-
Medical journals
- Career
Molecular genetic cause of achromatopsia in two patients of Czech origin
Authors: L. Hlavatá 1; Ľ. Ďuďáková 1; J. Moravíková 1; A. Zobanová 2; B. Kousal 1,3; P. Lišková 1,3
Authors‘ workplace: Klinika dětského a dorostového lékařství, 1. lékařská fakulta, Univerzita Karlova a Všeobecná fakultní nemocnice v Praze, Přednosta: doc. MUDr. Tomáš Honzík, Ph. D. 1; Soukromá oční ordinace MUDr. Anna Zobanová, Poliklinika Barrandov v Praze 2; Oční klinika, 1. lékařská fakulta, Univerzita Karlova a Všeobecná fakultní nemocnice v Praze, Přednostka: prof. MUDr. Jarmila Heissigerová, MBA, Ph. D. 3
Published in: Čes. a slov. Oftal., 75, 2019, No. 5, p. 272-276
Category: Case Report
doi: https://doi.org/10.31348/2019/5/5Overview
Introduction: Achromatopsia is an autosomal recessive retinal disorder with an estimated prevalence ranging from 1 in 30.000 to 50.000. The disease is caused by mutations in six different genes. The aim of the study was to perform molecular genetic analysis in 11 unrelated probands with a clinical diagnosis of achromatopsia and to describe clinical findings in those that were found to carry biallelic pathogenic mutations.
Methods: All probands and their parents underwent ophthalmic examination. Mutation detection was performed using Sanger sequencing of CNGB3 exons 6, 7, 9-13, which have been found to harbour most disease-causing mutations in patients with achromatopsia of European origin.
Results: Three known pathogenic variants in CNGB3 were identified in 2 probands. Proband 1 was a compound heterozygote for the c.819_826del; p.(Arg274Valfs*13) and c.1006G>T; p.(Glu336*). Proband 2 carried the c.1148del; p.(Thr383Ilefs*13) in a homozygous state. The best corrected visual acuity in proband 1 (aged 19 years) was 0.1 in both eyes, in proband 2 (aged 8 years) 0.05 in the right eye and 0.1 in the left eye. Both individuals had nystagmus, photophobia, and absence of colour discrimination. Fundus examination appeared normal however spectral-domain optical coherence tomography revealed subtle bilaterally symmetrical structural changes in the fovea.
Conclusion: Molecular genetic analysis of Czech patients with achromatopsia was performed for the first time. Identification of disease-causing mutations in achromatopsia is important for establishing an early diagnosis, participation in clinical trials assessing gene therapies and may be also used for preimplantation genetic diagnosis.
Keywords:
achromatopsia – CNGB3 – the Czech population
INTRODUCTION
Achromatopsia is a congenital autosomal recessive pathology of the retina, with an estimated prevalence of 1 per 30 000 to 50 000 of the population. It is clinically manifested by nystagmus, photophobia, colour sense disorder, eccentric fixation and reduced visual acuity, which in the majority of cases is within the range of purblindness (23, 36).
The biomicroscopic finding on the retina appears normal in the majority of affected individuals, only in some cases is it possible to detect slight pigment shifts or atrophic changes in the macula (31). Upon electroretinographic examination, the cone responses are substantially reduced or missing, whereas the function of the rods is normal or only slightly reduced (3, 25).
Until recently, achromatopsia was considered a stationary pathology (26), but more recent studies incline toward the opinion that this constitutes a slowly progressing disease (1, 13, 30-33). Analysis with the aid of spectral domain optical coherence tomography (SD-OCT) has defined 5 different stages (9, 27). Mild forms are characterised by a breach of continuity to absence of the ellipsoid layer of the internal segments of photoreceptors in the fovea, which gradually progresses to more pronounced atrophy, in which the most severe stage is accompanied by atrophy of the pigment epithelium (9, 27).
A correlation with the finding on SD-OCT was demonstrated with the aid of further imaging methods of fundus autofluorescence (1, 9). In the first stages, the signal is more intensive, probably as a sign of increased metabolic turnover accompanying the transitional stage before cell death. In the more advanced phases there is a decline of autofluorescence in the fovea as a consequence of atrophic changes. There was no significant demonstration of a correlation between structural and functional changes and age (1, 27).
Achromatopsia is caused by mutations in a total of 6 genes: ATF6 (19), CNGA3 (17, 34), CNGB3 (14, 28), GNAT2 (2, 15), PDE6C (29) and PDE6H (16).The gene CNGB3 (cyclic nucleotide gated channel beta 3) predominates, in which there is a causal mutation in approximately 50% of patients of European origin (18). CNGB3 codes the sub-unit modulating the function of CNG (cyclic nucleotide gated) of the channels in the cytoplasmatic membrane of the cones, which contribute to the process of phototransduction (18, 21).
In the Czech population, the molecular genetic cause of achromatopsia has not yet been studied. The aim of this study was to conduct screening for the presence of the most common causal mutations on 11 mutually unrelated patients with suspected clinical achromatopsia, as well as to describe in detail the clinical ocular findings in individuals with achromatopsia confirmed on the level of DNA.
METHODS
The genome DNA of 11 probands of Czech origin with a clinical finding of achromatopsia was isolated with the aid of a Gentra Puregene kit (QIAGEN, Hilden, Germany) from a sample of venous blood. With the aid of the conventional method of direct sequencing, screening of exons 6, 7, 9-13 of gene CNGB3 (referential sequence NM_019098.4) was performed. This targeted selection was conducted on the basis of the study by Mayer et al. (22), which tested 1074 probands of European origin with achromatopsia, and determined that in 41.5% of patients the illness originated upon a background of only 12 mutations in the gene CNGB3, located in the regions we selected. The used primers and detailed conditions of the reactions are available with the authors upon request. The description of the mutations corresponded to the recommendations of the Human Genome Variation Society, in which the first nucleotide coding sequences is in position 1 (5). The research was conducted in accordance with the Helsinki declaration, the probands or their legal representatives signed an informed consent form. The study was approved by the Ethical Commission of the General University Hospital in Prague.
We conducted a detailed ophthalmological examination on probands with confirmed diagnosis of achromatopsia on the level of DNA. Best corrected visual acuity was determined with the aid of ETDRS (Early Treatment Diabetic Retinopathy Study) Optotypes or Snellen charts, and converted to decimal values. Testing of colour perception was conducted with the aid of pseudoisochromatic tables or an HRR (Hardy-Rand-Rittler) test, the visual field was tested with a static perimeter (M-700, Medmont International, Nunawading, Australia). Within the framework of a biomicroscopic examination of the retina in artificial mydriasis, the ocular fundus was photographed and autofluorescence of the fundus was performed on proband 1 (Visucam 200, Carl Zeiss Meditec AG, Germany), in proband 2 it was not possible to perform this due to pronounced nystagmus. The individual layers of the macula were displayed with the aid of SD-OCT (RTVue, Optovue, Inc, Fremont, USA or Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany).
In the case of the parents, only a basic ocular examination was conducted, consisting in measurement of best corrected visual acuity and biomicroscopy of the fundus. The parents of proband 2 also underwent an examination with the aid of SD-OCT.
RESULTS
Direct sequencing determined that proband 1 was a compound heterozygote for mutations in the gene CNGB3: in exon 6 c.819_826del; p.(Arg274Valfs*13) and in exon 9 c.1006G>T, p.(Glu336*), whereas c.819_826del was inherited from the mother, c.1006G>T from the father (Fig. 1a). In proband 2, the scan determined in homozygote state mutation c.1148del; p.(Thr383Ilefs*13), meaning that both parents were therefore a heterozygote carrier for this variant (Fig. 1b).
1. Genealogies of both families and segregation of identified mutations in gene CNGB3, wt indicates referential sequence (wild type). a) family of proband 1, b) family of proband 2 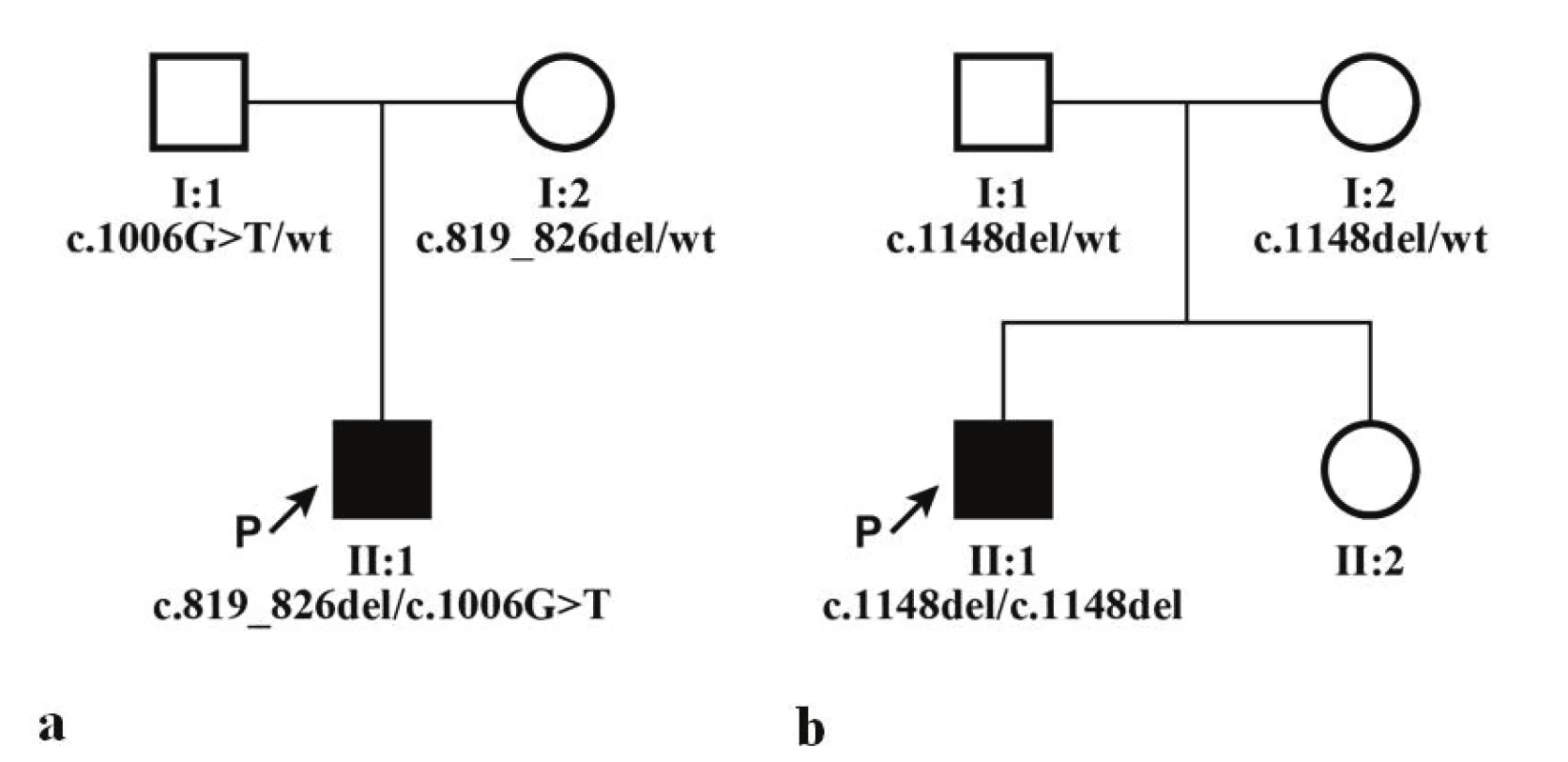
Proband 1 was observed from the first months of life for nystagmus and pronounced photophobia. At the age of 19 years, the best corrected visual acuity bilaterally was 0.1 with the use of edge filters and correction in the right eye of +1.50D = -1.00 Dcyl ax 170° and in the left eye plan = -0.75 Dcyl ax 180°. Testing of colour sense confirmed a total dysfunction of colour perception. An examination of the visual field determined bilateral central relative scotomas, though the validity of the conducted examination was low due to pronounced loss of fixation (Fig. 2c). Biomicroscopically the posterior pole of the fundus appeared physiological (Fig. 2a). The intensity of autofluorescence in the macula was also within the norm (Fig. 2b). The quality of the images obtained with the aid of SD-OCT was worse due to nystagmus, nonetheless they unequivocally demonstrated breach of continuity of the photoreceptor layer (Fig. 2d), therefore a finding corresponding to stage 1 according to Greenberg et al. (9). An examination of both parents did not demonstrate any ocular abnormalities.
2. Clinical finding in both probands with achromatopsia. a) photograph of fundus of right eye of proband 1, b) autofluorescence of fundus of right eye of proband 1, c) perimeter of right and left eye of proband 1, d) optical coherence tomography with spectral domain (SD-OCT) of left eye of proband 1, d) SD-OCT of left eye of proband 2 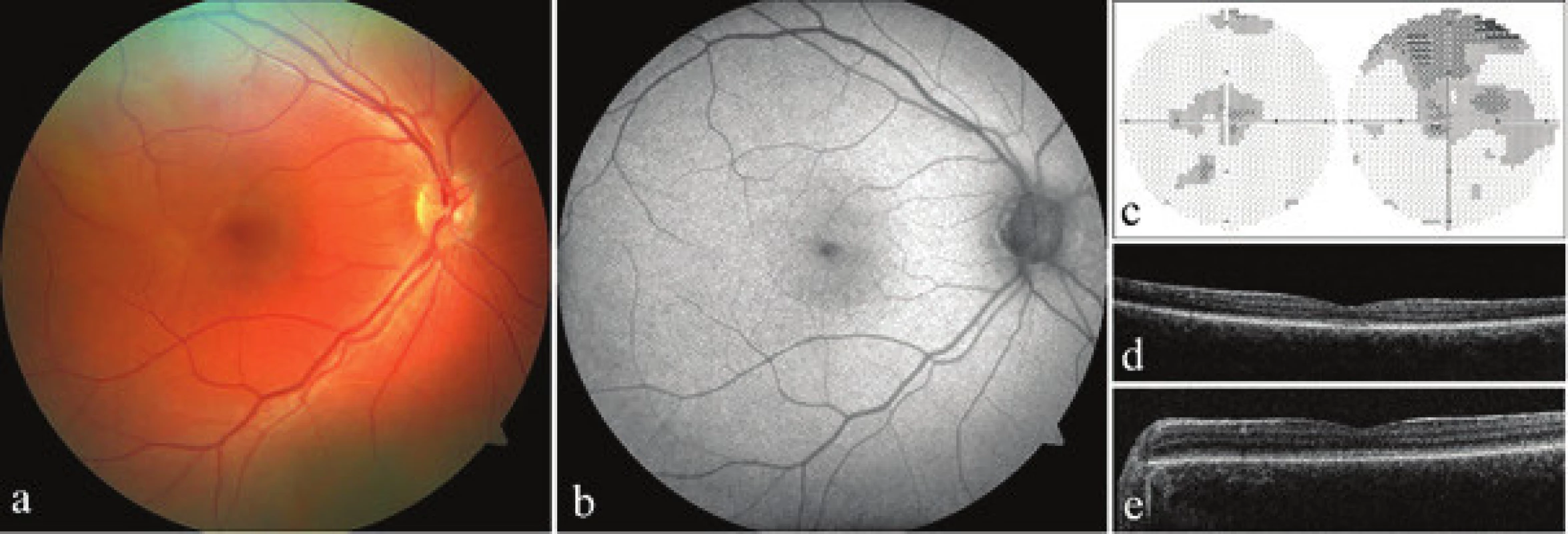
In proband 2 the pathology was also manifested in the form of photophobia and nystagmus from the first months of life. A diagnosis of achromatopsia was determined at the age of 4 years. Visual acuity at that time was 0.1 in both eyes without correction, and cycloplegic refraction was +3.25 D = -3.00 Dcyl ax 6°in the right eye and +3.00 D = -1.50 Dcyl ax 13° in the left eye, but this did not improve visual acuity. At the age of 8 years uncorrected visual acuity in the right eye was 0.05 and 0.1 in the left eye. There was no improvement even after the use of edge filters, nonetheless these at least significantly reduced photophobia. An examination of colour sense confirmed total colour blindness bilaterally. The biomicroscopic finding on the retina was without perceptible structural changes, however due to nystagmus and photophobia it was not possible to obtain a quality photograph of the ocular fundus, and it was also not possible to document autofluorescence of the fundus. The quality of the SD-OCT images of the macula was reduced (Fig. 2e), nevertheless the layer of ellipsoids was present, and we therefore evaluated the stage of the pathology as 1 (9). The ocular finding of the parents was within the norm.
DISCUSSION
We conducted a detection of causal mutations in patients of Czech origin with a clinical diagnosis or suspicion of achromatopsia for the first time. Determination of the diagnosis on the level of the gene has a substantial benefit in the case of achromatopsia, since especially in early childhood it is not possible to perform a range of essential objective and subjective examinations in a valid manner, such as SD-OCT, fundus autofluorescence, examination of colour sense or electroretinographic examination.
No significant correlation between phenotype and genotype was demonstrated in achromatopsia (8, 31), and as a result it is not possible according to the clinical finding to determine a gene in which it would be possible to locate pathogenic variants. According to the available literature, causal mutations in the gene CNGB3 contribute most to the occurrence of the pathology (18). Out of the 10 most common pathogenic variants in this gene occurring in individuals with achromatopsia, who were predominantly of Western European origin (22), in 11 patients of Czech origin we located only three: c.1148del, c.819_826del, and c.1006G>T. Mutation of c.1148del, which was determined in proband 2, is the most common pathogenic alelle of all, in homozygote state it was present in an extensive study numbering 1074 probands in more than one fifth of the families (22).
The fact that we demonstrated both pathogenic variants only in two probands out of 11 (18%), as against an expected 42%, indicates that in Czech patients the spectrum and frequency of causal mutations is different from that in the previously studied populations of other European countries. However, this finding may be distorted by the small cohort of patients.
The clinical ocular finding was typical in our probands. Achromatopsia was manifested already in the first months of life in the form of nystagmus and photophobia, and in both probands visual acuity was also within the zone of severe visual defects, which is defined by a range of visual acuity from 0.05 to 0.1 (11). Although the biomicroscopic finding on the retina appeared to be within the norm, nevertheless with the aid of SD-OCT it was possible to demonstrate discrete structural changes in the macula in both probands.
As yet no effective treatment of achromatopsia exists, patients are referred only for visual correction aids. Photophobia can be partially eliminated by wearing special absorption lenses known as edge filters. If causal mutations are known, in the case of a further pregnancy the parents of the affected child have the option of a pre-implantation genetic diagnosis (10, 35). At present clinical gene therapy tests are taking place on patients with mutations in the genes CNGA3 and CNGB3 (6, 7, 12). Their potential effect was supported in pre-clinical trials on animal models, which demonstrated an improvement of the electroretinographic findings and of visual acuity (4, 20, 24).
CONCLUSION
Although achromatopsia is a rare pathology, it may be encountered in clinical practice. The diagnostic difficulties that frequently accompany achromatopsia in small children often represent a considerable psychological burden for the family. In the case of suspicion of this clinical unit, we recommend genetic testing, which may substantially shorten the diagnostic process.
Thanks to Ing. Martin Melišek for performing an examination with the aid of SD-OCT and processing the results. The study was supported by grants from the Charles University PROGRESS Q26, UNCE 204064 and SVV 260367/2017.
Received: 28. 8. 2019
Accepted: 21. 10. 2019
Available on-line: 17. 2. 2020
MUDr. Lucia Hlavatá
Klinika dětského a dorostového lékařství
1. lékařská fakulta,
Univerzita Karlova a VFN v Praze
Ke Karlovu 2,
128 00 Praha 2
Sources
1. Aboshiha, J., Dubis, AM., Cowing, J., et al.: A prospective longitudinal study of retinal structure and function in achromatopsia. Invest Ophthalmol Vis Sci, 55 (9); 2014 : 5733-5743.
2. Aligianis, IA., Forshew, T., Johnson, S., et al.: Mapping of a novel locus for achromatopsia (ACHM4) to 1p and identification of a germline mutation in the alpha subunit of cone transducin (GNAT2). J Med Genet, 39 (9); 2002 : 656-660.
3. Andreasson, S., Tornqvist, K.: Electroretinograms in patients with achromatopsia. Acta Ophthalmol (Copenh), 69 (6); 1991 : 711-716.
4. Carvalho, LS., Xu, J., Pearson, RA., et al.: Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum Mol Genet, 20 (16); 2011 : 3161-3175.
5. Den Dunnen, JT., Dalgleish, R., Maglott, DR., et al.: HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum Mutat, 37 (6); 2016 : 564-569.
6. Dudakova L., Kousal B., Kolarova H., et al.: Gene Therapy for inherited retinal and optic nerve disorders: Current Knowledge. Cesk Slov Oftalmol. 72(4); 2016 : 128-136.
7. Fu, X., Huu, VAN., Duan, Y., et al.: Clinical applications of retinal gene therapies. Precis Clin Med, 1 (1); 2018 : 5-20.
8. Genead, MA., Fishman, GA., Rha, J., et al.: Photoreceptor structure and function in patients with congenital achromatopsia. Invest Ophthalmol Vis Sci, 52 (10); 2011 : 7298-7308.
9. Greenberg, JP., Sherman, J., Zweifel, SA., et al.: Spectral-domain optical coherence tomography staging and autofluorescence imaging in achromatopsia. JAMA Ophthalmol, 132 (4); 2014 : 437-445.
10. Hlavata L., Dudakova L., Trkova M., et al.: Preimplantation genetic diagnosis and monogenic inherited eye diseases. Cesk Slov Oftalmol.72(5); 2016 : 167-171.
11. ICD-11 for Mortality and Morbidity Statistics [online]. [cit. 2019-07-04]. Dostupné z: https://icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1103667651.
12. Kahle, NA., Peters T., Zobor D., et al.: Development of Methodology and Study Protocol: Safety and Efficacy of a Single Subretinal Injection of rAAV.hCNGA3 in Patients with CNGA3-Linked Achromatopsia Investigated in an Exploratory Dose-Escalation Trial. Hum Gene Ther Clin Dev, 29 (3); 2018 : 121-131.
13. Khan, NW., Wissinger, B., Kohl, S., et al.: CNGB3 achromatopsia with progressive loss of residual cone function and impaired rod-mediated function. Invest Ophthalmol Vis Sci, 48 (8); 2007 : 3864-3871.
14. Kohl, S., Baumann, B., Broghammer, M., et al.: Mutations in the CNGB3 gene encoding the beta-subunit of the cone photoreceptor cGMP-gated channel are responsible for achromatopsia (ACHM3) linked to chromosome 8q21. Hum Mol Genet, 9 (14); 2000 : 2107-2116.
15. Kohl, S., Baumann, B., Rosenberg, T., et al.: Mutations in the cone photoreceptor G-protein alpha-subunit gene GNAT2 in patients with achromatopsia. Am J Hum Genet, 71 (2); 2002 : 422-425.
16. Kohl, S., Coppieters, F., Meire, F., et al.: A nonsense mutation in PDE6H causes autosomal-recessive incomplete achromatopsia. Am J Hum Genet, 91 (3); 2012 : 527-532.
17. Kohl, S., Marx, T., Giddings, I., et al.: Total colourblindness is caused by mutations in the gene encoding the alpha-subunit of the cone photoreceptor cGMP-gated cation channel. Nat Genet, 19 (3); 1998 : 257-259.
18. Kohl, S., Varsanyi, B., Antunes, GA., et al.: CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet, 13 (3); 2005 : 302-308.
19. Kohl, S., D. Zobor, W.C. Chiang, et al.: Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat Genet, 47 (7); 2015 : 757-65.
20. Komaromy, AM., Alexander, JJ., Rowlan, JS., et al.: Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet, 19 (13); 2010 : 2581-2593.
21. Lamb, TD., Pugh, EN. Jr.: Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci, 47 (12); 2006 : 5137-5152.
22. Mayer, AK., Van Cauwenbergh, C., Rother, C., et al.: CNGB3 mutation spectrum including copy number variations in 552 achromatopsia patients. Hum Mutat, 38 (11); 2017 : 1579-1591.
23. Michaelides, M., Hunt, DM., Moore, AT.: The cone dysfunction syndromes. Br J Ophthalmol, 88 (2); 2004 : 291-297.
24. Michalakis, S., Muhlfriedel, R., Tanimoto, N., et al.: Restoration of cone vision in the CNGA3-/ - mouse model of congenital complete lack of cone photoreceptor function. Mol Ther, 18 (12); 2010 : 2057-2063.
25. Moskowitz, A., Hansen, RM., Akula, JD., et al.: Rod and rod-driven function in achromatopsia and blue cone monochromatism. Invest Ophthalmol Vis Sci, 50 (2); 2009 : 950-958.
26. Simunovic, MP., Moore, AT.: The cone dystrophies. Eye (Lond), 12 ( Pt 3b) 1998 : 553-6.
27. Sundaram, V., Wilde, C., Aboshiha, J., et al.: Retinal structure and function in achromatopsia: implications for gene therapy. Ophthalmology, 121 (1); 2014 : 234-245.
28. Sundin, OH., Yang, JM., Li, Y., et al.: Genetic basis of total colourblindness among the Pingelapese islanders. Nat Genet, 25 (3); 2000 : 289-293.
29. Thiadens, AA., den Hollander, AI., Roosing, S., et al.: Homozygosity mapping reveals PDE6C mutations in patients with early-onset cone photoreceptor disorders. Am J Hum Genet, 85 (2); 2009 : 240-247.
30. Thiadens, AA., Slingerland, NW., Roosing, S., et al.: Genetic etiology and clinical consequences of complete and incomplete achromatopsia. Ophthalmology, 116 (10); 2009 : 1984-1989.e1.
31. Thiadens, AA., Somervuo, V., van den Born, LI., et al.: Progressive loss of cones in achromatopsia: an imaging study using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci, 51 (11); 2010 : 5952-5957.
32. Thomas, MG., Kumar, A., Kohl, S., et al.: High-resolution in vivo imaging in achromatopsia. Ophthalmology, 118 (5); 2011 : 882-887.
33. Thomas, MG., McLean, RJ., Kohl, S., et al.: Early signs of longitudinal progressive cone photoreceptor degeneration in achromatopsia. Br J Ophthalmol, 96 (9); 2012 : 1232-1236.
34. Wissinger, B., Jagle, H., Kohl, S., et al.: Human rod monochromacy: linkage analysis and mapping of a cone photoreceptor expressed candidate gene on chromosome 2q11. Genomics, 51 (3); 1998 : 325-331.
35. Yahalom, C., Macarov, M., Lazer-Derbeko, G., et al.: Preimplantation genetic diagnosis as a strategy to prevent having a child born with an heritable eye disease. Ophthalmic Genet, 39 (4); 2018 : 450-456.
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology
2019 Issue 5-
All articles in this issue
- Vodiace psy sa cvičia na Slovensku už 25 rokov
- The long-term monitoring of sympathetic ophthalmia in the diagnostic and terapeutic view. Review Department of Ophthalmology
- The optic nerve drusen and haemodynamics
- Sensitivity and specificity of spectral OCT in patients with early glaucoma.
- Nanolaser in cataract surgery and its impact on corneal endotelium
- Molecular genetic cause of achromatopsia in two patients of Czech origin
- INTRAVITREAL THERAPY OF ENDOGENOUS ENDOPHTALMITIS DUE TO UROSEPSIS – A CASE REPORT
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Molecular genetic cause of achromatopsia in two patients of Czech origin
- INTRAVITREAL THERAPY OF ENDOGENOUS ENDOPHTALMITIS DUE TO UROSEPSIS – A CASE REPORT
- Sensitivity and specificity of spectral OCT in patients with early glaucoma.
- The long-term monitoring of sympathetic ophthalmia in the diagnostic and terapeutic view. Review Department of Ophthalmology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


