. doi: 10.1097/j. jcrs.0000000000000091
18. Galvis V, Tello A, Guerra A, Acuña MF, Villarreal D. Sensibilidad antibiótica de bacterias obtenidas de queratitis e infecciones intraoculares en la Fundación Oftalmológica de Santander (FOSCAL), Floridablanca, Colombia [Antibiotic susceptibility patterns of bacteria isolated from keratitis and intraocular infections at Fundación Oftalmológica de Santander (FOSCAL), Floridablanca, Colombia]. Biomedica. 2014;34 Suppl 1 : 23‐33. Spanish. doi:10.1590/S0120 - 41572014000500004
19. Galvis V, Parra MM, Tello A, et al. Perfil de resistencia antibiótica en infecciones oculares en un centro de referencia en Floridablanca, Colombia. [Antibiotic resistance profile in eye infections in a reference centre in Floridablanca, Colombia]. Arch Soc Esp Oftalmol. 2019;94(1):4‐11. Spanish. doi: 10.1016/j. oftal.2018.07.003
20. Li AL, Wykoff CC, Wang R, et al. Endophthalmitis after intravitreal injection: Role of Prophylactic Topical Ophthalmic Antibiotics. Retina. 2015; doi: 10.1097/IAE.0000000000000901
21. Leng T, Miller D, Flynn HW Jr, Jacobs DJ, Gedde SJ. Delayed-onset bleb-associated endophthalmitis (1996-2008): Causative organisms and visual acuity outcomes. Retina. 2011; doi: 10.1097/ IAE.0b013e3181e09810
22. Zheng CX, Moster MR, Khan MA, et al. Infectious Endophthalmitis After Glaucoma Drainage Implant Surgery. Retina. 2017; doi: 10.1097/IAE.0000000000001329
23. Al-Torbak AA, Al-Shahwan S, Al-Jadaan I, Al-Hommadi A, Edward DP. Endophthalmitis associated with the Ahmed glaucoma valve implant. Br J Ophthalmol. 2005; doi: 10.1136/bjo.2004.049015
24. Eifrig CWG, Scott IU, Flynn HW, Smiddy WE, Newton J. Endophthalmitis after pars plana vitrectomy: Incidence, causative organisms, and visual acuity outcomes. Am J Ophthalmol. 2004; doi: 10.1016/j. ajo.2004.06.035
25. Ramakrishnan R, Bharathi MJ, Shivkumar C, et al. Microbiological profile of culture-proven cases of exogenous and endogenous endophthalmitis: a 10-year retrospective study. Eye (Lond). 2009; doi: 10.1038/eye.2008.197
26. Gokce G, Sobaci G, Ozgonul C. Post-Traumatic Endophthalmitis: A Mini-Review. Semin Ophthalmol. 2015; doi: 10.3109/08820538.2013.877939
27. Faghihi H, Hajizadeh F, Esfahani MR, et al. Posttraumatic Endophthalmitis: Report No. 2. Retina. 2012; doi: 10.1097/ IAE.0b013e3182180087
28. Rishi E, Rishi P, Koundanya VV, Sahu C, Roy R, Bhende PS. Post-traumatic endophthalmitis in 143 eyes of children and adolescents from India. Eye (Lond). 2016; doi: 10.1038/eye.2016.9
29. Chaparro Tapias TA, Rangel Gualdron CM, Rodriguez HA, Rodriguez LM, Flores de Los Reyes L, Sánchez España JC. Bilateral enucleation due to multi-bacterial fulminant endogenous panophthalmitis. Enucleación bilateral por panoftalmitis endógena polimicrobiana fulminante. Arch Soc Esp Oftalmol. 2020;95(1):34‐37. doi: 10.1016/j.oftal.2019.10.005
30. Kelkar AS, Kelkar JA, Barve PM, Mulay A, Sharma S, Amoaku W. Post-clear corneal phacoemulsification endophthalmitis: profile and management outcomes at a tertiary eye care center in western India. J Ophthalmic Inflamm Infect. 2016; doi: 10.1186/s12348 - 016-0115-y
31. Gower EW, Keay LJ, Stare DE, Arora P, Cassard SD, Behrens A, Tielsch JM, Schein OD. Characteristics of Endophthalmitis after Cataract Surgery in the United States Medicare Population. Ophthalmology. 2015; doi: 10.1016/j.ophtha.2015.04.036
32. Danielescu C, Anton N, Stanca HT, Munteanu M. Endogenous Endophthalmitis: A Review of Case Series Published between 2011 and 2020. J Ophthalmol. 2020 Oct 23;2020 : 8869590. doi: 10.1155/2020/8869590. PMID: 33149945; PMCID: PMC7603614.
33. Yannuzzi NA, Patel NA, Relhan N, Tran KD, Si N, Albini TA, Berrocal AM, Davis JL, Smiddy WE, Townsend J, Miller D, Flynn HW Jr. Clinical Features, Antibiotic Susceptibilities, and Treatment Outcomes of Endophthalmitis Caused by Staphylococcus epidermidis. Ophthalmol Retina. 2018 May;2(5):396-400. doi: 10.1016/j. oret.2017.08.025. Epub 2017 Nov 2. PMID: 31047321.
34. Chiquet C, Maurin M, Altayrac J, Aptel F, Boisset S, Vandenesch F, Cornut PL, Romanet JP, Gain P, Carricajo A. Correlation between clinical data and antibiotic resistance in coagulase-negative Staphylococcus species isolated from 68 patients with acute post-cataract endophthalmitis. Clin Microbiol Infect. 2015 Jun;21(6):592.e1-8. doi: 10.1016/j.cmi.2015.01.028. Epub 2015 Feb 11. PMID: 25680315.
35. Gentile RC, Shukla S, Shah M, Ritterband DC, Engelbert M, Davis A, Hu DN. Microbiological spectrum and antibiotic sensitivity in endophthalmitis: a 25-year review. Ophthalmology. 2014 Aug;121(8):1634-42. doi: 10.1016/j.ophtha.2014.02.001. Epub 2014 Apr 2. PMID: 24702755.
36. Liu C, Ji J, Wang Z, Chen H, Cao W, Sun X. Microbiological Isolates and Antibiotic Susceptibilities in Cases of Posttraumatic Endophthalmitis: A 15-Year Review. J Ophthalmol. 2020 Apr 29;2020 : 5053923. doi: 10.1155/2020/5053923. PMID: 32411430; PMCID: PMC7210529.
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology

2022 Issue 4-
All articles in this issue
- VITAMIN D AND OPHTHALMOPATHIAS. A REVIEW
- EFFECT OF RANIBIZUMAB AND AFLIBERCEPT ON RETINAL PIGMENT EPITHELIAL DETACHEMENT, SUBRETINAL AND INTRARETINAL FLUID IN AGE-RELATED MACULAR DEGENERATION
- COINCIDENCE OF IDIOPATHIC INTRACRANIAL HYPERTENSION AND LEBER HEREDITARY OPTIC NEUROPATHY. A CASE REPORT
- EN BLOC RESECTION OF RETINAL VASOPROLIFERATIVE TUMOR USING 23G VITRECTOMY. A CASE REPORT
- ENDOPHTHALMITIS IN OPHTHALMOLOGICAL REFERRAL CENTRE IN COLOMBIA: AETIOLOGY AND MICROBIAL RESISTANCE
- DETERMINATION OF FACTORS ASSOCIATED WITH LONG-TERM ENDOTHELIAL LOSS AND REFRACTIVE RESULT IN PATIENTS WITH ARTISAN PHAKIC LENS
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- EN BLOC RESECTION OF RETINAL VASOPROLIFERATIVE TUMOR USING 23G VITRECTOMY. A CASE REPORT
- VITAMIN D AND OPHTHALMOPATHIAS. A REVIEW
- COINCIDENCE OF IDIOPATHIC INTRACRANIAL HYPERTENSION AND LEBER HEREDITARY OPTIC NEUROPATHY. A CASE REPORT
- EFFECT OF RANIBIZUMAB AND AFLIBERCEPT ON RETINAL PIGMENT EPITHELIAL DETACHEMENT, SUBRETINAL AND INTRARETINAL FLUID IN AGE-RELATED MACULAR DEGENERATION
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career




