-
Medical journals
- Career
Sonographic evaluation of sciatic nerve in individuals with S1 radicular symptoms
Authors: S. Kurková 1; K. Mezian 2; M. Kynčl 3; S. Machač 1
Authors‘ workplace: Department of Rehabilitation and, Sports Medicine, Second Faculty, of Medicine, Charles University and, University Hospital Motol, Prague, Czech Republic 1; Department of Rehabilitation, Medicine, First Faculty of Medicine, Charles University and General, University Hospital in Prague, Czech Republic 2; Department of Radiology, Second, Faculty of Medicine, Charles, University and University Hospital, Motol, Prague, Czech Republic 3
Published in: Cesk Slov Neurol N 2020; 83/116(5): 526-530
Category: Original Paper
doi: https://doi.org/10.14735/amcsnn2020526Overview
Aim: This study aimed to evaluate, using ultrasonography, the morphological changes of the sciatic nerve in the dorsal thigh in terms of its cross-section area (CSA) in patients with unilateral S1 radicular symptoms.
Methods: This observational study was performed in 15 participants (12 females; mean age 46.9 ± 9.9 years; mean body mass index 26.5 ± 4.7 kg/m2) diagnosed with unilateral S1 radiculopathy. The CSA of the sciatic nerve was measured and evaluated bilaterally by ultrasound imaging at three levels of the dorsal thigh; at the gluteal fold, proximal quarter-thigh, and mid-thigh. The values for the symptomatic and asymptomatic sides of each patient were compared.
Results: The mean CSA values of the symptomatic (39.6 ± 15.6 mm²) and asymptomatic (32.9 ± 11.2 mm²) sciatic nerves at the level of the GF were measured. A significant increase of the CSA at this level on the patients‘ symptomatic side was observed (P = 0.02; d = 0.49, i.e., medium size of the effect). At proximal quarter-thigh and mid-thigh levels, the side-to-side difference did not reach a level of significance.
Conclusion: An enlargement of the nerve CSA at the gluteal fold level was observed on the symptomatic side with sciatica, while those changes were generally present during the period when the patient complained about the subjective symptoms. Ultrasound imaging may be a useful, available tool to assess morphological changes of the sciatic nerve in unilateral S1 radicular symptomatic patients.
Keywords:
ultrasonography – sciatica – radiculopathy – sciatic nerve – cross-section area
Introduction
Radicular symptomatology at the lumbosacral level is a frequent complaint of patients in the offices of musculoskeletal medicine practitioners, and a common reason for the patient‘s incapacity to work. Especially in patients with irritating pain from the lumbosacral region to the lower limb, irritation is believed to be caused mainly by herniation of the lumbar disc [1]. Therefore, imaging methods such as MRI or CT focus predominantly on the lumbar spine region [2–4]. However, some intraspinal or extraspinal pathologic processes along the sciatic nerve (SN) may also cause non-discogenic sciatica [5]. Imaging of the SN alone to assess morphology using MR-neurography is possible but is not commonly done in subjects with symptoms of radicular irritation [6,7]. Its use is relatively expensive, has limited availability, and may be contraindicated in some cases [8].
Due to technological advances and the availability of ultrasonography (US) in the physician‘s or physiotherapist‘s office as a complementary diagnostic tool, US imaging appears to be a promising method even allowing the quantification of the rate of nerve structure swelling in patients with assumed radiculopathy. It has been established that radicular intraspinal and non-radicular extraspinal forms of sciatica may cause SN swelling. Studies reported SN edema due to radicular and discogenic pathology and also due to entrapment in cases of deep gluteal syndrome [9,12]. So far, only a few anecdotal studies with a heterogeneous US methodology have been reported on this topic [9,11,13,14].
The aim of this study was to evaluate any morphological changes in the SN in terms of an alteration of its cross-section area (CSA) in patients with unilateral radicular symptom S1 using US imaging. The purpose was to compare the CSA of the SN at three different locations of the dorsal thigh on the symptomatic and asymptomatic sides.
An increase of the SN CSA on the affected side was expected, especially at the gluteal fold level due to nerve edema close to the site of compression and/or irritation. This non-invasive assessment of neural tissue may be useful for a better understanding of sciatica and may be clinically relevant to monitor the effect of therapy for future studies or in clinical practice.
Materials and methods
Participants
Fifteen probands (12 females; mean age 46.9 ± 9.9 years; mean body mass index 26.5 ± 4.7 kg/m2) with a neurological diag-nosis of unilateral sciatica lasting for more than 1 month were chosen for the study. Criteria for sciatica were: pain irritation in the S1 dermatome longer than 1 month as a dominant symptom, numbness and paresthesias in the same distribution, and leg pain worse than back pain. Inclusion criteria were unilateral S1 radicular symptoms longer than 1 month. Radiating pain in the S1 dermatome with numbness or paresthesias had the same distribution, and leg pain was worse than back pain. Probands with any concomitant disease affecting the peripheral nerves (e. g., diabetes mellitus, or any other type of neuropathy), probands with non-S1 radicular irritation or with irritation in multiple dermatomes, severe lower limb injuries, neurogenic claudication, sacroiliac or facet joint pain, and probands who underwent lumbar spine surgery were excluded. Individuals with bilateral radiculopathy were excluded due to the impossibility of comparing findings in symptomatic and asymptomatic limbs. All participants underwent static US examination of SN, bilaterally at the same level, from which the data were further statistically processed and analyzed. The values of the unaffected limb of each subject were used as individual controls. All subjects were informed about the procedure and consented to participate in the study and to the processing of their personal data. The study protocol was approved by the local ethics committee.
Protocol of ultrasound examination
An Alpinion, E-Cube 11 US machine and Alpinion, L3-12H High density linear transducer, 3-12MHz (Alpinion Medical Systems Co., Seoul, Korea) was used for the scanning of the SN. A frequency of 12 MHz was used to achieve optimal musculoskeletal imaging. The focal zone was set slightly below the level of the SN, which differs at different levels of the thigh. Parameters such as gain and depth were individually adjusted during the examination according to the volume of soft tissues in the area of interest. The region of interest was always verified according to the surrounding structures – the long head of the biceps femoris muscle laterally and the semitendinosus muscle medially. The settings and protocols recommended by the European Society of Musculoskeletal Radiology were used [15].
Each patient was examined by the same ultrasound practitioner experienced in musculoskeletal US (in the presence of the second examiner) in a relaxed prone position on the examination bed. Individual dorsal distances from the gluteal fold, mid-thigh (half-distance measured from the gluteal fold to the popliteal crease) and proximal quarter-thigh (proximal quarter-distance measured from the gluteal fold to the popliteal crease) were marked on the dorsal thigh by an indelible marker bilaterally to ensure the same thigh level while measuring all subjects.
The probe was placed in the short axis on the dorsal thigh where the SN was recognized. The CSA parameters were analyzed by manually tracking the nerve fiber outlines just below the epineurium. In the case the nerve had small vessels adjacent to the nerve, they were manually excluded from CSA tracking. Tracking the nerve and calculating CSA values were always performed directly during the examination whenever both examiners agreed to capture the optimal image at each level (Fig. 1). The US record was not used for measuring any of the cases. Values were taken at the same anatomical location in all probands, in the gluteal fold, mid-tigh, and also in the proximal quarter-thigh. At each defined level, three measurements were taken bilaterally, for a total of 18 measurements for each proband. The individual median values were used for analysis. The asymptomatic limb was used as a reference, based on the Chen study, where no differences between the sides were found analyzing the CSAs of the SNs in 200 healthy subjects [14]. Data were statistically processed, compared, and the rate of side asymmetry calculated.
1. Transverse ultrasound image of the sciatic nerve and muscles in the dorsal thigh.
Obr. 1. Příčný UZ obraz sedacího nervu a svalů v dorzální části stehna.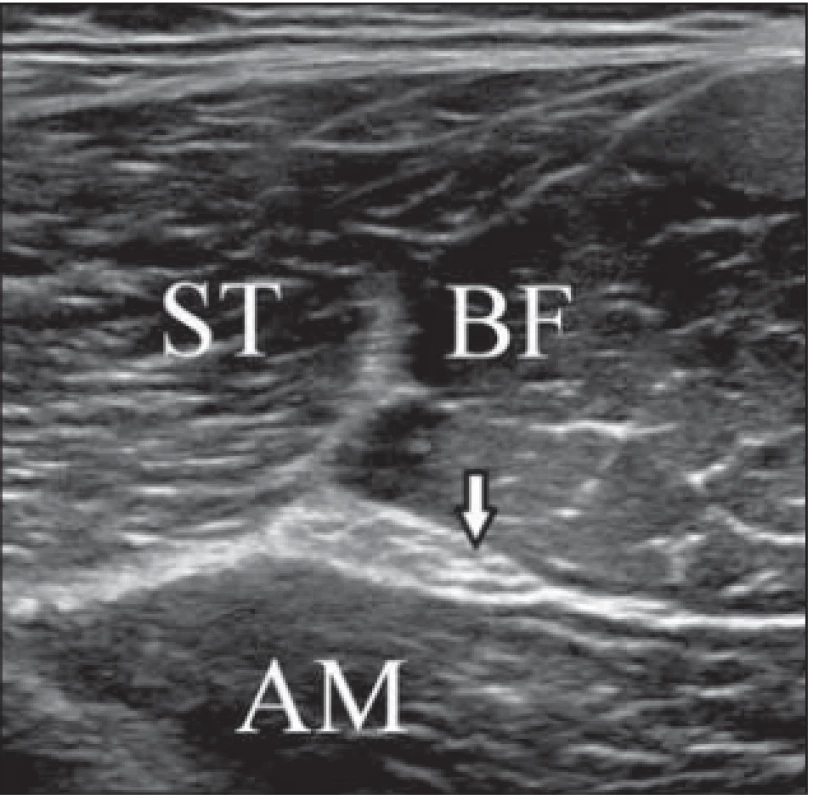
AM – adductor magnus muscle; arrow – sciatic nerve; BF – biceps femoris muscle; ST – semitendinosus muscle
AM – musculus adductor magnus; BF – musculus biceps femoris; ST – musculus semitendinosus; šipka – sedací nervStatistical analysis
Statistical analysis was performed using Graph Pad Prism5 (GraphPad, San Diego, CA, USA) for statistical data processing and graphing. The results are presented as mean ± standard deviation (SD). The data distribution was evaluated by the D’Agnostino Pearson normality test. The arithmetic mean with SD was determined from the values for each subject. Paired values for subgroups of the symptomatic and asymptomatic limbs were compared by t-test. In addition to statistical significance, a size effect was always evaluated as a Cohen’s d value. An interval of 0.2–0.4 was taken as the value for a small effect, 0.4–0.8 for the medium effect, and > 0.8 as the value for a large effect. Correlations between values were evaluated via the Pearson correlation coefficient. P < 0.05 was considered statistically significant.
Results
Fifteen patients (12 females) with a neurological diagnosis of unilateral sciatica were examined in this study. The dominant symptom was pain irritation in the S1 dermatome lasting for more than 1 month (average 42.1 ± 53.4 months). Based on the values obtained, we defined mean CSA SN values of the symptomatic side at the gluteal fold level of 39.6 ± 15.6 mm² and at the asymptomatic side of 32.9 ± 11.2 mm². At the proximal quarter-thigh level of the symptomatic side, we measured a CSA of 34.6 ± 14.6 mm² and 34.0 ± 13.8 mm² on the asymptomatic side. At mid-thigh level on the symptomatic side, we measured a CSA of 39.1 ± 13.5 cm² and 36.6 ± 15.7 mm² on the asymptomatic side. At the gluteal fold level, the CSA comparison showed a statistically significant difference between the symptomatic and asymptomatic limbs (P = 0.02), with a medium effect size (d = 0.49). At the proximal quarter-thigh, almost identical bilateral values had no significant difference in CSA on both sides. The values at the mid-thigh level are not considered statistically important (Fig. 2). Clinical and demographic characteristics of the patients are given in Tab. 1. The results are summarized in Tab. 2.
2. CSA values of the sciatic nerve in the defi ned three levels of the dorsal thigh in symptomatic and asymptomatic limb.
Obr. 2. Hodnoty CSA nervus ischiadicus v defi novaných třech úrovních dorzálního stehna symptomatické a asymptomatické končetiny.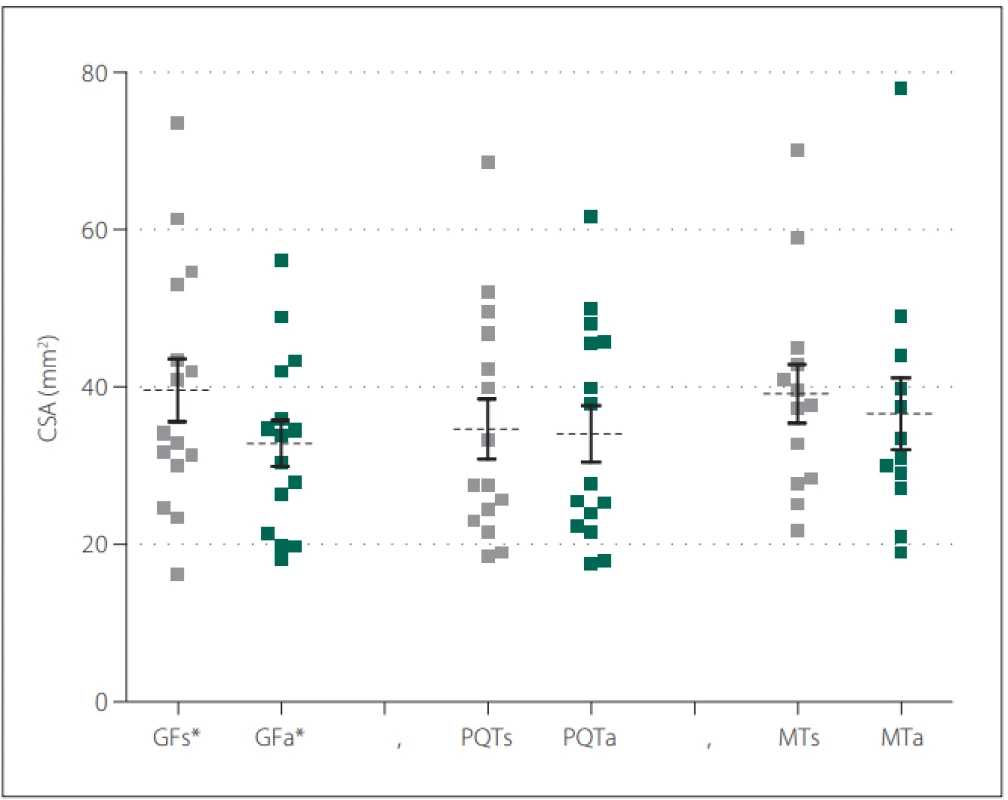
*statistically signifi cant P-values
a – asymptomatic side; CSA – cross-section area; GF – gluteal fold; MT – mid-thigh; PQT – proximal quarter of the thigh; s – symptomatic side
* statisticky signifi kantní p-hodnota
a – asymptomatická strana; CSA – průřezová plocha; GF – gluteální rýha; MT – střed stehna; PQT – proximální čtvrtina stehna; s – symptomatická strana1. Clinical and demographic characteristics of the subjects (N = 15). 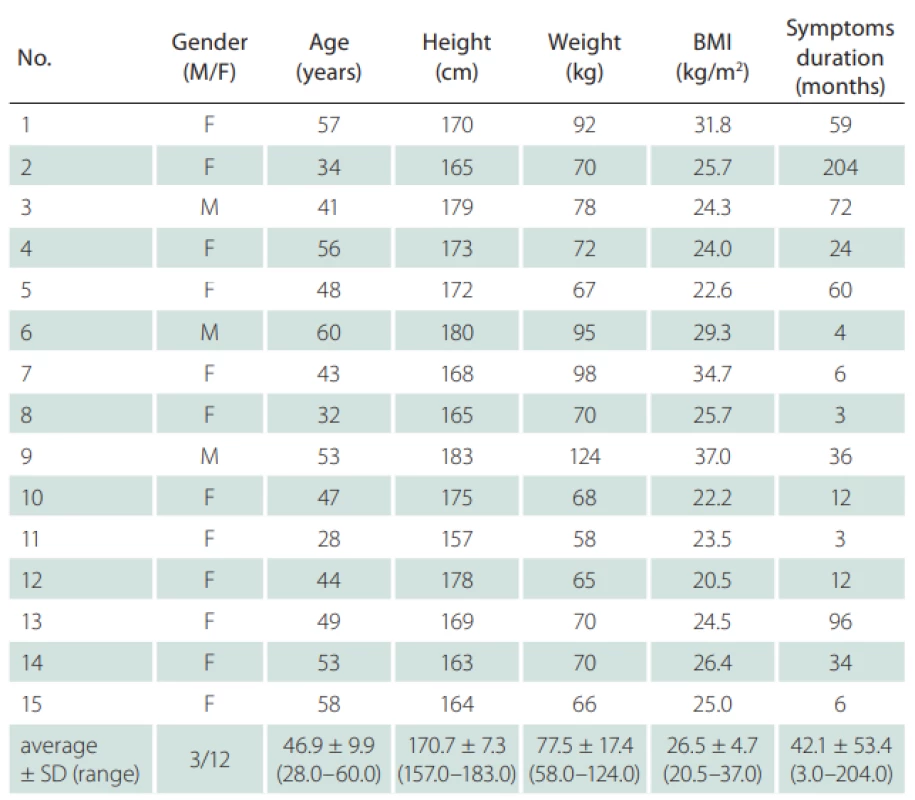
BMI – body mass index; F – female; M – male; N – number; SD – standard deviation 2. Ultrasonographic measurements cross-section area of the sciatic nerve at the defi ned three levels of the dorsal thigh in symptomatic and asymptomatic limb. 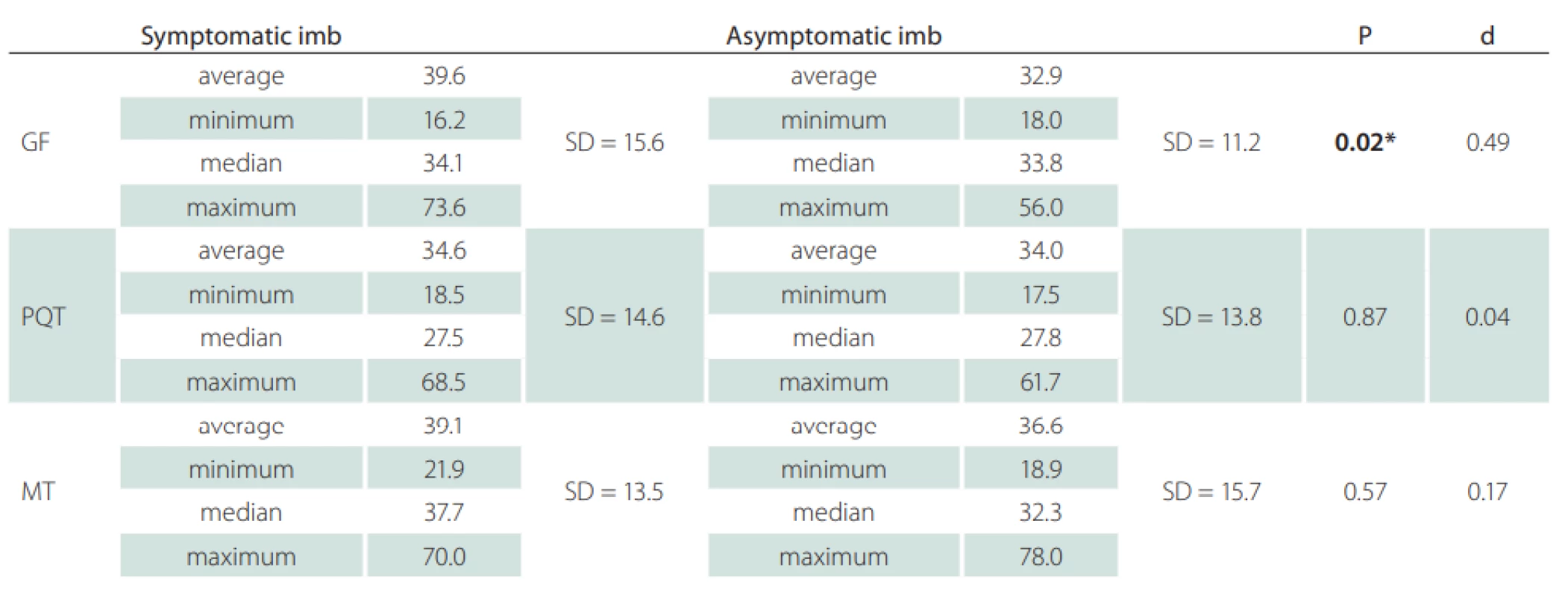
*statistically signifi cant P-values
d – eff ect size; GF – gluteal fold; MT – mid-thigh; PQT – proximal quarter of the thigh; SD – standard deviationDiscussion
In this study, we found increases in CSA of the ipsilateral SN probably due to edema in S1 symptomatic subjects at the gluteal fold level. In the case of spinal etiology, nerve edema is present distally from the intervertebral foramen, but the first site where edema can be measured sonographically is in the gluteal fold. Edema can be caused not only by local inflammation, but also by a disorder in axoplasm flow in both directions – antegrade and retrograde. Swelling can spread longitudinally along the spaces between the layers of the nerve sheath. Neurogenic inflammation caused by the release of inflammatory mediators may create nerve swelling and mid-axonal activation may explain the spreading of neurogenic inflammation along the SN branches [16]. SN edema may be caused also by histamine-induced vascular permeability, which allows an influx of serum albumin, which is formed between SN fibers [17].
Some studies were performed to examine swelling of the nerves of the upper limb due to compression and/or irritation. Studies suggested that symptomatic nerve roots were wider than asymptomatic nerve roots due to the presence of edema [11,18,19]. Peripheral nerves have also been shown to develop edema, fibrosis, and changes distally to the affected nerve as a result of mechanical compression [11,12,20–22]. The peripheral nerves are more sensitive to pressure, and a proximal nerve lesion makes the distal segment of the nerve more susceptible to anatomic deterioration by causing an interruption in axoplasmic conduction due to the compression [23].
There are very few studies to date which evaluate the unilateral change of SN morphology in S1 radicular symptomatic probands [9,11–14,24]. This study demonstrated the suitability of US CSA measurements to confirm SN edema in response to nerve irritation and compression. The results are in contrast with previously published studies that evaluated the level of the mid-thigh as very suitable for US imaging and measurement and reported statistically significant different values [13,24]. We speculate that this disagreement could be caused by the occasional higher SN bifurcation and the poor visibility of the epineurial boundaries. The mid-thigh CSA difference in the measured mean values of the previous studies compared to ours can also be attributed to a different method of manual nerve tracking. Tracking in the current study was performed solely inside the visible epineurium, excluding the adjacent artery, which is considered standard in assessing the CSA of nerve structures [25]. Kara et al, who were the first to asses SN using US in symptomatic subjects, described the SN CSA meassurement as using the manual tracking [9]. Frost and Brown applied the boundary over the epineurium, and in addition, the data were evaluated by another examiner offline from the record [11]. Sarafraz et al also performed an offline analysis from the record [12]. In our opinion, offline analysis with a time lag may increase the error rate of measurements. Echogenicity is not evaluated in the current study, because changes in nerve echogenicity are related to the pathology of the nerve structures, but also to the manipulation of the probe and the surrounding structures. Compared to previous work, the current study focused on more levels of SN, but statistically significant values were found only at the gluteal fold level. This is attributed to the assumption that the swelling is manifested at the most proximal level to the site of compression, as a result of either the true S1 radiculopathy or any of the subtypes of pseudoradicular manifestations, like deep gluteal syndrome. It should be noted that edema at higher levels than the gluteal fold can be clearly seen using MRI neurography [26,27].
An increase of the CSA along the SN was found during the period of subjective irritative symptoms, regardless of whether the patient was acute or chronic. We assume that the influence of gravity and worsened conditions of axoplasmic return and fluid drainage of edema plays a role in this case.
A limitation of all US assessments is examiner dependency, especially with regard to transducer placement and manual tracking of the nerve during image analysis. We tried to eliminate measurement error by matching the opinion of two examiners. In this study, the relatively small number of cases was also a limitation. Further studies with more patients would provide a broader understanding of the changes in SN morphology and related radicular symptoms. In a larger group of patients with sciatica, it is necessary to define differences between acute and chronic subgroups, and subgroups with different severity of neurological findings. The lack of intra - and inter-observer blindness, absence of randomizing and comparison with other imaging techniques may be further limitations of the study.
Despite these limitations, this study demonstrated the possibility of US imaging of the SN and quantitative evaluation of its CSA. Based on our results, we can conclude that in patients with unilateral sciatica, there is usually a unilateral morphological change at the gluteal fold level in terms of increasing the CSA of the SN on the symptomatic limb compared to the asymptomatic one, presumably due to intraneural edema. According to our observations, this enlargement is generally present during the time of the patient‘s subjective symptoms.
Ultrasonographic analysis appears to be a promising and useful complementary tool for diagnostic quantification of nerve edema. It allows monitoring of the development or regression of morphological changes in the SN during the affliction caused by true radicular syndrome or a symptomatic imitation of radiculopathy due to SN involvement. This opens the door to quantitative monitoring of the effect of different approaches to therapy in patients with sciatica, which to the best of our knowledge, has not been used in any research to date. Technological advances and further refinement of imaging methods, such as US, are likely to lead to a wider application of this technique as a diagnostic tool in the clinical practice of musculoskeletal medicine.
Conclusion
This work confirms a significant unilateral morphological change in the SN in patients with S1 radicular symptoms. The change is significant in terms of enlargement of the nerve CSA at the gluteal fold level. This information may change therapeutic approaches from focusing only on the lumbar spine but to also focusing on the nerve structures themselves. Potentially, this can be used further to monitor the rate of nerve swelling as a quantitative parameter of therapeutic efficacy.
Ethical statements
All procedures followed were in accordance with the ethical standards of the relevant committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The study was approved by the ethics committee at Motol University Hospital, 4.12.2019 (EK-1310/19). Informed consent was obtained from all patients for being included in the study.
Acknowledgement
Project was supported by PROGRES Q41.
Conflict of Interest
All authors declare that they have no conflict of interest.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Simona Kurková, PT, MSc
Department of Rehabilitation and Sports Medicine
Second Faculty of Medicine Charles University and University Hospital Motol
V Úvalu 84
150 06 Prague
Czech Republic
e-mail: kurkova22@gmail.com
Accepted for review: 10. 4. 2020
Accepted for print: 8. 10. 2020
Sources
1. Koes B, Van Tulder M, Peul W. Diagnosis and treatment of sciatica. BMJ 2007; 334 (7607): 1313–1317. doi: 10.1136/bmj.39223.428495.BE.
2. Maus T. Imaging the back pain patient. Phys Med Rehabil Clin N Am 2010; 21 (4): 725–766. doi: 10.1016/j.pmr. 2010.07.004.
3. Kuijper B, Tans JTJ, van der Kallen BF et al. Root compression on MRI compared with clinical findings in patients with recent onset cervical radiculopathy. J Neurol Neurosurg Psychiatry 2011; 82 (5): 561–563. doi: 10.1136/jnnp.2010.217182.
4. Wassenaar M, Van Rijn RM, Van Tulder MW et al. Magnetic resonance imaging for diagnosing lumbar spinal pathology in adult patients with low back pain or sciatica: a diagnostic systematic review. Eur Spine J 2012; 21 (2): 220–227. doi: 10.1007/s00586-011-2019-8.
5. Kulcu DG, Naderi S. Differential diagnosis of intraspinal and extraspinal non-discogenic sciatica. J Clin Neurosci 2008; 15 (11): 1246–1252. doi: 10.1016/j.jocn.2008.01.017.
6. Zhang Z, Song L, Meng Q et al. Morphological analysis in patients with sciatica. Spine 2009; 34 (7): 245–250. doi: 10.1097/BRS.0b013e318197162e.
7. Noguerol MT, Barousse R, Socolovsky M et al. Quantitative magnetic resonance (MR) neurography for evaluation of peripheral nerves and plexus injuries. Quant Imaging Med Surg 2017; 7 (4): 398–421. doi: 10.21037/qims.2017.08.01.
8. Juliano AF, Policeni B, Agarwal V et al. ACR Appropriateness Criteria ® Ataxia. J Am Coll Radiol 2019; 16 (5S): S44–S56. doi: 10.1016/j.jacr.2019.02.021.
9. Kara M, Özçakar L, Tiftik T et al. Sonographic evaluation of sciatic nerves in patients with unilateral sciatica. Arch Phys Med Rehabil 2012; 93 (9): 1598–1602. doi: 10.1016/j.apmr.2012.03.013.
10. Filler A, Haynes J, Jordan S et al. Sciatica of nondisc origin and piriformis syndrome: diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome study of resulting treatment. J Neurosurg Spine 2005; 2 (2): 99–115. doi: 10.3171/spi.2005.2.2.0099.
11. Frost LR, Brown SHM. Neuromuscular ultrasound imaging in low back pain patients with radiculopathy. Man Ther 2016; 21 : 83–88. doi: 10.1016/j.math.2015.05.003.
12. Sarafraz H, Hadian MR, Ayoobi YN et al. Neuromuscular morphometric characteristics in low back pain with unilateral radiculopathy caused by disc herniation: an ultrasound imaging evaluation. Musculoskelet Sci Pract 2019; 40 : 80–86. doi: 10.1016/j.msksp.2019.01.016.
13. Shen Su-hong, LÜ Hai-xia, Zhan-sen E et al. High-frequency ultrasound research on the normal adult sciatic nerve. Zhongguo Gu Shang 2013; 26 (2): 107–110.
14. Chen J, Liu J, Zeng J et al. Ultrasonographic reference values for assessing normal sciatic nerve ultrasonography in the normal population. J Med Ultrasound 2018; 26 (2); 85–89. doi: 10.4103/JMU.JMU_6_17.
15. Klauser AS, Tagliafico A, Allen GM et al. Clinical indications for musculoskeletal ultrasound: a Delphi-based consensus paper of the European Society of Musculoskeletal Radiology. Eur Radiol 2012; 22 (5): 1140–1148. doi: 10.1007/s00330-011-2356-3.
16. Sorkin LS, Eddinger KA, Woller SA et al. Origin of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin Immunopathol 2018; 40 (3): 237–247. doi: 10.1007/s00281-017-0669-2.
17. Olsson Y. Studies on vascular permeability in peripheral nerves. Acta Neuropathol 1966; 7 (1): 1–15. doi: 10.1007/BF00686605.
18. Takeuchi M, Wakao N, Hirasawa A et al. Ultrasonography has a diagnostic value in the assessment of cervical radiculopathy: A prospective pilot study. Eur Radiol 2017; 27 (8): 3467–3473. doi: 10.1007/s00330-016-4704-9.
19. Kim E, Yoon JS, Kang HJ. Ultrasonographic cross-sectional area of spinal nerve roots in cervical radiculopathy. Am J Phys Med Rehabil 2015; 94 (2); 159–164. doi: 10.1097/PHM.0000000000000212.
20. Ahlawat S, Belzberg AJ, Fayad LM. Utility of magnetic resonance imaging for predicting severity of sciatic nerve injury. J Comput Assist Tomogr 2018; 42 (4); 580–587. doi: 10.1097/RCT.0000000000000730.
21. Chhabra A, Chalian M, Soldatos T et al. 3-T High-resolution MR neurography of sciatic neuropathy. Am J Roentgenol 2012; 198 (4); 357–364. doi: 10.2214/AJR.11.6981.
22. Garwood ER, Duarte A, Bencardino JT. MR imaging of entrapment neuropathies of the lower extremity. Radiol Clin North Am 2018; 56 (6); 997–1012. doi: 10.1016/j.rcl.2018.06.012.
23. Ökmen M, Ökmen K, Lale A. Investigation of the effect of cervical radiculopathy on peripheral nerves of the upper extremity with high-resolution ultrasonography. Spine 2018; 43 (14): E798–E803. doi: 10.1097/BRS.0000000000002539.
24. Bruhn J, Van Geffen GJ, Gielen MJ et al. Visualization of the course of the sciatic nerve in adult volunteers by ultrasonography. Acta Anaesthesiol Scand 2008; 52 (9): 1298–1302. doi: 10.1111/j.1399-6576.2008.01695.x.
25. Wu WT, Chang KV, Mezian K et al. Basis of shoulder nerve entrapment syndrome: an ultrasonographic study exploring factors influencing cross-sectional area of the suprascapular nerve. Front Neurol 2018; 9 : 902. doi: 10.3389/fneur.2018.00902.
26. Neufeld EA, Shen PY, Nidecker AE et al. MR imaging of the lumbosacral plexus: a review of techniques and pathologies. J Neuroimaging 2015; 25 (5): 691–703. doi: 10.1111/jon.12253.
27. Flug JA, Burge A, Melisaratos D et al. Post-operative extraspinal etiologies of sciatic nerve impingement. Skeletal Radiol 2018; 47 (7): 913–921. doi: 10.1007/s00256-018-2879-7.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2020 Issue 5-
All articles in this issue
- Diffuse low grade gliomas
- Respiratory rehabilitation in patients with amyotrophic lateral sclerosis
- Socio-economic impact of headache disorders –reasons and improvement possibilities
- Neurorehabilitation in patients with amyotrophic lateral sclerosis
- Surgical treatment of degenerative scoliosis
- Functional and structural cortical changes in patients with non-specific low back pain
- Cognitive-motor interference after stroke
- Sonographic evaluation of sciatic nerve in individuals with S1 radicular symptoms
- Taste strips – the method of a self-administered taste function test
- The efficiency of micro-vascular decompression versus micro-vascular decompression with partial sensory rhizotomy for classical trigeminal neuralgia – a retrospective analysis of 58 patients
- Patient with Parkinson‘s disease in data sources of the National Health Information System
- Treatment with intravenous trombolysis out of stroke center
- Decompressive craniectomy in malignant middle cerebral artery infarction – monoinstitutional retrospective analysis of a set of 33 patients
- Consecutive low-flow and high-flow EC-IC bypass in ischemia prevention during internal carotid artery sacrifice in intracavernous aneurysm
- Venous sinus stenting in idiopathic intracranial hypertension
- Posterior reversible encephalopathy syndrome associated with Neisseria elongata meningitis
- Hyperbarická oxygenoterapie & mozek; přehled z výroční konference EUBS 2019
- Amnesia Light and Brief Assessment (ALBA) test – the second version and repeated examinations
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Diffuse low grade gliomas
- Respiratory rehabilitation in patients with amyotrophic lateral sclerosis
- Amnesia Light and Brief Assessment (ALBA) test – the second version and repeated examinations
- Neurorehabilitation in patients with amyotrophic lateral sclerosis
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

