-
Medical journals
- Career
A comparison of perioperative pressure measurements in the aneurysm sac and parent artery in ruptured and unruptured aneurysm
Authors: S. Kanbagli 1; S. Men 2; O. Kaya 3; A. Taylan 4; E. Ozer 5
Authors‘ workplace: Department of Radiology, Burdur State Hospital, Burdur, Turkey 1; Interventional Neuroradiology Section, Department of Radiology, Dokuz Eylul University, Medical School, Izmir, Turkey 2; Department of Radiology, Ceyhan State Hospital, Adana, Turkey 3; Department of Radiology, University of Health Sciences, Samsun Training and Research Hospital, Samsun, Turkey 4; Department of Neurosurgery, Dokuz Eylul University, Medical School, Izmir, Turkey 5
Published in: Cesk Slov Neurol N 2019; 82(6): 644-648
Category: Original Paper
doi: https://doi.org/10.14735/amcsnn2019644Overview
Aim: The purpose of this study is to compare the pressure differences between the aneurysm sac and the parent artery in both ruptured and unruptured aneurysms to investigate the effect of pressure differences on the aneurysm rupture.
Material and methods: Patients treated for ruptured and unruptured aneurysms were included in the study. All the cases were imaged using catheter angiography and the aneurysms were filled with coils. The measurement was made just before and after the microcatheter was advanced into the aneurysm. The aneurysm‘s height-width, maximum dome diameter, neck diameter and entrance angle were measured in three-dimensional images.
Results: Endovascular treatment of 40 aneurysms was performed. Of these aneurysms, 17 (42.5%) were unruptured; 23 (57.5%) were ruptured. There was no statistically significant difference between patient groups with ruptured and unruptured aneurysms in terms of the demographic data and risk factors, the perioperative pressure measurements in the aneurysm sac and parent artery and the aneurysm morphological measurement.
Conclusion: In our study, peroperative pressure measurements in the aneurysm sac and parent artery were similar in both ruptured and unruptured aneurysms, and no statistically significant difference was found in any result between the groups. A wider series of studies about aneurysm’s characteristics such as shape or wall properties will contribute to our knowledge to clarify the mechanisms that play a role in the formation, growth and rupture of intracranial aneurysms.
破裂性和非破裂性动脉瘤的动脉瘤囊和亲代动脉围手术期压力测量的比较
目的:本研究的目的是比较破裂动脉瘤和未破裂动脉瘤中动脉瘤囊与亲代动脉之间的压力差,探讨压力差对动脉瘤破裂的影响。
材料和方法:本研究包括接受动脉瘤破裂和未破裂治疗的患者。所有病例均行血管造影及弹簧圈造影。在将微导管推进到动脉瘤之前和之后进行测量。在三维图像中测量动脉瘤的高度,宽度,最大圆顶直径,颈部直径和进入角。
结果:进行了40例动脉瘤的血管内治疗。在这些动脉瘤中,有17例(42.5%)未破裂; 23例(57.5%)破裂。在人口统计学数据和危险因素,动脉瘤囊和亲代动脉的围手术期压力测量以及动脉瘤形态学测量方面,动脉瘤破裂和未破裂的患者组之间在统计学上无显着差异。
结论:在我们的研究中,破裂动脉瘤和未破裂动脉瘤的动脉瘤囊和亲代动脉的围手术期压力测量结果相似,两组之间的任何结果均无统计学差异。更广泛的关于动脉瘤特征的一系列研究,如形状或壁的特性,将有助于我们阐明在颅内动脉瘤形成、生长和破裂过程中起作用的机制。
关键词:压力测量–动脉瘤囊–动脉瘤破裂–动脉高压–父母动脉
Keywords:
arterial hypertension – pressure measurements – aneurysm sac – aneurysm rupture – parent artery
Introduction
Intracranial aneurysms are cerebrovascular lesions with high mortality and morbidity. Various studies show that 3 – 6% of the population over 30 years of age have an aneurysm. Subarachnoid haemorrhage (SAH) with aneurysm rupture has a mortality of up to 50%. Many factors such as smoking, alcohol consumption, cocaine use, ethnicity, gender, age, family history and most importantly, arterial hypertension may play a role in the formation of aneurysms. However, the mechanisms about the formation, growth and rupture of intracranial aneurysms are not fully elucidated [1]. Arterial hypertension and haemodynamic stress are accepted as the oldest and most widely accepted factors for aneurysm formation and rupture [2,3]. The flow pattern in the aneurysm is thought to be affected by the geometry of the dilatation, the relationship between the aneurysm and the parent artery, the volume of the aneurysm and the aspect ratio [4,5]. The morphological relationship between the aneurysm and the parent artery is important because of its effect on the aneurysmal flow pattern [6]. There are differences in the internal structures and clinical features of ruptured and unruptured aneurysms. Due to this reason, many investigators try to identify the risk factors for intracranial aneurysm rupture. The current risk assessment is based on the size of the aneurysm, and the risk of rupture is higher in large aneurysms [7,8]. However, small aneurysms may also rupture and aneurysm size alone is insufficient to determine the risk of rupture [9]. Therefore, attempts are being made to explain the risk of rupture with more complicated geometric measurements; these attempts focus on the size, location and shape of the aneurysm [10]. In experimental aneurysms, pressure measurements in the parent artery and aneurysm show similarities [11]. This finding highlights the role of hypertension in aneurysm rupture.
Material and methods
A total of 37 patients treated for intracranial aneurysm were included in the study between May and December 2012 in the Interventional Radiology Unit of Dokuz Eylül University Hospital. The patients were evaluated for demographic data and risk factors such as age, gender, smoking, arterial hypertension and familial history of aneurysm. The ages of the patients ranged from 40 to 66 (mean 53) years. Pediatric patients were excluded from the study. 24 patients were smokers, 11 patients had arterial hypertension and 4 patients had a family history of aneurysm. Imaging and measurements with catheter angiography digital subtraction angiography) were performed for 40 aneurysms including 1 for 35 patients, 2 for 1 patient, and 3 for 1 patient. An informed consent form was obtained from the patients before the treatment. Cases with vasospasms due to SAH were excluded from the study. The patient and aneurysm data are given in Tab. 1.
1. Descriptive data. 
N – number Perioperative pressure measurement
Perioperative pressure measurement was performed just before and after the microcatheter entered into the aneurysm sac during microcatheterization. The measurements were performed while the microcatheter tip was inside the parent artery (at the neck of the aneurysm) and in the aneurysm sac (in the middle of the sac). All patients cannulated from the radial artery during general anaesthesia and simultaneous radial arterial pressures were recorded.
Morphological measurements of the aneurysm
Three-dimensional (3D) cerebral angiography images were obtained using software and reconstruction systems. The aneurysm height-width (H/ W), neck diameter, maximum dome diameter and entrance angle were measured in 3D images (Fig. 1). The image ratio, H/ W ratio and aneurysm average volume were calculated using this volumetric measurement system.
1. (A) Example of aneurysm morphological parameters; (B) aneurysm input angle measurement.
Obr. 1. (A) Příklad morfologických parametrů aneuryzmatu; (B) měření angulace aneuryzmatu.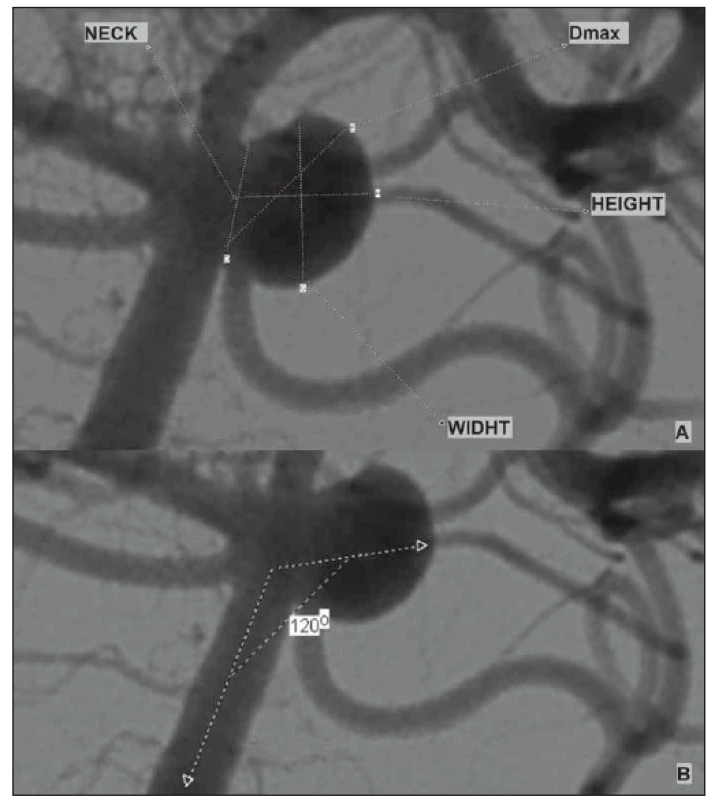
Statistical analysis
The data and statistical analysis were evaluated with SPSS 20.0 (IBM, Armonk, NY, USA). The variables are indicated by mean and distributions. The chi-square test and Fisher‘s exact test were used for the values specified by counting in statistical analysis. For measured values, correlation analysis and the Wilcoxon test were used for intra-group comparison and the Mann-Whitney U test was used for comparison between groups. Receiver operating characteristic (ROC) analysis was used to evaluate sensitivity and specificity in aneurysm morphological measurements. P < 0.05 was accepted for statistical significance.
Results
When comparing patient groups with ruptured and unruptured aneurysms, no statistically significant difference was found between the groups in terms of demographic data and risk factors. The patients showed a homogeneous distribution in both groups. Demographic data about the findings and patients are presented in Tab. 1.
No statistically significant difference was found between the ruptured and non-ruptured aneurysm groups in the comparison of perioperative parent artery and aneurysm pressures (Tab. 2).
2. Comparison of parent artery and intra-aneurysmal pressures in ruptured and unruptured aneurysms 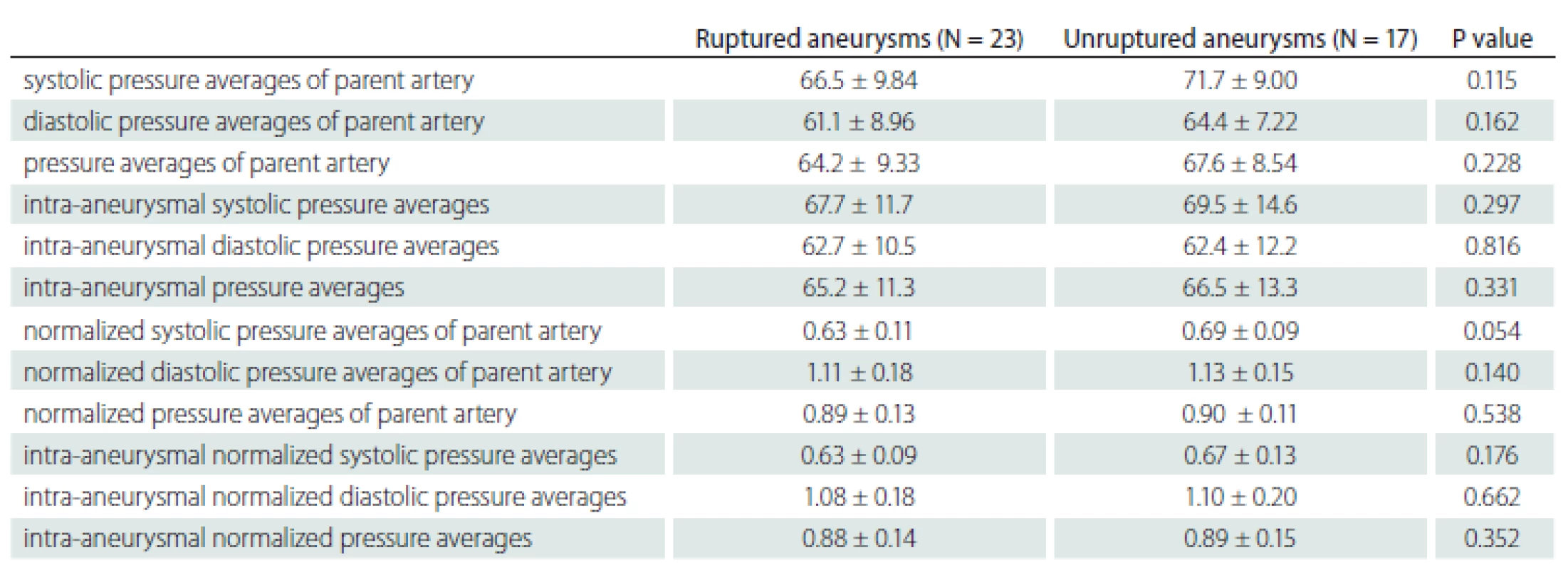
N – number Significant correlation was found between the pressure of the parent artery and aneurysm sac (Tab. 3).
3. Evaluation of the relationship between aneurysm sac and parent artery pressures 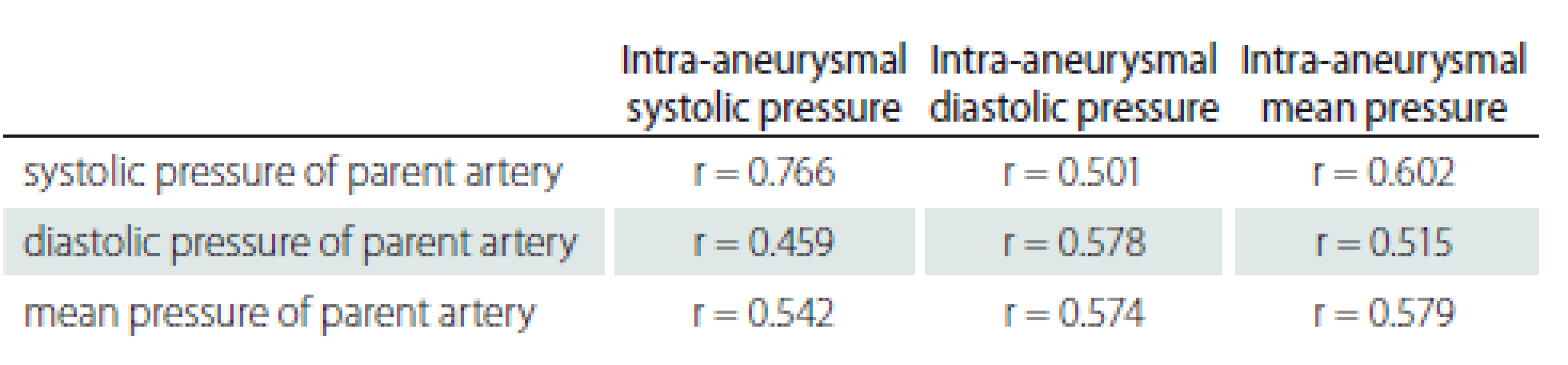
In the ruptured and unruptured aneurysm groups, there was no significant difference in the systolic and diastolic pressure values between the parent artery and aneurysm sac, measured simultaneously (Tab. 4).
4. Comparison of pressures in ruptured and unruptured aneurysms. 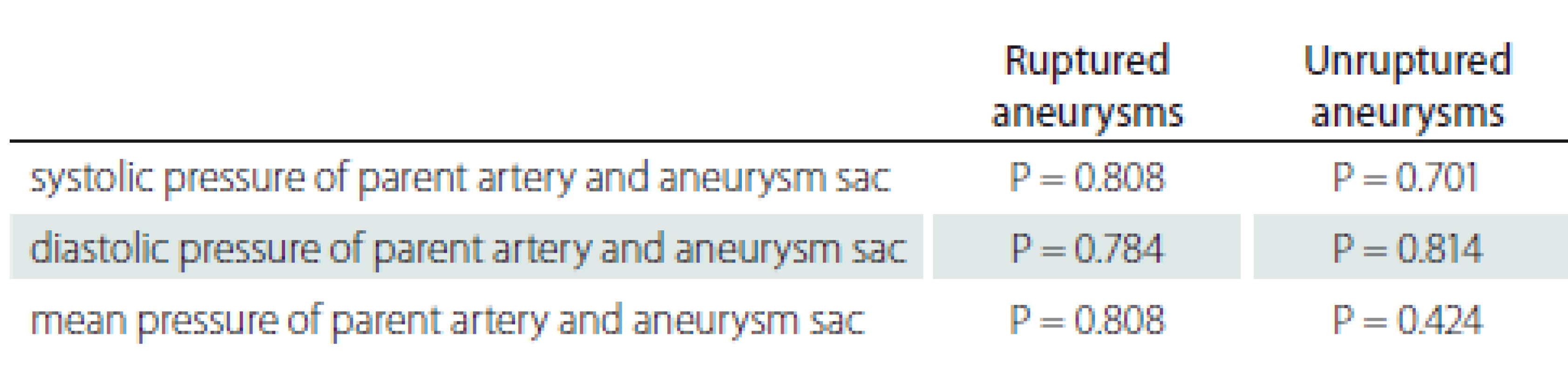
There were differences between the ruptured and unruptured aneurysms in terms of internal structures and clinical features. However, there was no statistically significant difference between the groups in the aneurysm morphological measurements. In addition, no significant difference was found between morphological differences according to rupture status. The morphological data are shown in Tab. 5 and 6.
5. Evaluation of rupture status according to the morphological characteristics of the aneurysm. 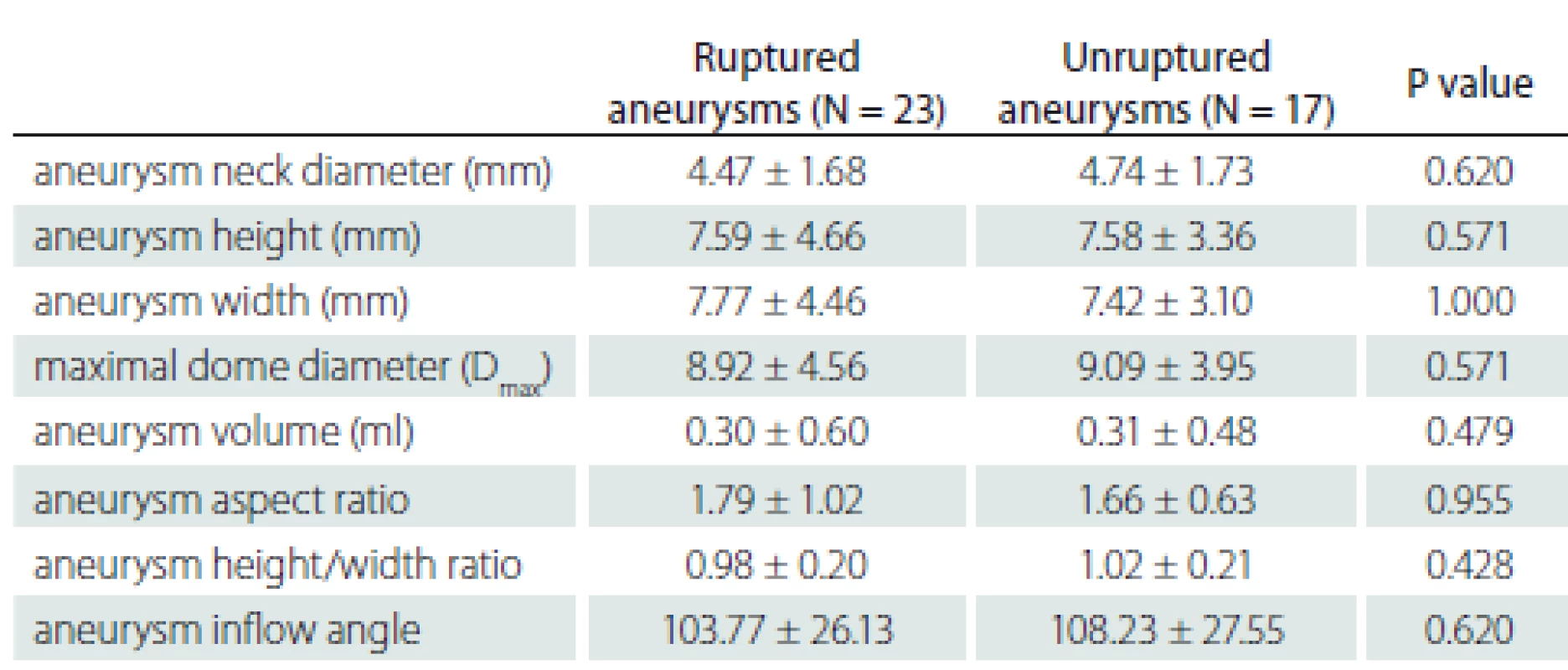
N – number 6. Evaluation of aneurysm morphological parameters according to rupture status. 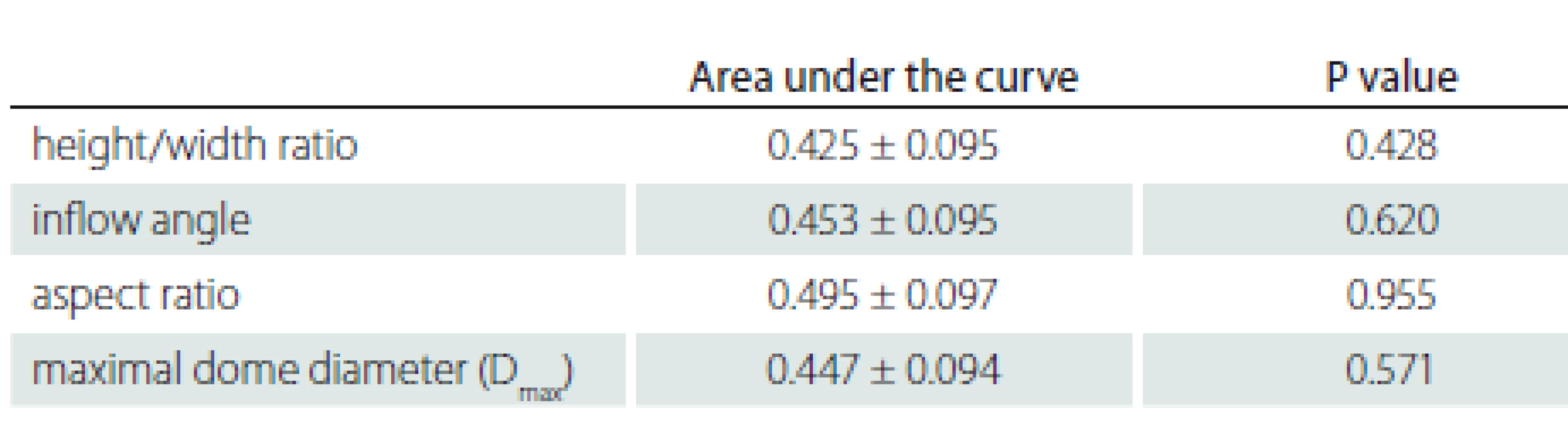
According to the ROC analysis, the areas under the curve are 0.495 ± 0.097 for the aspect ratio; 0.447 ± 0.094 for the maximum dome diameter (Dmax); 0.454 ± 0.095 for the inflow angle; 0.425 ± 0.095 for the H/ W ratio; and there was no statistical significance (P > 0.05).
Discussion
The most widely accepted reasons for aneurysm formation and rupture are arterial hypertension and haemodynamic stress [2,3,12]. Factors such as transmural pressure changes and wall shear stress in the aneurysm and parent artery can be determinant for an aneurysm rupture. These variables may also be affected by the morphology and flow velocity of the parent artery and aneurysm. The flow pattern in the aneurysm is thought to be associated with the shape of the dilatation, the relationship between the aneurysm and the parent artery, the volume of the aneurysm and the aspect ratio. A large aspect ratio is an indicator of intra-aneurysmal stagnation and may be the cause of SAH. In addition, turbulence due to atypical shape of the vessel wall may also cause stress in the aneurysm wall and transmural pressure increase [4,5].
Genetic factors, perianeurysmal and vascular wall structure, and haemodynamic stress can be determinant in the growth and rupture of aneurysms [13]. The growth of aneurysms depends on the interaction between haemodynamic loads and the mechano-biological responses of cell elements in the wall, resulting in a weakening of the vessel wall [14]. Rupture occurs when the wall stress exceeds the wall strength of the aneurysm. However, the mechanisms causing growth and rupture have not been fully elucidated. Defining the haemodynamic variables most associated with clinical progression and rupture may help to explain the effects of these mechanisms and improve the treatment approaches.
In a study performed by Sekhar et al [11], experimental models of saccular aneurysms in dogs were created and intra-aneurysmal pressures were measured. Intracarotid and intra-aneurysmal pressures were similar in this study and showed a linear relationship with mean arterial pressure. The results are similar to those in our study, and it is understood that the findings in humans are similar to the experimental aneurysms in animals.
Studies based on intra-aneurysmal and parent artery pressure measurements are limited. In a study conducted by Sorteberg et al [15], no statistical difference in pressure was found in the aneurysm and in the parent artery in the experimental silicone aneurysm models. In another experimental study, aneurysm pressures were similar to those of the parent arterial pressures [16]. The experimental silicon aneurysm models also have similar findings to our results and support the idea that intra-aneurysm pressure alone cannot cause rupture.
There are differences between ruptured and unruptured aneurysms in terms of internal structures and clinical features. Therefore, attempts are being made to explain the risk of rupture using complicated geometric measurements. In a study by Hoh et al, the aneurysm aspect ratio and the H/ W width ratio were higher in the ruptured aneurysms [17]. In another study, the inflow angle was defined as an independent separator for the rupture. The inflow angle was significantly higher in ruptured aneurysms in comparison with unruptured aneurysms. Also in this study, Dmax, H/ W ratio and aspect ratio were significantly higher in ruptured aneurysms. The inflow angle and H/ W ratio were defined as an independent and significant separator for the rupture [6]. In another study, however, Dmax, aspect ratio and H/ W ratio were high in ruptured aneurysms. But only the inflow angle and H/ W ratio were found as independent separators and stated as more important than the size of the aneurysm and more reliable in the risk classification of aneurysm morphological features [18]. In our study, the inflow angle, H/ W ratio, aneurysm neck diameter and Dmax were higher in ruptured aneurysms in comparison with unruptured aneurysms, but these differences were not statistically significant. A study with more cases may change the statistical significance.
As a result of many studies and our study, differences in pressure measurements alone cannot explain aneurysm rupture and we understand from the results of our study that pressure measurements cannot be used as a treatment planning indicator and cannot guide the treatment algorithm even if done before treatment. On the other hand, pressure measurement is not a practical method because it is performed using catheter angiography, which is an interventional procedure.
Conclusion
In our study, no significant pressure difference was detected in the aneurysm sac and parent artery between unruptured and ruptured aneurysms, and we think that the rupture can be a process related to the multifactorial effect of all conditions that cause stress in an aneurysm, especially the aneurysm shape and vessel wall properties. On the other hand, studies based on intra-aneurysmal and parent artery pressure measurements are limited and we need studies with a wider series based on geometric measurements such as the aneurysm shape and wall properties; this will contribute to our knowledge to better understand the rupture mechanisms.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Omer Kaya, MD, PhD
Department of Radiology
Ceyhan State Hospital
Ulus District
Hospital Street
Adana, Turkey
e-mail: dr.omerkaya@gmail.com
Accepted for review: 8. 11. 2018
Accepted for print: 4. 9. 2019
Sources
1. Penn DL, Komotar RJ, Sander Connolly E. Hemodynamic mechanisms underlying cerebral aneurysm pathogenesis. J Clin Neurosci 2011; 18(11): 1435 – 1438. doi: 10.1016/ j.jocn.2011.05.001.
2. Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats. Surg Neurol 1978; 10(1): 3 – 8.
3. Crompton MR. Mechanism of growth and rupture in cerebral berry aneurysms. Br Med J 1966; 1(5496): 1138 – 1142. doi: 10.1136/ bmj.1.5496.1138.
4. Weir B, Amidei C, Kongable G et al. The aspect ratio (dome/ neck) of ruptured and unruptured aneurysms. J Neurosurg 2003; 99(3): 447 – 451. doi: 10.3171/ jns.2003. 99.3.0447.
5. Ujiie H, Tamano Y, Sasaki K et al. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery 2001; 48(3): 495 – 503. doi: 10.1097/ 00006123-200103000-00007.
6. Baharoglu MI, Schirmer CM, Hoit DA et al. Aneurysm inflow-angle as a discriminant for rupture in sidewall cerebral aneurysms: morphometric and computational fluid dynamic analysis. Stroke 2010; 41(7): 1423 – 1430. doi: 10.1161/ STROKEAHA.109.570770.
7. Nishioka H, Torner JC, Graf CJ et al. Cooperative study of intracranial aneurysms and subarachnoid hemorrhage: a long-term prognostic study. II. Ruptured intracranial aneurysms managed conservatively. Arch Neurol 1984; 41(11): 1142 – 1146. doi: 10.1001/ archneur.1984.04050220036011.
8. White PM, Wardlaw JM. Unruptured intracranial aneurysms. J Neuroradiol 2003; 30(5): 336 – 350.
9. Nixon AM, Gunel M, Sumpio BE. The critical role of hemodynamics in the development of cerebral vascular disease. J Neurosurg 2010; 112(6): 1240 – 1253. doi: 10.3171/ 2009.10.JNS09759.
10. Wiebers DO. Unruptured intracranial aneurysms: natural history and clinical management. Update on the international study of unruptured intracranial aneurysms. Neuroimag Clin N Am 2006; 16(3): 383 – 390. doi: 10.1016/ j.nic.2006.04.005.
11. Sekhar LN, Sclabassi RJ, Sun M et al. Intra-aneurysmal pressure measurements in experimental saccular aneurysms in dogs. Stroke 1988; 19(3): 352 – 356. doi: 10.1161/ 01.str.19.3.352.
12. Gao L, Hoi Y, Swartz DD et al. Nascent aneurysm formation at the basilar terminus induced by hemodynamics. Stroke 2008; 39(7): 2085 – 2090. doi: 10.1161/ STROKEAHA.107.509422.
13. Sforza DM, Putman CM, Cebral JR. Hemodynamics of cerebral aneurysms. Annu Rev Fluid Mech 2009; 41 : 91 – 107. doi: 10.1146/ annurev.fluid.40.111406.102126.
14. Socci L, Pennati G, Gastaldi D et al. Modeling and mechanobiology of cerebral aneurysms. J Appl Boimater Biomech 2008; 6(2): 63 – 71.
15. Sorteberg A, Farhoudi D. The Influence of aneurysm configuration on ıntra-aneurysmal pressure and flow. Interv Neuroradiol 2006; 12(3): 203 – 214. doi: 10.1177/ 159101990601200302.
16. Ozdamar N, Celebi G. Pressure distribution on the wall of experimental aneurysms. Acta Neurochir (Wien) 1978; 45(1 – 2): 27 – 34. doi: 10.1007/ bf01774381.
17. Hoh BL, Sistrom CL, Firment CS et al. Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery 2007; 61(4): 716 – 723. doi: 10.1227/ 01.NEU.0000298899.77097.BF.
18. Wiebers DO, Whisnant JP, Huston J 3rd et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003; 362(9378): 103 – 110. doi: 10.1016/ s0140-6736(03)13860-3.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2019 Issue 6-
All articles in this issue
- Gunshot injury of the brain
- Are ticagrelor and prasugrel an alternative in the antiplatelet treatment of ischemic stroke? YES
- Are ticagrelor and prasugrel an alternative in the antiplatelet treatment of ischemic stroke? NO
- Are ticagrelor and prasugrel an alternative in the antiplatelet treatment of ischemic stroke?
- Cervical plexus lesions in clinical practice
- Neurorehabilitation of gait impairment using functional electrical stimulation – current findings from randomized clinical trials
- Difficulty in respecting autonomy in patients with Parkinson’s disease
- Current management of patients with degenerative cervical spine compression
- Surgical treatment of bilateral drug-resistant Menière’s disease
- Mechanical thrombectomy in the treatment of acute ischemic stroke in childhood
- Doporučení pro mechanickou trombektomii akutního mozkového infarktu – verze 2019
- Osmdesátiny doc. MU Dr. Jiřího Bauera, CSc.
- Intraplaque hemorrhage in symptomatic and asymptomatic progressive internal carotid artery stenosis – a pilot study
- Significant fall risk factors in the personal history of in-patients with neurological disease
- Spinal meningiomas – 92 patients operated at our department
- Civilian and military gunshot wounds to the head
- Epidural application of steroids Part 1 – Patient profile before application
- Facial nerve reconstruction with great auricular nerve graft following resection of recurrent basal cell carcinoma in parotidomasseteric region
- A comparison of perioperative pressure measurements in the aneurysm sac and parent artery in ruptured and unruptured aneurysm
- A systematic review of the clinical efficacy of sacroiliac joint stabilization in the treatment of lower back pain
- Does choroidal thickness change in Parkinson’s disease?
- Conservative management of a ruptured Galassi III middle fossa arachnoid cyst
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Cervical plexus lesions in clinical practice
- Doporučení pro mechanickou trombektomii akutního mozkového infarktu – verze 2019
- Mechanical thrombectomy in the treatment of acute ischemic stroke in childhood
- Gunshot injury of the brain
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

