-
Medical journals
- Career
Neuroradiological features and clinical outcomes in methanol intoxication
Authors: H. B. Onan 1; F. C. Piskin 1; T. Demir 2; H. T. Ballı 1; N. R. Disel 3
Authors‘ workplace: Department of Radiology, Balcali, Hospital, Medical Faculty Cukurova, University, Adana, Turkey 1; Department of Neurology, Balcali, Hospital, Medical Faculty Cukurova, University, Adana, Turkey 2; Department of Emergency Medicine, Balcali Hospital, Medical Faculty Cukurova, University, Adana, Turkey 3
Published in: Cesk Slov Neurol N 2021; 84(5): 442-448
Category: Original Paper
doi: https://doi.org/10.48095/cccsnn2021442Overview
Aim: The aim of this study was to identify the early radiological findings detected in patients diagnosed with methanol intoxication and to investigate the relationship between the presence and extent of these findings and the prognosis. Methods: The clinical and early radiological findings of patients who presented at the emergency department with sudden-onset complaints and were diagnosed with methanol intoxication were evaluated. The patients were grouped according to the radiological findings and compared in terms of methanol levels at presentation, severity of metabolic acidosis and clinical outcomes. Results: In this study, 45 patients (41 [91.1%] males) with a mean age of 51.6 ± 13.6 years were evaluated. The most common symptom was vision loss (N = 25; 55.6%). Pathological effects were detected in 18 (40%) patients (Group I) using CT and MRI, whereas 27 patients (60%) (Group II) had normal imaging findings. The number of patients with sequelae or death in Group I (N = 18; 100%) was significantly higher than in Group II (N = 9; 33.3%) (P < 0.001). Conclusion: Radiological imaging findings obtained in the early period can reveal pathology and predict prognosis in methanol intoxication. Patients with radiological findings have a worse prognosis than those without positive imaging findings.
Keywords:
Prognosis – orbit – methanol intoxication – neuroradiological findings – brain MRI
Introduction
Methanol is a colourless and odourless substance used in the production of industrial solvents and antifreeze solutions, and it tastes similarly to ethanol [1]. Methanol intoxication occurs due to oral intake, inhalation or transdermal exposure to methanol by accident or for suicide purposes [2,3]. Methanol itself is non-toxic substance and when taken orally, it is quickly absorbed through the intestines within 30–60 min, in a similar way to ethanol. Substances (formic acid and formaldehyde) that are produced by the liver as a result of methanol toxicity cause metabolic acidosis within 24 h [4]. Symptoms in the early period of methanol intoxication are non-specific and can include mild or severe headache, nausea and/or vomiting, and abdominal pain. The metabolization of methanol and the increase in the blood levels of toxic substances lead to the development of confusion, vision impairment, loss of consciousness and eventually death due to respiratory system depression [5]. Formic acid and formaldehyde may cause degenerative demyelization of the optic nerve and retinal toxicity. Therefore, optic nerve damage and related neurological symptoms are common in methanol intoxication [6].
Some patients are discharged with full recovery at the end of their treatment, but in others, despite the use of appropriate treatment methods, loss of vision, neurological sequelae (Parkinsonism symptoms such as tremor, hypokinesia, rigidity and paralysis) or even death may occur. From the studies in the literature, a number of poor prognostic factors have been identified and it has been concluded that the early diagnosis and treatment are important for favourable clinical outcomes in methanol intoxication [7,8]. According to the literature, necrosis in the basal ganglia (especially the putamen and globus pallidus) may be evidence that the toxic metabolite of methanol tends to accumulate and is the most commonly detected radiologic finding in methanol intoxication. In addition, necrosis of other structures (e. g., white and grey matter) in the brain has been observed in severe cases. There is no clear consensus on the role of radiological imaging in the early period (within the first week) in determining prognosis in methanol intoxication [9,10]. This study aimed to identify the early radiological findings in patients diagnosed with methanol poisoning and to investigate the relationship between the presence and extent of these findings and the clinical outcomes.
Materials and methods
Participants
In this study, the clinical and early radiological findings (within the first week of presentation) of 45 consecutive patients who presented at the emergency department of our hospital with sudden-onset complaints and were diagnosed with methanol intoxication between April 2014 and July 2019 were evaluated retrospectively. Patients who had received any treatment for methanol intoxication before the imaging process were excluded from the study.
Clinical evaluation
At the time of presentation, all patients were questioned about their alcohol consumption (within the past week) before the development of their complaints. Symptoms that may be associated with methanol intoxication (headache, dizziness, nausea, vomiting, abdominal pain, vision loss and loss of consciousness) were recorded. During laboratory tests, an arterial blood gas analysis was performed and blood methanol levels were measured. The diagnosis of methanol intoxication was undertaken based on clinical findings accompanied by a blood methanol level of ≥ 10 mg/dL. Other toxic conditions (e. g., carbon monoxide inhalation and acute cyanide intoxication) that could lead to the clinical manifestation were ruled out by laboratory tests.
Imaging methods
All patients in the study underwent non-contrast-enhanced CT of the brain (Toshiba Aquilion Prime, Otawara, Japan) at the emergency department at the time of presentation. The patients with pathologies detected on the CT images and those without pathology but with symptoms that did not subside within the first week of treatment also underwent 3.0 T (Ingenia, Philips Medical Systems, Best, Amsterdam, the Netherlands) or 1.5 T (Signa, GE Medical Systems, Milwaukee, WI, USA) MRI of the brain and orbit without contrast enhancement. Any abnormal signal intensity in the brain and orbit were evaluated by an experienced neuroradiologist in conventional sequences (T1-weighted images [T1WI], T2-weighted images [T2WI] and fluid attenuated inversion recovery [FLAIR]) and diffusion weighted imaging [DWI]).
Treatment and follow-up
All patients were placed under observation at the emergency department at presentation. Fomepizole was administered to all patients. A haemodialysis was applied in patients in whom metabolic acidosis with the increased anion gap (cut-off value of pH: < 7.35 and anion gap: > 12 ± 2 meq/L) was detected in the blood gas test. The patients were treated and followed up at the intensive care service. After discharge following treatment, the patients were clinically evaluated by a neurologist and an ophthalmologist. The clinical data of the patients were screened from the hospital’s patient information system and recorded.
Patient grouping
The patients were grouped according to the pathological findings obtained from the CT and MRI. The patients with putamen necrosis and additional accompanying pathologies (globus pallidus, thalamus, white matter and cortex necrosis, and parenchymal and/or subarachnoid haemorrhage) were included in Group I, while those with normal imaging findings were included in Group II. The patients in Group I and Group II were compared in terms of methanol levels at presentation, severity of metabolic acidosis and clinical outcomes.
Statistical analysis
The demographic information and clinical characteristics of the patients were expressed as descriptive statistical data. Quantitative data showing normal distribution were obtained as mean and standard deviation, while median and maximum-minimum values were used to present quantitative data without normal distribution. Qualitative data were summarized as numbers and percentages. The variables were comparatively evaluated between Groups I and II using the chi-square (Fisher’s exact) test. A P-value of less than 0.05 was considered statistically significant. TURCOSA software (Turcosa Analytics Ltd. Co., Kayseri, Turkey) was used for statistical analyses.
Results
The set consisting of 45 patients (41 [91.1%] males) with a mean age of 51.6 ± 13.6 years was evaluated. When the clinical histories of the patients over the past 24 h were questioned, 43 (95.6%) had consumed alcohol, 1 (2.2%) had ingested spirit by an accident, and 1 (2.2%) had ingested antifreeze solution for the purpose of suicide. The symptoms at presentation were headache (N = 4; 8.9%), nausea and/or vomiting (N = 3; 6.7%), vision loss (N = 25; 55.6%) and loss of consciousness (N = 13; 28.9%). On the CT and MRI, the pathological findings were compatible with methanol intoxication in 18 (40%) patients (Group I), whereas 27 patients (60%) (Group II) had normal imaging findings.
The first treatment was undertaken in all patients at the emergency department; haemodialysis was performed in 38 patients (84.4%) (Group I: N = 18; 100% and Group II: N = 19; 70.4%) due to metabolic acidosis. The arterial blood gas analysis conducted at presentation revealed the mean pH value of 7.05 ± 0.09 for Group I and 7.22 ± 0.24 for Group II. There was a significant difference between the two groups in terms of the pH values (P = 0.003). The mean pCO2 value was 37.6 ± 16.2 kPa for Group I and 41.5 ± 16.2 kPa for Group II. There was no significant difference between the two groups in terms of the pH values (P = 0.184). The median methanol levels of Groups I and II were 44.9 (8.9–202) mg/dL and 17 (10–289) mg/dL, respectively, indicating no significant difference (P > 0.05). The demographic information and clinical characteristics of the patients are summarized in Tab. 1. In Group I, 14 (77.8%) of the patients were imaged using MRI of the brain and orbit in addition to the brain CT. The remaining four patients (22.2%) could not be evaluated by MRI due to their unstable clinical condition. The imaging of Group I revealed isolated putamen necrosis (increased signal in T1WI, T2WI and FLAIR images) in 3 patients (16.7%) (Fig. 1), basal ganglia necrosis (increased signal in T1WI, T2WI and decreased diffusion in DWI especially in the lentiform nucleus) in four patients (22.2%) and diffuse cerebral necrosis (increased signal in T1WI, T2WI and decreased diffusion in DWI in basal ganglia necrosis and subcortical white matter) in 10 patients (55.6%) (Fig. 2). Three patients with diffuse cerebral necrosis (16.7%) were also found to have subarachnoid haemorrhage on the brain CT (Fig. 3). Isolated diffusion restriction of the optic nerve was found in one patient (5.6%), while other patients with vision loss had normal findings for the optic nerves in DWI (Fig. 4).
1. Demographic and clinical characteristics of patients treated and followed up due to methyl alcohol intoxication. 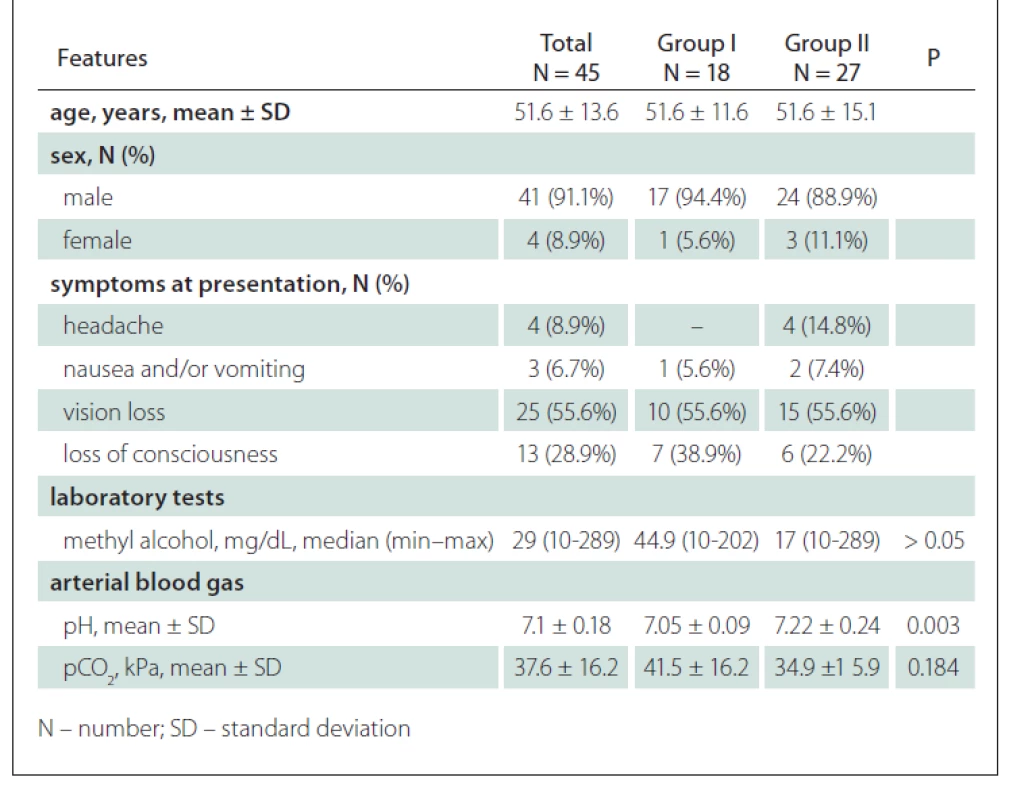
Fig. 1. The brain MRI images of a 37-year-old male patient who developed vision loss after alcohol consumption. The T1-weighted (A), T2-weighted (B), and FLAIR (C) sequences reveal bilateral putamen necrosis and increased signal at the putamen level (methanol level: 58.3 mg/dL, arterial blood gas pH: 7.01, pCO2: 28 kPa). Vision loss was present at the time of discharge from the hospital. FLAIR – fluid attenuated inversion recovery
Obr. 1. Snímky z MR mozku 37letého pacienta, u kterého došlo ke ztrátě zraku po požití alkoholu. T1 vážená sekvence (A), T2 vážená sekvence (B) a sekvence FLAIR (C) ukazují oboustrannou nekrózu putamen a zvýšený signál v oblasti putamen (hladina metanolu: 58,3 mg/dl, vyšetření arteriálních krevních plynů – pH: 7,01, pCO2: 28 kPa). Při propuštění u pacienta přetrvávala ztráta zraku. FLAIR – fluid attenuated inversion recovery
Fig. 2. The brain MRI images of a 58-year-old male patient that presented to the emergency department due to headache that developed after alcohol consumption. At the level of the basal ganglia, findings were normal in the T2-weighted (A) sequence but clearly distinguished in the diffusion-weighted image (B) and ADC mapping (C) (methanol level: 9. 7 mg/dL, arterial blood gas pH: 7.33, pCO2: 31.4 kPa). Vision loss was present at the time of discharge.
ADC – apparent diffusion coefficient
Obr. 2. Snímky z MR mozku 58letého pacienta, který se dostavil na pohotovost kvůli bolesti hlavy po požití alkoholu. Nálezy v oblasti bazálních ganglií byly v T2 vážené sekvenci (A) normální, ale na difúzí vážených obrazech (B) a v mapách ADC (C) měla bazální ganglia restrikci difúze (hladina metanolu: 9,7 mg/dl, vyšetření arteriálních krevních plynů – pH: 7,33, pCO2: 31,4 kPa). Při propuštění u pacienta přetrvávala ztráta zraku.
ADC – aparentní difuzní koeficient
Fig. 3. The brain CT images of a 67-year-old male patient who developed vision loss after alcohol consumption. Diffuse parenchymal hypodensity in the basal ganglion and subcortical white matter, parenchymal and subarachnoid hemorrhage revealed by CT images (methanol level: 202 mg/dL, arterial blood gas pH: 6.95, pCO2: 49.7 kPa). The patient died during treatment.
Obr. 3. Snímky z CT mozku 67letého pacienta, u kterého došlo ke ztrátě zraku po požití alkoholu. Snímky ukazují difúzní parenchymální hypodenzity v oblasti bazálních ganglií a subkortikální bílé hmoty, parenchymální a subarachnoidální krvácení (hladina metanolu: 202 mg/dl, vyšetření arteriálních krevních plynů – pH: 6,95, pCO2: 49,7 kPa). Pacient v průběhu léčby zemřel.
Fig. 4. The brain MRI images of a 59-year-old male patient referred to the emergency department due to loss of consciousness after alcohol consumption. The hypointensity in the basal ganglia revealed by the T1-weighted (A) image and hyperintensity and diffusion restriction in the basal ganglia and subcortical white matter revealed by FLAIR (B) and diffusion-weighted (C) images are consistent with diffuse cerebral necrosis (methanol level: 23.4 mg/dL, arterial blood gas pH: 7.21, pCO2: 31.9 kPa). Neurological sequelae and vision loss were present at the time of discharge.
FLAIR – fluid attenuated inversion recovery
Obr. 4. Snímky z MR mozku 59letého pacienta odeslaného na pohotovost z důvodu ztráty vědomí po požití alkoholu. Hypointenzity v oblasti bazálních ganglií zjištěné v T1 vážené sekvenci (A) a hyperintenzity spolu s restrikcí difúze v oblasti bazálních ganglií a subkortikální bílé hmotě zjištěné v sekvenci FLAIR (B) a na difúzí vážených obrazech (C) odpovídají difúzní mozkové nekróze (hladina metanolu: 23,4 mg/dl, vyšetření arteriálních krevních plynů – pH: 7,21, pCO2: 31,9 kPa). Při propuštění u pacienta přetrvávaly ztráta zraku a neurologické následky.
FLAIR – fluid attenuated inversion recovery
Death occurred in seven (38.9%) patients in Group I during their treatment at the emergency department. Another seven patients (38.9%) were discharged with isolated vision loss and four (22.2%) with vision loss and neurological sequelae at the end of their treatment.
All three patients with isolated putamen necrosis were found to have vision loss at the end of their treatment. Of the patients with detected putamen and globus pallidus necrosis, three (75%) had visual impairment and one had neurological sequelae (25%) at the end of treatment.
Seven of the patients with diffuse cerebral necrosis (70%) died during their treatment at the emergency department and three (30%) had visual loss and neurological sequelae at the end of their treatment. All three patients with diffuse cerebral necrosis and subarachnoid haemorrhage died. The imaging findings of the patients in Group I and their clinical status at the time of discharge are summarized in Tab. 2.
2. Radiological findings and clinical outcomes of patients with pathological findings on radiological imaging performed due to methyl alcohol poisoning. 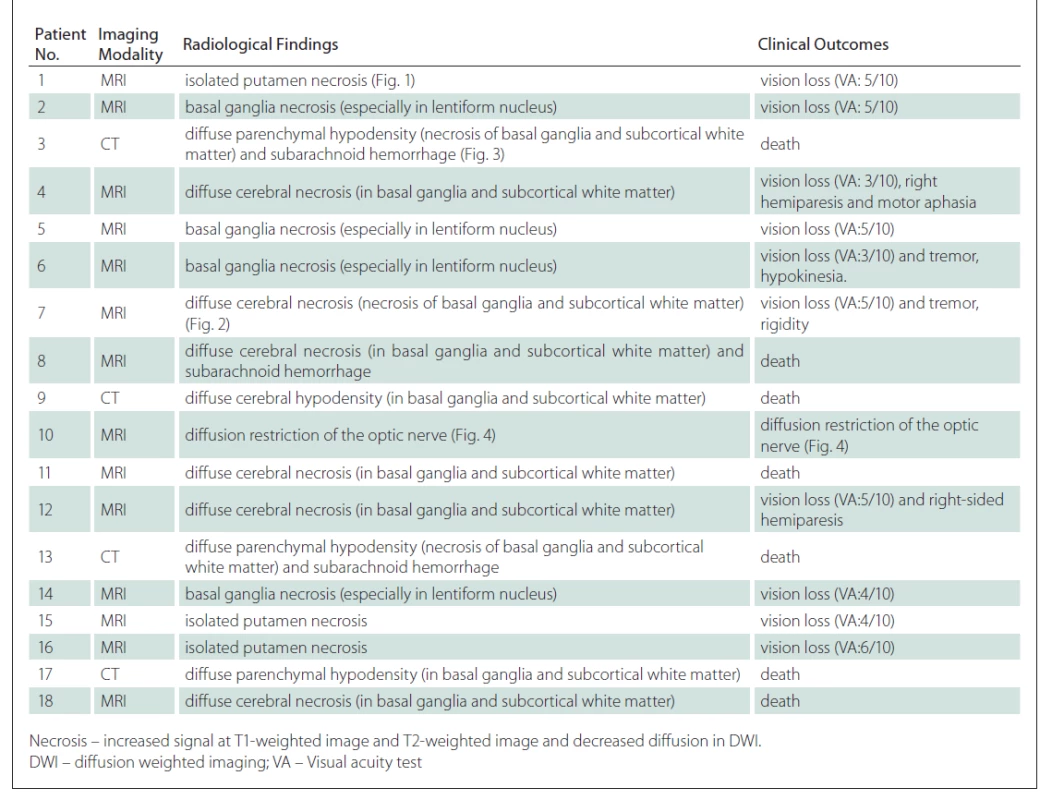
In Group II, three of the patients (11.1%) died during the treatment at the emergency department. Six (22.2%) patients had vision loss at the end of their treatment. In these patients, in addition to CT, MRI was performed but did not reveal any findings consistent with methanol intoxication or any pathology that could have caused vision loss. Eighteen of the patients in Group II (66.7%) recovered fully and were discharged.
Among the methanol intoxication patients that were treated and followed up, sequelae (N = 17; 37.8%) or death (N = 10; 22.2%) occurred in 27 patients (60%), regardless of the group. When the patients were evaluated according to groups, the number of patients with sequelae or death in Group I (N = 18; 100%) was significantly higher than in Group II (N = 9; 33.3%) (P < 0.001).
Discussion
In this study, 45 patients with methanol intoxication were evaluated by radiological imaging in the early period. When the patients were divided into two groups according to the presence/absence of pathological findings on radiological imaging, there was a significant difference between the two groups in terms of the clinical prognosis of patients (P < 0.001).
The majority of the patients with methanol intoxication (95.6%) had consumed alcohol within the last 24 h. According to studies in the literature, the aetiology of methanol intoxication in Turkey is mostly due to the consumption of alcohol produced illegally. In Turkey, the price of methanol is much lower than that of ethanol and therefore it is often used for the illegal production of liquor. Since illegal liquor is batch-produced and distributed to a certain region, methanol intoxication is generally seen in the form of epidemics [11,12]. The most common symptom was vision loss (55.6%). Even at very low blood methanol levels, such as 10 mg/dL, methanol intoxication may cause visual loss due to degenerative demyelization in the optic nerve and retinal toxicity. Therefore, optic nerve damage and related neurological symptoms are common in methanol intoxication [6].
In a multicentre study, Vaneckova et al evaluated 46 patients who were treated and followed up due to methanol intoxication. For the evaluation of sequelae in the late period, brain imaging was performed in 42 patients (91.3%) using MRI and 4 patients (8.7%) using CT. According to the imaging results, 21 patients (45.6%) had pathological findings [13]. In the current study, 18 patients (40%) with methanol intoxication had pathological findings revealed by early radiological imaging. Sefidbakht et al evaluated nine patients, who were treated and followed up due to methanol intoxication, by radiological imaging in the early period. According to the imaging results, haemorrhagic or non-haemorrhagic necrosis in the putamen was present in four patients; diffuse cerebral hypodensity and concomitant subarachnoid haemorrhage in one patient; and necrosis in the bilateral basal ganglia, white matter and bilateral occipital cortex in another patient [9]. The distribution of pathological findings detected in the current study was similar, with the most common finding being basal ganglia necrosis. The most common pathological finding in methanol intoxication is necrosis of the basal ganglia, especially that of the putamen. This is because formic acid, which is the toxic metabolite of methanol, tends to accumulate in the basal ganglia and inhibits the cytochrome C oxidase enzyme, causing a hypoxic effect [14]. Necrosis of the basal ganglia detected in different diseases, including other intoxications (carbon monoxide inhalation), metabolic diseases (renal dysfunction), mitochondriopathies (Leigh’s disease) and neurodegenerative changes (Wilson’s disease), is non-specific finding. However, differential diagnosis can easily be made with the detection of acute metabolic acidosis together with a history of alcohol consumption [9].
In autopsy studies on methanol intoxication patients, necrosis can be seen in the white matter, especially in the subcortical fibres [15]. In their case report, Server et al described a patient who was diagnosed with methanol intoxication and reported that the DWI findings in the early period revealed the presence of the diffusion restriction of the putamen and subcortical white matter. The authors attributed this to the termination of ATP cytotoxic oedema caused by the impairment of the Na + / K + ATPase pump, which is responsible for diffusion in the cell membrane. Similarly, in the current study, of the patients with pathological findings, 10 (55.6%) had cytotoxic oedema extending to the subcortical fibres in the white matter, and MRI revealed diffuse restriction in these sections. Interestingly, one patient (5.6%) with pathological findings had bilateral diffusion restriction in the retrobulbar segment of the optic nerve. In the literature, this was previously described in only one case report [16]. In another study, Elkhamary et al defined the changes in the optic nerve as atrophy and increased enhancement in patients with methanol intoxication; however, since radiological images were obtained in the late period (from 12 weeks to 48 months), no diffusion restriction was detected in any of the patients [17].
In this study, three patients with pathological findings (16.7%) had subarachnoid haemorrhage. Studies have shown that in methanol intoxication, parenchymal haemorrhage is frequently detected in patients with necrosis, but subarachnoid haemorrhage is extremely rare. Although there is no clear consensus, some authors argue that the use of heparin during haemodialysis triggers bleeding in the presence of necrosis [18]. In this study, three patients had subarachnoid haemorrhage detected before the application of haemodialysis treatment. The cause of the subarachnoid haemorrhage may be extended haemorrhagic parenchymal necrosis. However, more study is needed to understand the reason for this.
Vaneckova et al divided the patients into two groups: those with and without pathological findings according to radiological imaging. There was a statistically significant difference between these groups in terms of the arterial blood gas pH and blood methanol values at the time of presentation (P < 0.001 and P = 0.022, respectively) [13]. In the present study, a statistically significant difference was observed between the arterial blood gas pH values of the patients with and without pathological findings on radiological imaging (P = 0.003); however, there was no statistical difference in relation to the blood methanol levels (P > 0.05).
In the literature, studies have shown that severe metabolic acidosis resulting from methanol intoxication is a poor prognostic factor, but the blood methanol level has no effect on the prognosis in this patient group [5,7]. This can be explained by the genetically varying speed and amount of the alcohol dehydrogenase enzyme, which metabolizes methanol into its toxic metabolites. Thus, the toxicity threshold value of methanol differs from one person to another [19]. Furthermore, the ingestion of methanol together with ethanol, the latter being the antidote of the former, reduces the toxic effect, despite high blood methanol levels. This was demonstrated in a patient in our set with the highest blood methanol level (289 mg/dL) at the time of diagnosis being discharged without sequelae at the end of the treatment. On the other hand, it was determined that the blood levels of formic acid, can be used to determine the prognosis of patients intoxication. However, tests including the measurement of formic acid in blood are not frequently performed by laboratories [20]. For example, it is not available in our hospital.
Patankar et al evaluated the brain CT imaging findings and treatment results of four patients who developed methanol intoxication. The authors detected isolated bilateral putamen and globus pallidus necrosis in one patient, diffuse cerebral necrosis in one patient, parenchymal bleeding accompanying putamen and globus pallidus necrosis in one patient and no pathological findings in the last patient. While death occurred in a patient with diffuse cerebral necrosis, the remaining patients were discharged with mild sequelae or complete recovery. Patankar et al interpreted the results they obtained as an indication that there was no relationship between the prevalence of early radiological findings and prognosis in methanol intoxication [10]. In their early radiological examination of nine methanol intoxication patients, Sefidbakht et al detected radiological findings in six patients. Of these patients, two with developed diffuse cerebral necrosis died during treatment. In two of the patients with normal radiological findings, sequelae as vision loss were detected at the end of treatment, while one patient recovered completely. Sefidbakht et al interpreted this as the presence of a relationship between the extent of the radiological findings and the clinical outcomes [9].
In this study, the detection of pathological findings on early radiological imaging was found to be a poor prognostic factor (P < 0.001). All patients with pathological findings who survived were discharged with sequelae. Among the patients with radiological findings, diffuse cerebral necrosis was present in all patients who died. This shows that radiological findings are poor prognostic factors; there may also be a relationship between the extent of the radiological findings and the prognosis in methanol intoxication.
Similarly, to the study by Sefidbakht et al [9], the current set also included patients who did not have radiological findings but developed sequelae or died. In this group, respiratory depression associated with deep metabolic acidosis may have been the cause of death. In addition, we consider that for patients presenting with no radiological findings who develop sequelae such as vision loss, radiological images cannot reveal the pathology in the optic nerve and visual pathways. Therefore, early radiological imaging may not be sufficient to predict the development of sequelae such as vision loss.
To the best of our knowledge, this study presents the largest number of patients in whom early radiological findings were identified in methanol intoxication. In addition, this is the first study demonstrating that radiological findings detected in the early period of methanol intoxication are a poor prognostic factor.
There were several important limitations to this study. Since it was conducted retrospectively, the appropriate sample size was not calculated in advance. The clinical background of the patients was not taken into consideration, and therefore previously existing vision loss or neurological sequelae in patients may have potentially affected our results. However, the records we obtained through the registration system of our hospital did not show any visual loss or neurological sequelae in the clinical histories of the patients. Lastly, the response rate of the patients was evaluated based on the complete recovery and development of sequelae, but patient response to treatment was not graded.
In conclusion, although the clinical course may vary in methanol intoxication, clinical outcomes are quite poor. Therefore, prognostic factors must be determined for early diagnosis and treatment. Radiological imaging findings obtained in the early period can reveal any pathology and help predict prognosis. Patients with radiological findings have a worse prognosis than those with more common symptoms. However, even with an absence of radiological findings in methanol intoxication, there are patients who develop sequelae or die, and larger-scale prospective studies are needed to explain this situation.
Acknowledgements
The authors thank Nebile Daglıoglu and Toygun Anıl Ozesen, Department of Forensic Medicine, Balcali Hospital, Medical Faculty Cukurova University, Adana, Turkey for their assistance in the preparation of this manuscript.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Ethical principles
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008).
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.Ferhat Can Piskin, MD
Department of Radiology
Balcali Hospital
Medical Faculty Cukurova University
01330 Sarıçam/Adana
Turkey
e-mail: ferhatcpiskin@gmail.com
Accepted for review: 23. 4. 2021
Accepted for print: 18. 8. 2021
Sources
1. Casarett LJ, Doull J, Klaassen CD et al. Toxicology: the basic science of poisons. 8th Ed. New York: Maxwell-MacMillian-Pergamon 2013.
2. Sahin S, Solak S, Akyol O et al. Transdermal methyl alcohol intoxication cause of pain relief. West Indian Med J 2013; 62 (1): 84–86.
3. Kumar P, Gogia A, Kakar A et al. An interesting case of characteristic methanol toxicity through inhalational exposure. J Fam Med Prim Care 2015; 4 (3): 470–473. doi: 10.4103/2249-4863.161359.
4. McMartin KE, Ambre JJ, Tephly TR. Methanol poisoning in human subjects: role for formic acid accumulation in the metabolic acidosis. Am J Med 1980; 68 (3): 414–418. doi: 10.1016/0002-9343 (80) 90113-8.
5. Hovda KE, Hunderi OH, Tafjord AB et al. Methanol outbreak in Norway 2002–2004: epidemiology, clinical features and prognostic signs. J Intern Med 2005; 258 (2): 181–190. doi: 10.1111/j.1365-2796.2005.01521.x.
6. Sharpe JA, Hostovsky M, Bilbao JM et al. Methanol optic neuropathy: a histopathological study. Neurology 1982; 32 (10): 1093–1100. doi: 10.1212/wnl.32.10.1093.
7. Hassanian-Moghaddam H, Pajoumand A, Dadgar SM et al. Prognostic factors in methanol poisoning. Hum Exp Toxicol 2007; 26 (7): 583–586. doi: 10.1177/096 0327106080077.
8. Liu JJ, Daya MR, Carrasquillo O et al. Prognostic factors in patients with methanol poisoning. J Toxicol Clin Toxicol 1998; 36 (3): 175–181. doi: 10.3109/15563659809028937.
9. Sefidbakht S, Rasekhi AR, Kamali K et al. Methanol poisoning: acute MR and CT findings in nine patients. Neuroradiology 2007; 49 (5): 427–435. doi: 10.1007/s00234-007-0210-8.
10. Patankar T, Bichile L, Karnad D et al. Methanol poisoning: brain computed tomography scan findings in four patients. Australas Radiol 1999; 43 (4): 526–528. doi: 10.1046/j.1440-1673.1999.00723.x.
11. Yayci N, Agritmis H, Turla A et al. Fatalities due to methyl alcohol intoxication in Turkey: an 8-year study. Forensic Sci Int 2003; 131 (1): 36–41. doi: 10.1016/s0379-0738 (02) 00376-6.
12. Celik S, Karapirli M, Kandemir E et al. Fatal ethyl and methyl alcohol-related poisoning in Ankara: a retrospective analysis of 10,720 cases between 2001 and 2011. J Forensic Leg Med 2013; 20 (3): 151–154. doi: 10.1016/j.jflm.2012.05.009.
13. Vaneckova M, Zakharov S, Klempir J et al. Imaging findings after methanol intoxication (cohort of 46 patients). Neuro Endocrinol Lett 2016; 36 (8): 737–744.
14. Comoglu S, Ozen B, Ozbakır S. Methanol intoxication with bilateral basal ganglia infarct. Australas Radiol 2001; 45 (3): 357–358. doi: 10.1046/j.1440-1673.2001.00937.x.
15. Kurtas O, Imre K, Ozer E et al. The evaluation of deaths due to methyl alcohol intoxication. Biomedical Research 2017; 28 (8): 1–7.
16. Server A, Hovda KE, Nakstad PH et al. Conventional and diffusion-weighted MRI in the evaluation of methanol poisoning. Acta Radiol 2003; 44 (6): 691–695. doi: 10.1046/j.1600-0455.2003.00138.x.
17. Elkhamary SM, Fahmy DM, Galvez-Ruiz A et al. Spectrum of MRI findings in 58 patients with methanol intoxication: long-term visual and neurological correlation. Egypt J Radiol Nucl Med 2016; 47 (3): 1049–1055. doi: 10.1016/j.ejrnm.2016.06.011.
18. Zakharov S, Kotikova K, Vaneckova M et al. Acute methanol poisoning: prevalence and predisposing factors of haemorrhagic and non-haemorrhagic brain lesions. Basic Clin Pharmacol Toxicol 2016; 119 (2): 228–238. doi: 10.1111/bcpt.12559.
19. Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health 2007; 30 (1): 5–13.
20. Liu JJ, Daya MR, Carrasquillo O et al. Prognostic factors in patients with methanol poisoning. J Toxicol Clin Toxicol 1998; 36 (3): 175–181. doi: 10.3109/15563659809028937.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2021 Issue 5-
All articles in this issue
- Ofatumumab – a new high-efficacy treatment for relapsing forms of multiple sclerosis
- Hereditary gelsolin amyloidosis – clinical symptoms and molecular genetic cause
- Do initial clinical symptoms affect the outcome of ischemic stroke patients with recanalization treatment?
- Analgesic-muscle relaxant infusion in back pain therapy – technological and clinical aspects
- Comparison of the influence of the first and the second wave of COVID-19 pandemic on numbers of admitted ischemic stroke patients, on their diagnostics, treatment, and prognosis
- The first experience with the use of direct monitoring of the auditory nerve in vestibular schwannoma surgery in the Czech Republic
- Ultrasound-guided sacroiliac joint injection
- Aphasia in migraine with aura – video case report
- A syndrome of progressive ataxia and palatal tremor in a patient with mild bilateral idiopathic hypertrophic olivary degeneration
- Recenze knihy
- Cenu J. E. Purkyně 2021 obdržel neurochirurg prof. MUDr. Eduard Zvěřina, DrSc., FCMA
- Neuroradiological features and clinical outcomes in methanol intoxication
- Report of an epicranial arteriovenous malformation
- Large-vessel occlusion in a patient with Emery-Dreifuss muscular dystrophy
- Meningeal Form of Rosai-Dorfman Disease
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Analgesic-muscle relaxant infusion in back pain therapy – technological and clinical aspects
- Ofatumumab – a new high-efficacy treatment for relapsing forms of multiple sclerosis
- Ultrasound-guided sacroiliac joint injection
- A syndrome of progressive ataxia and palatal tremor in a patient with mild bilateral idiopathic hypertrophic olivary degeneration
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

