-
Medical journals
- Career
Thermal Management in Patients Undergoing Elective Spinal Surgery in Prone Position – a Prospective Randomized Trial
Authors: V. Dostálová 1; J. Schreiberová 1; M. Bartoš 2; T. Česák 2; J. Habalová 2; V. Dostálová jr. 1; P. Dostál 1
Authors‘ workplace: Department of Anesthesiology and Intensive Care Medicine, Faculty of Medicine Hradec Kralove, Charles University and University Hospital Hradec Kralove, Czech Republic 1; Department of Neurosurgery, Faculty of Medicine Hradec Kralove, Charles University and University Hospital Hradec Kralove, Czech Republic 2
Published in: Cesk Slov Neurol N 2017; 80/113(5): 552-559
Category: Original Paper
doi: https://doi.org/10.14735/amcsnn2017552Overview
Background:
Perioperative hypothermia is a common complication of general anesthesia and surgery. The hypothesis that the new self-warming blanket (Barrier® EasyWarm®, Mölnlycke Health Care, Gothenburg, Sweden) is better at reducing the incidence of perioperative hypothermia in spinal surgery compared to passive insulation techniques, was tested.Methods:
In this prospective randomized study, 100 patients with American Society of Anesthesiologists physical status I – III who were scheduled to undergo spinal surgical procedures with an expected duration of surgery < 2 hours were enrolled. Patients were assigned to either the control group that received standard perioperative care, or to the group that received preoperative, intraoperative and postoperative use of the active self-warming blanket. The recorded measures included preoperative and postoperative axillary temperatures, intraoperative esophageal temperatures, duration of surgery, blood loss, hemodynamic instability, postoperative shivering, thermal comfort satisfaction, wound complications, and hospital days.Results:
The axillary body temperatures were not different at baseline but were significantly lower in the control group at the time of departure to the operating theater (36.0 ± 0.5 vs. 36.3 ± 0.4; P = 0.0086). Patients in the self-warming blanket group had higher esophageal temperatures intraoperatively, higher axillary temperatures in the recovery room, and fewer episodes of postoperative shivering (1/46 vs. 8/46; P = 0.0352). No significant differences were observed in other recorded measures.Conclusion:
The use of the active self-warming blanket provided more satisfactory body temperature control and reduced the number of episodes of postoperative shivering.Key words:
hypothermia – prewarming – self-warming blanket – spinal surgery
Chinese summary - 摘要
俯卧位脊柱择期手术患者的热管理:一项前瞻性随机试验
背景:
围手术期低体温症是全身麻醉和外科手术的一种常见并发症。这个假设测试了:与被动绝缘技术相比,新型自热毯(Barrier® EasyWarm®, Mölnlycke Health Care, Gothenburg, Sweden)对于减少脊柱手术围手术期低体温的发生效果更好。
方法:
在这项前瞻性随机研究中,纳入了100名符合美国麻醉医师协会身体状态I-III型的患者,这些患者计划接受手术时间小于2小时的脊柱外科手术。患者被分配到接受标准围手术期护理的对照组,或者被分配到术前、术中和术后均使用主动自热毯护理的实验组中。记录的数据包括术前和术后腋窝温度,术中食管温度,手术持续时间,失血量,血流动力学不稳定性,术后寒战,热舒适满意度,伤口并发症和住院天数。
结果:
腋窝体温在基线时没有差异,但在出发到手术室时,对照组显著低于实验组(36.0±0.5 vs. 36.3±0.4;P=0.0086)。自热毯组患者术中食管温度更高,恢复室中腋窝温度较高,并且术后寒战发作次数较少(1/46 vs. 8/46; P = 0.0352)。 其它记录数据无显著差异。
结论:
活性自热毯的使用提供了更令人满意的体温控制,并减少了术后寒战发作的次数。
关键词:
低温 - 预热 - 自热毯 - 脊髓手术Introduction
Hypothermia, defined as a core body temperature < 36 °C, is a common and serious complication that affects 20–70% of patients undergoing surgery [1]. Even mild hypothermia (core temperature of 34 – 36 °C) prolongs the duration of action of inhaled and intravenous anesthetics, activity of neuromuscular drugs, increases thermal discomfort, and is associated with delayed post-anesthesia recovery [2,3]. Mild hypothermia increases perioperative blood loss significantly and increases the allogenic transfusion requirement. In addition, 1.9 °C core hypothermia triples the incidence of surgical wound infections following colon resection and increases the duration of hospitalization by 20% [2,4,5]. Furthermore, mild hypothermia triples the incidence of postoperative adverse myocardial events [6]. Thus, even mild hypothermia contributes significantly to patient care costs and should be avoided [5].
There are numerous suggested strategies to prevent inadvertent perioperative hypothermia in adults [7–9] based on the presence of risk factors and the extent and duration of surgery. Multiple techniques of intraoperative warming including the use of forced air devices, electric blankets, circulation water mattresses, radiant heat devices, water garments and warmed blankets have been tested clinically in different groups of patients. Currently, active intraoperative warming is recommended for all patients at a high risk for perioperative hypothermia. The use of forced air warming devices in combination with warmed intravenous fluids has been considered a method of choice for intraoperative warming [9]. Preoperative prewarming has been shown to effectively prevent or diminish the extent of perioperative hypothermia [7–9]. Recently, it has been suggested to actively warm patients preoperatively at a ward, if patient‘s preoperative temperature is below 36.0 °C, or at least 30 minutes before induction of anesthesia, if the patient‘s temperature is 36.0 °C or above, unless this should delay emergency surgery [9,10].
Intraoperative warming of patients undergoing spinal neurosurgical procedures in the prone position presents a specific problem. The effectiveness of forced air devices could potentially be diminished due to low covered body surface area. Leakage of warm air in an operating theater environment may lead to substantial thermal discomfort of surgical team members. Although current clinical evidence is not conclusive [11,12], there is a concern that the use of forced air warming systems increases the risk of surgical site infections [13] by acting as a vector or causing unwanted airflow disturbances. Therefore, surgeons may prefer to avoid using a forced air warming device and use alternative warming strategies.
Resistive heating is a newer warming modality with favorable characteristics such as silent operation, energy efficiency and re-usable components in some devices [14]. Depending on local prices, it could be potentially cheaper [9] and might offer advantages in terms of practicality and ease of use with regard to prewarming [15].
The aim of this study was to compare the efficacy of the preoperative, intraoperative and postoperative use of an active self-warming blanket with standard care based on passive insulation techniques in patients scheduled for a clean elective spinal surgery in the prone position with an expected duration of surgery of < 2 hours.
Patients and methods
This investigator-initiated, single-centre, prospective randomized study was performed at the University Hospital Hradec Kralove. Ethical approval for this study (Ethics Committee no. 201404 S14P) was granted by the Ethics Committee of the University Hospital. (Chairperson: Jiri Vortel, M.D.).
Adult patients scheduled for an elective spinal surgery in the prone position (lumbar laminectomy, hemilaminectomy, foraminotomy, or stabilization of lumbar vertebral fractures) with an expected length of surgery < 2 hours were considered for inclusion in this study. All patients gave consent to participate in the study and filled out a questionnaire regarding thermal comfort satisfaction. All subjects were recruited between October 10, 2013 and May 25, 2014.
The inclusion criteria were as follows: age 18–80 years, elective spinal surgery in the prone position with an expected length of surgery < 2 hours, and an American Society of Anesthesiologists physical status I–III. The exclusion criteria were pregnancy, preoperative temperature > 38 °C, and known hypo - and hyperthyreosis.
After simple randomization (a computer-generated random list of patients in sealed envelopes), patients were assigned to either the control group that received standard perioperative care (warmed infusion fluids, an operating theater temperature of 22–23 °C and the use of cotton blankets), or to the intervention group that received the preoperative, intraoperative, and postoperative use of the active self-warming blanket (Barrier® EasyWarm®, Mölnlycke Health Care, Gothenburg, Sweden). The blanket maintains the mean temperature of 44 °C for up to 10 hours, raising skin temperature to a maximum of 42 °C [16].
The self-warming blanket was removed from the sleeve 30 minutes before use and the activated blanket was administered to all patients randomized to the intervention group 90 minutes before the scheduled surgery. The blanket was used during transport, surgery, and recovery in a recovery room. Axillary temperature was measured at selected time points preoperatively (120 min, 90 min, and at the time of patient‘s transfer from a ward to an operating theatre) and postoperatively (upon arrival in a recovery unit and then at 15 minute intervals until discharge to a standard ward). Intraoperatively, body temperature was measured continuously using an esophageal thermometer probe (Aisys, GE Healthcare, Helsinki, Finland) inserted 30 to 35 cm into the distal esophagus after tracheal intubation. Intraoperative temperature was recorded at 5 minute intervals.
All patients were premedicated with oral midazolam (7.5 mg) approximately 90 minutes before the induction of anesthesia. General anesthesia was induced using a combination of propofol and sufentanil. Tracheal intubation was facilitated using 0.5 mg/kg bodyweight atracurium; no further boluses of muscle relaxants were used during surgery. Anesthesia was maintained with isoflurane (0.8–1 vol %) in 50% oxygen in nitrous oxide along with sufentanil boluses. Esophageal temperature probe was inserted in all patients. Warmed intravenous fluids (transfusion and infusion flow through the OTI 1A heating system, RTE, Prague, Czech Republic) were managed according to our standard practice: a baseline fluid intake of 5 ml/kg/hour was provided using a balanced crystalloid solution (Ringerfundin, B.Braun, Melsungen, Germany). The decision to give fluid boluses, colloids, or blood transfusion was at the discretion of the attending anesthesiologist. Hypotension was defined as a mean arterial pressure (MAP) < 70 mmHg or a drop in blood pressure > 20% from baseline that lasted > 5 minutes. Norepinephrine was indicated if hypotension persisted for more than 10 minutes; the dose of norepinephrine was adjusted to maintain MAP > b 70 mmHg.
The recorded variables included age, gender, type and location of surgery, hemodynamic variables (MAP and heart rate), preoperative and postoperative axillary temperature, intraoperative esophageal temperature, blood loss, duration of surgery, use of blood products, postoperative shivering, highest intensity of postoperative pain (expressed as a visual analog scale value; range 0–4), wound infections, and the length of hospital stay.
The primary outcome measure was defined as a difference in core body temperature between the two treatment groups during surgery. The secondary outcome measures were the incidence of intraoperative hypothermia, defined as an esophageal temperature < 36 °C, the incidence of postoperative shivering, thermal discomfort, the number of wound infections, and the number of hospital days.
A power analysis based on an a-error 0.01 and a b-error of 0.1 was performed using MedCalc 7.6.0. (MedCalc Software, Ostend, Belgium). Sample size needed for the independent samples t-test (with an expected difference between groups at the end of surgery of 0.5 °C and a standard deviation of 0.5 °C in both groups) was calculated. This calculation produced a sample size of 62 subjects (31 subjects per group). The sample size was increased to 50 patients per treatment group to compensate for potential dropouts and the possible inaccuracy of the predictions used for power analysis.
Data are presented as means ± standard deviations or medians and interquartile ranges (IQR) based on the results of D‘Agostino-Pearson tests. Differences between the groups were analyzed using chi-square tests with Yates’ correction for continuity (demographic variables) or Fisher’s exact test, as appropriate. Independent t-tests were used to compare other results between groups, and Mann-Whitney U-tests were used when the sample distribution was not normal. Time-dependent changes in esophageal and axillary temperatures were evaluated using one way analysis of variance (ANOVA) with Student-Newman-Keuls test for all pairwise comparisons. P < 0.05 was considered to be significant. All statistical analyses were performed using MedCalc 7.6.0.
Results
A total of 235 patients undergoing elective spinal surgery performed in prone position was screened. After excluding patients with contraindications, those involved in other studies, and those not recruited for logistic reasons, a total of 100 patients was enrolled (Scheme 1).
Four patients in the intervention group and four patients in the control group did not receive treatment due to a change in the operating plan. Baseline data of all the recruited patients and their preoperative axillary temperatures are summarized in Tab. 1. There were no significant differences between the two groups in terms of age, gender, bodyweight, ASA physical status, and type of surgery. The axillary body temperatures were not different at baseline but were significantly higher in the intervention group at the time of departure from a ward to an operating theatre (36.3 ± 0.4 °C vs. 36.0 ± 0.5 °C; P = 0.0086).
1. Baseline patient characteristics. 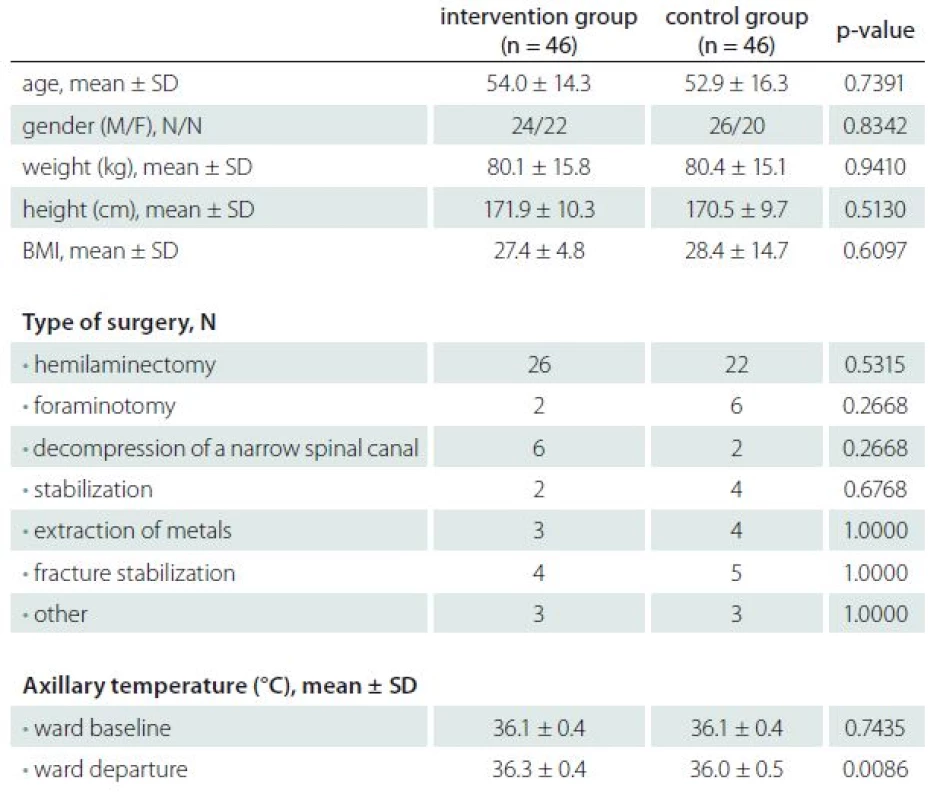
ASA – American Society of Anesthesiology, BMI – body mass index, M – male, F – female, SD – standard deviation, VAS – visual analog score, N – number of patients. Patients in the therapeutic group had, except at baseline, significantly higher intra-operative esophageal temperatures (Graph 1). The ANOVA did not reveal any differences between the different times in the intervention group (P = 0.996). In contrast, the ANOVA revealed a significantly lower esophageal body temperature after 90 minutes of anesthesia in the control group (P = 0.006). The proportion of patients with esophageal temperature < 36 °C was significantly higher in the control group after 90 and 120 minutes of anesthesia (Graph 2).
1. Intraoperative esophageal temperatures. N – number of patients in the group, * < 0.05 vs control group, # < 0.01 vs 0 min. 
2. Proportion of patients without intraoperative hypothermia. N – number of patients in the group, * < 0.05 vs intervention group, # < 0.01 vs 0 min. 
Compared to controls, patients in the treatment group had a similar mean rate of temperature change during the first hour of surgery (0.02 ± 0.27 vs. 0.04 ± 0.33 °C; P = 0.7061) and a lower mean rate of temperature change during the second hour of surgery (0.00 ± 0.10 vs. 0.08 ± 0.13 °C; P = 0.0497).
Patients in the intervention group had higher axillary temperatures in a recovery room (Tab. 2). The result of a post hoc analysis of relationship between BMI and effects of warming is shown in Graph 3.
3. Preoperative axillary and intraoperative esophageal temperatures in patients with body mass index (BMI) ≤ 25 or > 25. * P < 0.05 vs. control group/BMI > 25, ** P < 0.05 vs. control group/BMI > 25 and intervention group/BMI > 25, # P < 0.05 vs. control group/ BMI > 25 and control group/BMI ≤ 25. 
2. Postoperative axillary temperatures. 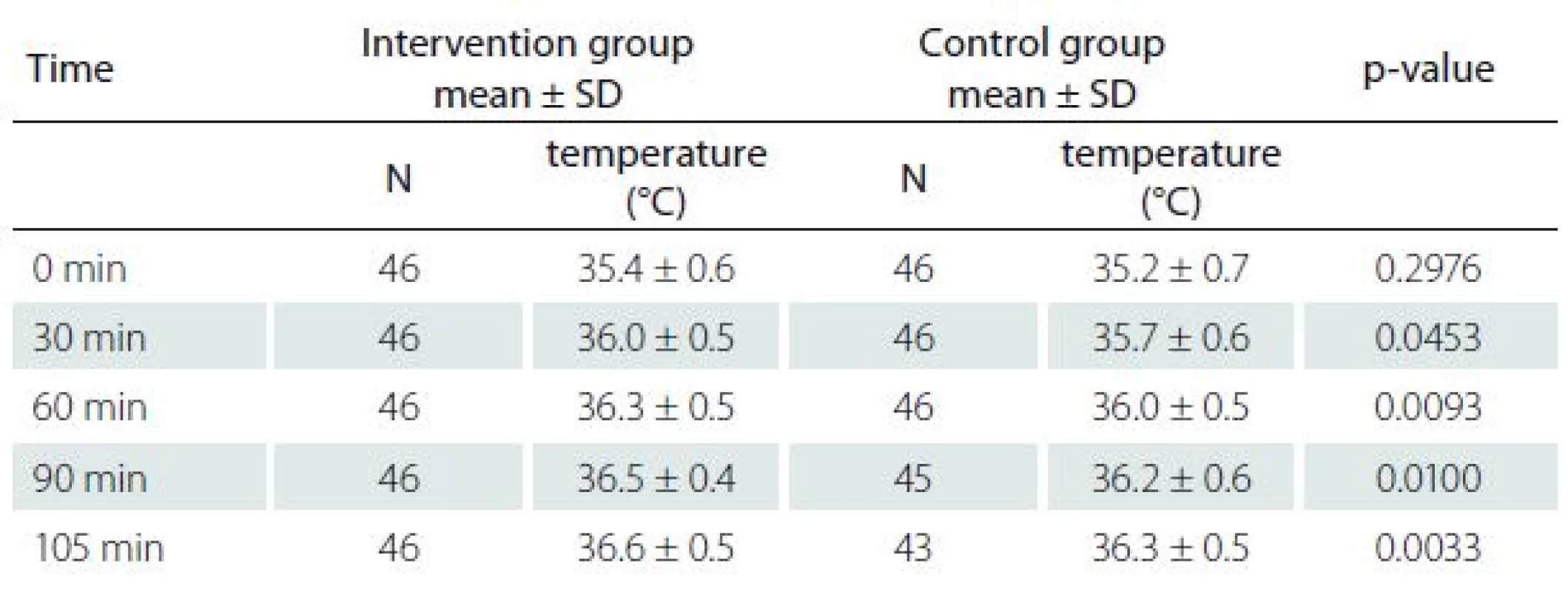
N – number of patients. Compared to the control group, patients in the intervention group had fewer episodes of postoperative shivering (1/46 vs. 8/46; P = 0.0352). No significant differences were observed between groups regarding the volume of blood loss, duration of surgery, number of episodes of hemodynamic instability, maximal postoperative pain, number of episodes of cold feeling, satisfaction with thermal care, number of wound complications, and the length of hospital stay (Tab. 3).
3. Perioperative and postoperative data for duration of surgery, blood loss, hemodynamic instability, maximal postoperative pain, feeling cold, postoperative shivering, satisfaction with thermal care, wound complications, and length of hospital stay. 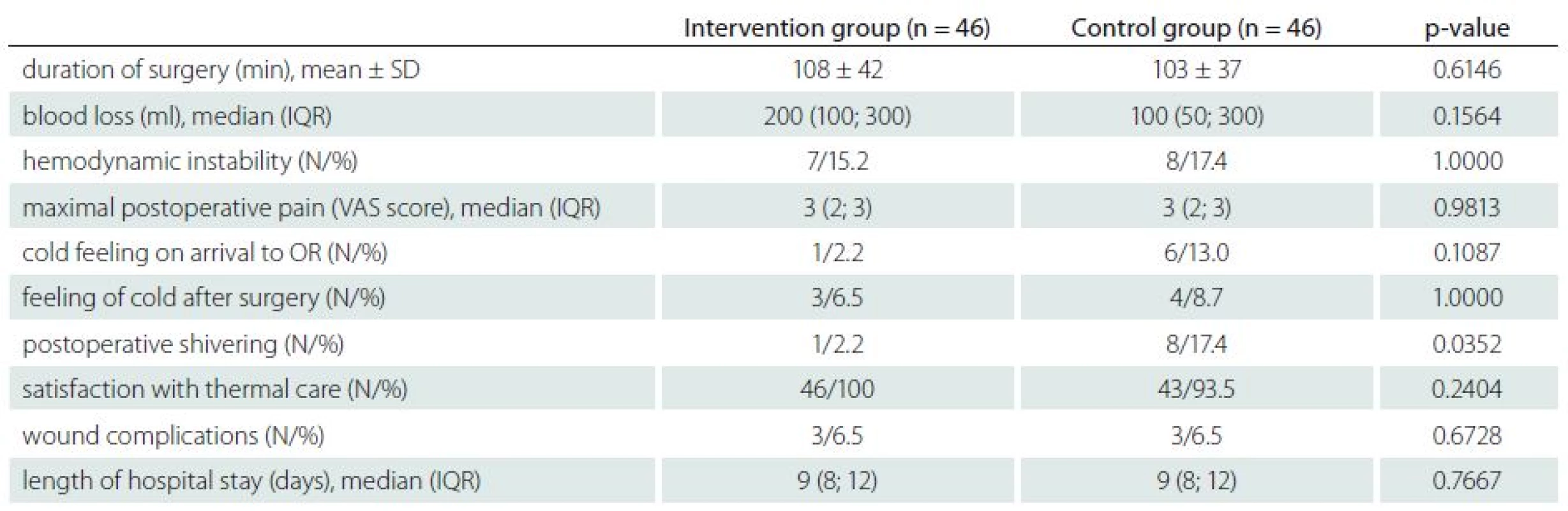
VAS – verbal analgesia scale (0 – none, 1 – mild, 2 – moderate, 3 – severe, 4 – very severe), N – number of patients, IQR – interquartile range. Discussion
The current study demonstrated that the use of an active self-warming blanket provided satisfactory body temperature control during a perioperative phase and decreased the number of episodes of postoperative shivering in patients undergoing elective spinal surgery in the prone position. The number of wound complications, patient satisfaction with the thermal care, and length of hospital stay were similar between the groups.
There are a limited number of clinical studies assessing frequency and severity of hypothermia and its prevention in patients undergoing elective spinal surgery that could be compared with this study.
Frequency of hypothermia in patients undergoing spinal surgery was recently published in a study comparing two methods of postoperative warming in a postanesthesia recovery room [17]. The study included patients undergoing elective spinal procedures between 3 – 6 hours in duration and the observed incidence of hypothermia, defined as tympanic temperature below 35.5 °C, was 16%. Methods of intraoperative warming were not reported. In our study, we defined hypothermia as esophageal temperature below 36.0 °C. We did not observe any episode of hypothermia in patients of the treatment group after two hours of surgery. Axillary temperature was measured upon arrival to the postanesthesia recovery unit. Axillary temperature correlates well with core body temperature in adults who are normothermic or who have slight fever. However, if the patient becomes hypothermic, peripheral vasoconstriction occurs, making axillary measurements unreliable as the core measurement [18]. Therefore, direct comparison with our results is not possible.
Recently, in a study testing the effectiveness of a newly designed fluid warming kit in patients undergoing spinal surgery, esophageal temperature at the end of surgery was 35.8 ± 0.3 °C in the group of patients with the tested warming kit and 34.8 ± 0.3 °C in a control group [19]. Comparison with our data is limited since the forced air warming device was only used in patients with esophageal temperatures < 35 °C.
Hypothermia and its relationship with blood loss and postoperative complications were previously evaluated in patients undergoing spinal surgery [20] for its possible neuroprotective effects. In that study, patients were not actively warmed until spinal cord decompression was completed, as determined by the operating surgeon; then warming was induced using a forced air-warming device. The nadir temperature was 35.3 ± 0.8 °C and the mean rate of temperature change without warming was 0.87 °C/the control group. In the majority of clinical studies [15,21,22], redistribution of heat after the induction of anesthesia leads to reduction in core temperature of ~0.3–0.8 °C during the first hour but it can reach up to 1.5 °C [23]. Our observation might be explained by the fact that the patients were kept comfortably warm during the entire preoperative period (i.e. in the ward, during transport to the operating theatre, and at the induction of anesthesia), as recommended by the recent National Institute for Health and Care Excellence (NICE) United Kingdom guidelines [9]. Prewarming before anesthesia prevents the initial decrease in body temperature by redistributing heat between the core and peripheral body compartment during the first hour of anesthesia [9,23]. During the linear phase of intraoperative hypothermia, radiation is considered to be the most important mechanism of heat loss [9,22,23]. Therefore, a combination of prewarming and intraoperative warming could be more effective than prewarming or intraoperative warming alone [9,23]; this concept was recently confirmed using a novel prewarming system [24] or a self-warming blanket [25].
We compared an active self-warming blanket during preoperative, intraoperative, and postoperative warming with standard passive insulation techniques for perioperative heat loss prevention. In contrast to a previously published study using the same blanket in patients undergoing ear and throat surgery [26] that failed to show any significant benefit of the device compared to passive insulation techniques, the present study examined the use of the device in patients undergoing spinal surgery. We used a longer preoperative warming phase of 90 minutes vs. “at least 30 minutes” and we did not exclude patients with a BMI < 20 or > 30. Other explanations of different findings include longer mean duration of surgery in this study and possibly lower efficacy of passive insulation techniques due to the lower cover body surface area in patients undergoing spinal surgery in comparison to ear and throat surgery.
The present study has several limitations. The study was not blinded during data acquisition as blinding of the investigator and patient was logistically impossible. In addition, different sites were used for temperature measurements intraoperatively and during preoperative and postoperative phases to avoid possible patient discomfort due to temperature measurements. Although sublingual or tympanic temperatures could have been used, they are also associated with significant deviations from the esophageal temperature Stanhope [20] and they are not well accepted by patients in our region.
The number of patients included in the study was also relatively small and did not have the statistical power to show differences in the numbers of postoperative complications.
In conclusion, the present study demonstrated that the use of an active self-warming blanket provided satisfactory body temperature control during a perioperative phase in patients undergoing elective spinal surgery in the prone position.
Supported by MZ ČR – RVO (FNHK, 00179906).
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
doc. MUDr. Pavel Dostál Ph.D., MBA
Department of Anesthesiology and Intensive Care Medicine
Faculty of Medicine, Charles University and University Hospital Hradec Kralove
Sokolska 581
500 05 Hradec Kralove Czech Republic
e-mail: pavel.dostal@fnuk.cz
Accepted for review: 2. 6. 2017
Accepted for print: 4. 7. 2017
Sources
1. Kurz A. Physiology of thermoregulation. Best PractRes Clin Anaesthesiol 2008;22(4):627 – 44. doi: 10.1016/ j.bpa.2008.06.004.
2. Reynolds L, Beckmann J, Kurz A. Perioperative complications of hypothermia. Best Pract Res Clin Anaesthesiol 2008;22(4):645 – 57. doi: 10.1016/ j.bpa.2008.07.005.
3. Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology 1997;87(6):1318 – 23. doi: 10.1097/ 00000542-199712000-00009.
4. Kurz A, Sessler D, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med 1996;334(19):1209 – 15. doi: 10.1056/ NEJM1996050933 41901.
5. Melling AC, Ali B, Scott EM, et al. Effects of preoperative warming on the incidence of wound infection after clean surgery: A randomised controlled trial. Lancet 2001;358(9285):876-80. doi: 10.1016/ S0140-6736(01)06071-8.
6. Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events: A randomized clinical trial. JAMA 1997;277(14):1127 – 34. doi: 10.1001/ jama.1997.03540380041029.
7. ASPAN. Clinical guideline for the prevention of unplanned perioperative hypothermia. J Perianesth Nurs 2001;16(5):305-14. doi: 10.1053/ jpan.2001.28060.
8. Hooper VD, Chard R, Clifford T, et al. ASPAN’s Evidence-Based Clinial Practice Guideline for the Promotion of Perioperative Normothermia: Second Edition. J PeriAnesthesia Nurs 2010;25(6):346-65. doi: 10.1016/ j.jopan.2010.10.006.
9. National Collaborating Centre for Nursing and Sup-portive Care (UK). The Management of Inadvertent Perioperative Hypothermia in Adults [accessed 24 October 2016]. Available form URL: www.ncbi.nlm.nih.gov/ books/ NBK53788.
10. Perl T, Peichl LH, Reyntjens K, et al. Efficacy of a novel prewarming system in the prevention of perioperative hypothermia. A prospective, randomized, multicenter study. Minerva Anestesiol 2014;80(4):436 – 43.
11. Kellam MD, Dieckmann LS, Austin PN. Forced-air warming device and the risk of surgical site infections. AORN J 2013; 98(4):354 – 66. doi: 10.1016/ j.aorn.2013.08.001.
12. Wood AM, Moss C, Keenan A, et al. Infection control hazards associated with the use of forced-air warming in operating theatres. J Hosp Infect 2014;88(3):132 – 40. doi: 10.1016/ j.jhin.2014.07.010.
13. McGovern PD, Albrecht M, Belani KG, et al. Forced-air warming and ultra-clean ventilation do not mix: an investigation of theatre ventilation, patient warming and joint replacement infection in orthopaedics. J Bone Joint Surg Br 2011;93(11):1537 – 44. doi: 10.1302/ 0301-620x.93b11.27124.
14. John M, Ford J, Harper M. Peri-operative warming devices: performance and clinical application. Anaesthesia 2014;69(6):623 – 38. doi:10.1111/ anae.12626.
15. Brandt S, Oguz R, Huttner H, et al. Resistive-polymer versus forced-air warming: comparable efficacy in orthopedic patients. Anesth Analg 2010;110(3):834 – 38. doi: 10.1213/ ane.0b013e3181cb3f5f.
16. Molnlycke Healthcare. Barrier EasyWarm. [accessed 22 November 2016]. Available from URL: www.molnlycke.com/ patient-warming/ barrier-easywarm.
17. Yang HL, Lee HF, Chu TL, et al. The comparison of two recovery room warming methods for hypothermia patients who had undergone spinal surgery. J Nurs Scholarsh 2012;44(1):2 – 10. doi: 10.1111/ j.1547-5069.2011.01426.x.
18. Stanhope N. Temperature measurement in the phase I PACU. J Perianesth Nurs 2006; 21 (1): 27 – 33. doi: 10.1016/ j.jopan.2005.11.004.
19. Jung KT, Kim SH, So KY, et al. Clinical evaluation of a newly designed fluid warming kit on fluid warming and hypothermia during spinal surgery. Korean J Anesthesiol 2015;68(5):462 – 8. doi: 10.4097/ kjae.2015.68.5.462.
20. Guest JD, Vanni S, Silbert L. Mild hypothermia, blood loss and complications in elective spinal surgery. Spine J 2004;4(2):130 – 7. doi: 10.1016/ j.spinee.2003.08.027.
21. De Witte JL, Demeyer C, Vandemaele E. Resist-ive-heating or forced-air warming for the prevention of redistribution hypothermia. Anesth Analg 2010;110(3):829 – 33. doi: 10.1213/ ane.0b013e3181cb3ebf.
22. Andrzejowski J, Hoyle J, Eapen G, et al. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br J Anaesth 2008;101(5):627 – 31. doi: 10.1093/ bja/ aen272.
23. Sessler DI, Todd M. Perioperative heat balance. Anesthesiology 2000;92 (2): 578 – 96. doi: 10.1097/ 00000542-200002000-00042.
24. Perl T, Rhenius A, Eich C, et al. Conductive warming and insulation reduces perioperative hypothermia. Cent Eur J Med 2012;7(3):284 – 89. doi: 10.2478/ s11536-011-0166-2.
25. Rosenkilde CH, Vamosi M, Lauridsen JT, et al. Efficacy of Prewarming With a Self-Warming Blanket for the Prevention of Unintended Perioperative Hypothermia in Patients Undergoing Hip or Knee Arthroplasty. J PeriAnesthesia Nurs 2016;32(4). doi: 10.1016/ j.jopan.2016.02.007.
26. Brandes IF, Müller C, Perl T, et al. Effektivität einer neuen Wärmedecke. Prospektive randomisierte Studie. Anaesthesist 2013;62(2):137 – 42. doi: 10.1007/ s00101-013-2140-7.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2017 Issue 5-
All articles in this issue
- Invasive Methods in the Treatment of Advanced Parkinson’s Disease
- Gait Neurorehabilitation in Stroke Patients
- Essential Tremor – Is There a New Nosological Concept?
- Leber Hereditary Optic Neuropathy
- Facial Nerve Function after Microsurgical Removal of the Vestibular Schwannoma
- Smoking Prevalence in Group of Central-European Patients with Narcolepsy-cataplexy, Narcolepsy without Cataplexy and Idiopathic Hypersomnia
- Long-term Postoperative Clinical Outcomes after Intramedullary Cavernoma Resection
- Statin-induced Necrotizing Autoimmune Myopathy
- Electrical Stimulation of the Suprahyoid Muscles in Post Stroke Patients with Dysphagia
- Intraventricular Meningiomas – a Retrospective Study on 19 Surgical Cases
- Single Nucleotide Polymorphism p.Val66Met in BDNF Gene in the Czech Population
- Two Cases of CNS Atypical Theratoid Rhabdoid Tumor and Review of Literature
- Thermal Management in Patients Undergoing Elective Spinal Surgery in Prone Position – a Prospective Randomized Trial
- Sub-chronic Intra-hippocampal Aminoguanidine Improves Passive Avoidance Task and Expression of Bcl-2 Family Genes in Diabetic Rats
- Case of Adult Escherichia Coli Meningitis
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Essential Tremor – Is There a New Nosological Concept?
- Leber Hereditary Optic Neuropathy
- Statin-induced Necrotizing Autoimmune Myopathy
- Invasive Methods in the Treatment of Advanced Parkinson’s Disease
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


