-
Medical journals
- Career
Detection of Anaplastic Foci within Infiltrative Gliomas with Nonsignificant Contrast Enhancement using 5-aminolevulic Acid – a Report of Five Cases
Authors: A. Šteňo 1; R. Illéš 1; B. Rychlý 2; M. Fabian 3; J. Šteňo 1
Authors‘ workplace: Department of Neurosurgery, Derer’s Hospital, Comenius University, Bratislava 1; Cytopathos s. r. o., Bratislava 2; Department of Rad-logy, Derer’s Hospital, Slovak Medical University, Bratislava 3
Published in: Cesk Slov Neurol N 2012; 75/108(2): 227-232
Category: Short Communication
Overview
Objective:
Detection of anaplastic areas within focally malignant infiltrative brain glioma is an important diagnostic step in preventing histopathological undergrading. The objective of this paper is to draw attention to the possibility of intraoperative detection of anaplastic foci within infiltrative gliomas using 5-aminolevulic acid (5-ALA).Material and methodology:
We prospectively studied a case series of five adult patients harboring infiltrative supratentorial gliomas with nonsignificant contrast enhancement (CE) on preoperative magnetic resonance imaging. The patients were operated on during a period of 10 months. All patients were administered 5-ALA before the surgery. After illuminating the operative field with violet-blue light (wavelength 400 nanometers), selective tissue samples were taken from fluorescing and non-fluorescing areas. Histopathological diagnosis was established according to World Health Organization (WHO) 2007 diagnostic consensus criteria. Cell proliferation was assessed immunohistochemically by Ki-67 labeling index (LI).Results:
Focal 5-ALA fluorescence was observed within four gliomas. All tissue samples taken selectively from fluorescing parts of these tumors were histopathologically evaluated as WHO grade III. The average Ki-67 LI in fluorescing parts was 14.13%. In contrast, all tissue samples taken selectively from nonfluorescing parts of these tumors corresponded to WHO grade II. Average Ki-67 LI in nonflourescing parts was 2.67%. In one glioma, no intraoperative 5-ALA fluorescence was observed; all tissue samples sent to histopathological examination were proven to be WHO grade II, the average Ki-67 LI was 3%.Conclusions:
Using 5-ALA may be a useful method in detecting anaplastic foci within infiltrative gliomas with nonsignificant CE.Key words:
glioma – 5-aminolevulic acid – anaplastic focusIntroduction
Until recently, intraoperative detection of anaplastic foci within infiltrative gliomas was mainly based on preoperative magnetic resonance imaging (MRI) or positron emission tomography (PET) investigations and subsequent intraoperative localization of potentially anaplastic tumor tissue with the use of neuronavigation [1]. However, brain shift may cause neuronavigation to become considerably inaccurate [2].
Recently, Widhalm et al [1] proposed a new method of intraoperative detection of anaplastic foci within infiltrative gliomas with nonsignificant contrast enhancement (CE) on preoperative MRI. The method is based on preoperative application of 5-aminolevulic acid (5-ALA), leading to accumulation of fluorescent porphyrins within malignant glioma tissue. Intraoperatively, on illumination of the operative field with violet-blue light (wavelength 400 nm), the anaplastic areas are seen as red-fluorescing spots. This method of anaplastic foci detection is unaffected by brain shift. In the present paper we describe our first experiences with this technique.
Material and methodology
From August, 2010 to June, 2011, five adult patients (four males, one female) harboring infiltrative supratentorial gliomas with nonsignificant CE were operated on at our department (Tab. 1). A written consent with proposed investigations and surgical strategy was obtained from all patients. All patients underwent routine MRI investigation comprising of axial, coronal and sagittal T1-weighted sequences, axial T2-weighted sequence, axial fluid-attenuated inversion recovery sequence and contrast-enhanced axial, coronal and sagittal T1-weighted sequences. The MRI scans were evaluated by a radiologist (M. F.), following the criteria published by Pallud et al [3]. All tumors had nonsignificant CE defined as patchy and faint on preoperative T1-weighted sequences (Fig. 1). No glioma with unequivocal (nodular-like or ring-like) CE was included into the case series. In addition to a routine MRI investigation, preoperative MRI spectroscopy and MRI perfusion-weighted imaging (PWI) was performed on three patients (patients 1, 2 and 3). The spectroscopic analysis and PWI corresponded to low-grade lesion in all three cases. However, because of the presence of contrast-enhancing areas, partial anaplastic transformation was regarded as likely in all five cases (according to the official MRI reports).
1. Operative and histopathological findings in patients harboring supratentorial gliomas with nonsignificant contrast enhancement on preoperative MRI. 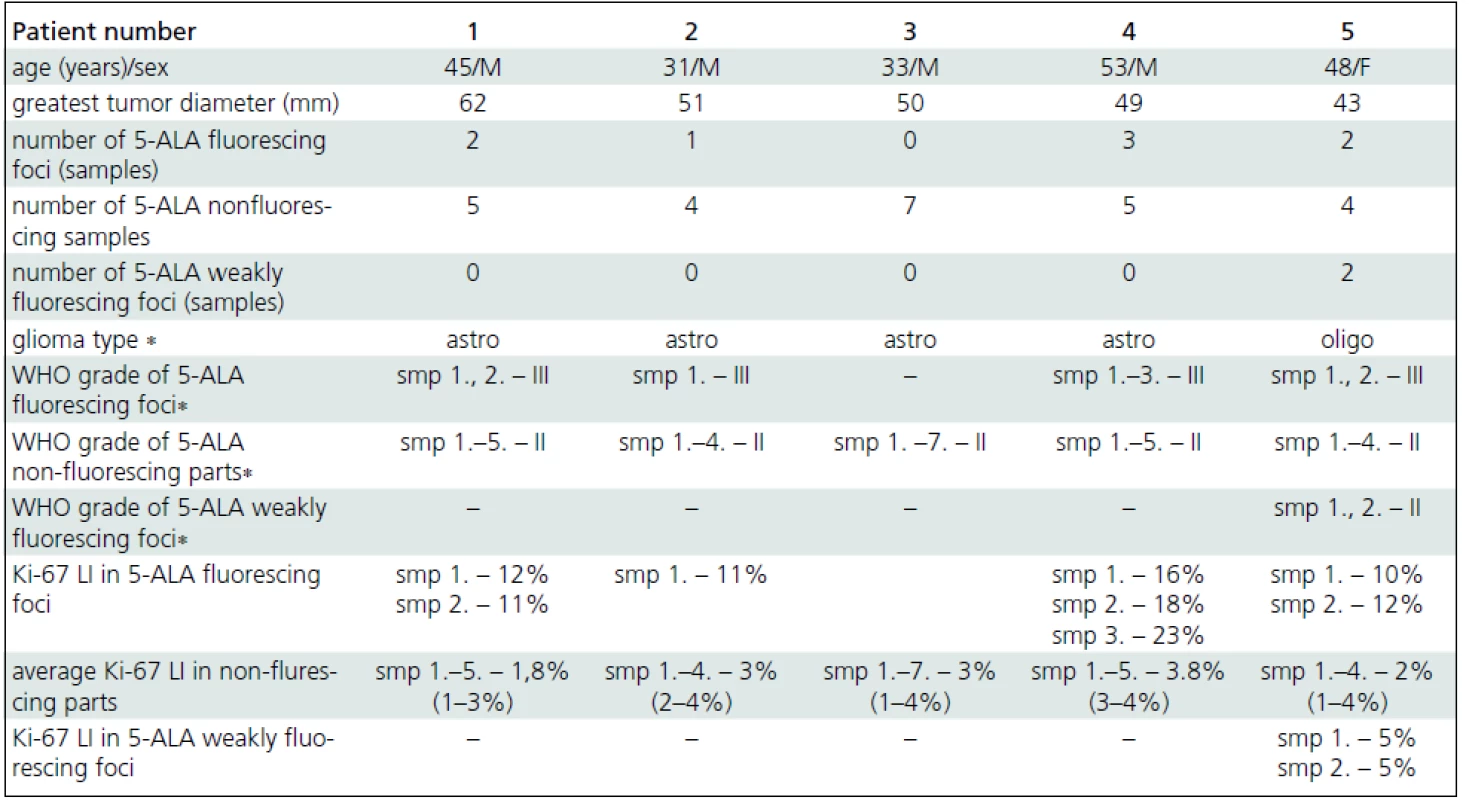
∗according to WHO 2007 criteria [4], MRI – Magnetic Resonance Imaging, M – Male, F – Female, mm – millimeters, 5-ALA – 5-aminolevulic acid, WHO – World Health Organization, astro – astrocytoma, oligo – oligodendroglioma, smp – sample, Ki-67 LI – Ki-67 labeling index. Histopathological diagnosis was established and Ki-67 LI was counted for each 5-ALA fluorescing, 5-ALA nonfluorescing and 5-ALA weakly fluorescing tissue sample. Because of high number of 5-ALA nonfluorescing samples, only average and boundary values of Ki-67 LI are presented in Tab. 1. Fig. 1. Examples of nonsignificant contrast enhancement. Fig. 1a) Patient 1. Contrast-enhanced axial T1-weighted sequence. Fig. 1b) Corresponding axial fluid-attenuated invers-n recovery (FLAIR) sequence of patient 1. Fig. 1c) Patient 5. Contrast-enhanced axial T1-weighted sequence. Fig. 1d) Corresponding axial FLAIR sequence of patient 5. 
Preoperative PET investigation using 18-fluorodeoxyglucose (18-FDG) was performed in four patients (patients 1, 2, 3 and 4). Tumors in patients 1, 2 and 3 appeared as areas of lower 18-FDG uptake, a small focus of increased 18-FDG uptake was found within the hypometabolic area in patient 4. PET was not performed in patient 5 due to technical reasons.
Three hours before the surgery, all patients were administered 5-ALA. After administration of 5-ALA, exposure of eyes and skin to strong light sources (e.g. operating illumination, direct sunlight or brightly focused indoor light) was avoided for 24 hours. All other steps of preoperative preparation were performed in the conventional manner.
Neuronavigation was used in all cases. Since all tumors were localized within or adjacent to eloquent areas, “awake” monitoring and/or direct electrical stimulation was used during all operations. After craniotomy and opening of the dura, tumor resection was performed in a standard fashion using microsurgical techniques, suctioning and cavitron ultrasonic surgical aspirator (CUSA).
During tumor resection, the operative field was regularly illuminated for approximately 10–15 seconds with a violet-blue light (wavelength 400 nm) to identify the areas with positive red fluorescence. The approximate violet-blue light illumination frequency was every three to five minutes (depending on the speed of resection; a more frequent illumination was used when the tumor consistence was soft and the resection was fast). A surgical microscope with integrated violet-blue light source was used (OPMI Pentero, Carl Zeiss Surgical GmbH, Oberkochen, Germany). The frequency of switching from conventional white light to violet-blue light was approximately every three to five minutes (depending on the speed of resection). After detection of the red-fluorescing foci, tumor tissue was selectively collected from these areas (Fig. 2). Different samples were taken from tumor parts with negative 5-ALA fluorescence. When an area of weak (pink) fluorescence was identified, a tissue sample was taken selectively from this part of the tumor. When no intraoperative fluorescence was detected, multiple samples were collected throughout entire tumor resection.
Fig. 2a) 5-aminolevulic acid (5-ALA) fluorescing focus visible within the surrounding nonfluorescing tumor tissue after illumination of the operating field with violet-blue light in patient 1 (arrow). Fig. 2b) Selective tissue sample taken from 5-ALA fluorescing area showed in Fig. 2a. Fig. 2c) 5-ALA weakly fluorescing foci (arrows) in patient 5. 
After the end of resection, all resected tumor tissue with negative 5-ALA fluorescence was carefully examined again under violet-blue light illumination in order to identify any minor areas of red fluorescence. Subsequently, all resected tissue samples were sent for histopathological examination. The operation was finished in a standard fashion.
Tissue samples from fluorescing, non-fluorescing and weakly fluorescing tumor areas of all five tumors were assessed by experienced pathologist (B. R.), who was blinded to the type of fluorescence of the samples. The histopathological diagnosis was established and the proliferation index was assessed separately for each tissue sample.
All samples were embedded in paraffin and stained routinely with hematoxilin-eosin. Histopathological diagnosis was established according to the World Health Organization (WHO) 2007 diagnostic consensus criteria [4]. In brief, grade II astrocytoma is defined as a diffusely infiltrating glial tumor composed of well differentiated neoplastic astrocytes. Mitotic activity is generally absent. The anaplastic (grade III) astrocytoma has increased cellularity as compared to the grade II equivalent, distinct nuclear atypia and mitotic activity. The presence of necrosis or microvascular proliferation within an astrocytic tumor corresponds to the diagnosis of glioblastoma. Diagnosis of grade II and anaplastic (grade III) oligodendroglioma are analogously defined, however necrosis and/or microvascular proliferation is still compatible with the diagnosis of the anaplastic oligodendroglioma. The oligoastrocytoma is a glioma composed of a conspicuous mixture of two distinct neoplastic cell types morphologically resembling the tumour cells in oligodendroglioma and astrocytoma.
Glioma cell proliferation was assessed immunohistochemically by detecting the Ki-67 marker, using the MIB-1 antibody (anti-Ki-67, 1 : 75; DAKO). Ki-67 antigen was apparent after MIB-1 binding as nuclear staining. The percentage of Ki-67 immunopositive cells (Ki-67 labeling index, Ki-67 LI) was assessed in hot spots (i.e. areas of highest density of Ki-67 immunopositive cells) of each specimen by evaluating a total of 500 tumor cell nuclei.
Results
The mean tumor resection time was 124 minutes (80–165 minutes). Focal 5-ALA fluorescence was observed in four patients; three fluorescing areas in one patient, two foci each in two patients, and one area in one patient (Tab. 1). All but one fluorescing areas were smaller than 10 milimeters (mm) in diameter; the maximum diameter of one of the areas in patient 4 reached 15 mm. All tissue samples taken selectively from fluorescing parts of these tumors corresponded to WHO grade III gliomas (Fig. 3a). In contrast, all tissue samples taken selectively from non--fluorescing parts of these tumors (five samples in the patient 1, four in patient 2, five samples in patient 4 and four in patient 5) corresponded to WHO grade II (Fig. 3b). The average proliferation rate assessed by counting the Ki-67 LI in fluorescing parts of tumors in patients 1, 2, 4, and 5 was 14.13% (SD 4.52; median 12). Average Ki-67 LI from nonflourescing part of these tumors was 2.67% (SD 1.58; median 3).
Fig. 3. 5-aminolevulic acid (5-ALA) fluorescing and nonfluorescing tissue taken from tumor in patient 2. Fig. 3a) 5-ALA nonfluorescing tissue, hematoxilin-eosin stained specimen (magnification 200×). Low tumor cell density, absence of mitotic activity. Fig. 3b) 5-ALA fluorescing tissue, hematoxilin-eosin stained specimen (magnification 400×). High tumor cell density, presence of mitotic activity (arrow). Fig. 3c) 5-ALA nonfluorescing tissue, immunohistochemically detected proliferation marker Ki-67 visible as nuclear staining (magnification 200×). Low proliferation rate. Fig. 3d) 5-ALA fluorescing tissue, immunohistochemically detected proliferation marker Ki-67 visible as nuclear staining (magnification 200×). Higher proliferation rate. 
In patient 5, two areas of weak (pink) fluorescence were found within the tumor in addition to intense red fluorescing foci. The samples taken from these parts were evaluated as WHO grade II. Ki-67 LI was 5% in both samples.
In patient 3, no intraoperative 5-ALA fluorescence was observed. All tumor tissue (seven samples) sent for histopathological examination proved to be grade II, including the sample taken selectively from the part with preoperative nonsignificant CE, localized with the use of neuronavigation (with obvious methodology-inherited risk of navigation inaccuracy). The mean Ki-67 LI from seven tissue samples taken from this tumor was 3% (range 1–4%).
Because of the presence of anaplastic foci, patients 1, 2, 4 and 5 were referred for oncological treatment after the surgery. Patient 3 is being managed expectantly with regular MRI follow up.
No new permanent postoperative neurological deficit and no adverse reaction after 5-ALA application were noted in our case series.
Discussion
Adult supratentorial infiltrative glioma is an incurable and finally fatal disease. However, the prognosis of various grades of supratentorial infiltrative gliomas differs substantially. Although adult supratentorial grade II gliomas (low-grade gliomas, LGG) ultimately undergo malignant transformation [5], they can remain as grade II for years. Patients harboring LGG can have relatively long life expectancy (10–15 years or more), and in majority of cases surgery is the treatment of choice [6], with no need for early oncological treatment. On the contrary, patients with high-grade gliomas (WHO grade III and IV, HGG) have significantly shorter life expectancy and they always require oncological treatment after tumor resection [7]. Hence, exact histopathological tumor grade determination is crucial for optimal treatment.
MRI CE in gliomas is traditionally explained as the result of blood-brain barrier disruption by neovascularization or direct tumor damage [8]. Gliomas with ring-like CE are usually HGG lesions [3]. Nodular--like MRI CE is a possible finding not just in HGG but also in LGG lesions. However, LGG with nodular-like CE were shown to have worse prognosis compared to LGG with nonsignificant or no CE [3].
Preoperative grade (and biological behavior) prediction in infiltrative gliomas with nonsignificant or no CE is challenging. Both LGG and HGG can have absent or nonsignificant CE [8], however, the probability of CE is higher in anaplastic than in grade II gliomas [8]. Newer MRI sequences may provide more accurate preoperative diagnosis – areas of elevated MRI perfusion on perfusion weighted image [9] or areas with high choline peak on MRI spectroscopy are usually suspicious for malignancy [10]. PET represents another possibility for preoperative tumor grade prediction [11]. However, despite advances in all aforementioned methods, the accurate preoperative differentiation between LGG and HGG (especially between grades II and III) is not always possible [8,11]. Therefore, preoperative identification of anaplastic foci can be arduous in some cases.
Postoperative histopathological diagnosis is hindered by the fact that gliomas are heterogeneous tumors and different parts of the same tumor can have different grade [12]. Tumors containing only a few small anaplastic foci within the bulk of LGG tissue are at a particular risk of histopathological undergrading (i.e. false negative evaluation as lower grade glioma) [5,12]. Undergrading is usually due to a sampling error. It is technically difficult to histopathollogically examine the entire specimen of glioma tissue, especially when the resected samples of tumor tissues are large. In such circumstances, a small size of anaplastic foci may be the reason for false negative evaluation as lower grade. The second reason for undergrading may be the fact, that some tumor tissue is almost always destroyed during surgery by suctioning or CUSA. If the small foci of anaplasia are not sent to histhopathological examination, the tumor may be misdiagnosed as LGG. Exact intraoperative localization based on preoperative investigation modalities during a surgery may be problematic even with the use of neuronavigation. Navigation inaccuracy caused by brain shift was reported to reach up to 2.4 centimeters [2].
Recently, a new method of intraoperative detection of anaplastic foci within infiltrative gliomas with nonsignificant CE was proposed by Widhalm et al [1]. The method is based on preoperative application of 5-ALA, and subsequent selective tissue collection from fluorescing areas throughout the tumor resection. After the oral ingestion, 5-ALA is taken up in HGG cells and converted into protoporphyrin IX. When illuminated under violet--blue light (wavelength 400 nm), the protoporphyrin IX in the HGG cells glows an intense red, while the normal brain tissue appears blue. This method of anaplastic foci detection is, unlike neuronavigation based on preoperative MR or PET investigation, unaffected by brain shift.
The positive effect of 5-ALA use during resection of HGG was shown in a randomized controlled multicenter phase III trial; its use leads to a significant increase of complete resections rate and improved prognosis [13]. In LGG, unlike in HGG, 5-ALA fluorescence was not reported. Stummer et al [14] reported a single patient with a partially anaplastic glioma. The tumor showed positive 5-ALA fluorescence in the central contrast-enhancing focus which was histologically proven to be anaplastic, but no 5-ALA fluorescence in the major tumor areas which corresponded to grade II [14]. However, the idea of routine using 5-ALA as a marker for detection of anaplastic foci within predominantly grade II infiltrative gliomas is new. In the pilot study of Widhalm et al, 17 patients were included [1]. Focal 5-ALA fluorescence was observed in eight of nine patients with gliomas postoperatively evaluated as WHO grade III (according to WHO 2007 criteria [4]). All eight of eight tumors postoperatively confirmed as WHO grade II were 5-ALA negative. Thus, all tumors with intraoperative 5-ALA fluorescence were classified as WHO grade III gliomas and all WHO grade II gliomas were intraoperatively found to be 5-ALA negative. Ki-67 LI was significantly higher in 5-ALA-positive foci than in nonfluorescent areas within the given tumor. Hence, the 5-ALA served as a marker for detecting anaplastic foci within diffusely infiltrating gliomas. One anaplastic oligodendroglioma did not show 5-ALA fluorescence. The autors speculated that, due to long tumor resection time, the photobleaching effect could be responsible for the 5-ALA negativity in this case. The positive predictive value of focal 5-ALA fluorescence for WHO grade III glioma was 100% (sensitivity 89%). The positive predictive value of a 5-ALA-negative tumor for WHO grade II glioma was 89% (sensitivity 100%).
As in the series published by Widhalm et al [1], histopathological examination of tissue samples collected from our small case series, revealed excellent correlation between tumor grade and tissue 5-ALA fluorescence. All tissue samples taken selectively from fluorescing tumor parts were evaluated as WHO grade III. In four cases exhibiting 5-ALA fluorescence, all samples taken from nonfluorescing tumor were evaluated as grade II. All tumor samples from the 5-ALA negative tumor (patient 3) were evaluated as grade II astrocytoma.
Since the finding of a solitary mitosis in a sample specimen does not confer grade III behaviour, separation of grade II from grade III tumors can be more reliably achieved by determination of Ki-67 LI [4]. The average Ki-67 LI are bellow 4% for grade II diffuse astrocytomas, bellow 5% for grade II oligodendrogliomas and bellow 6% for grade II oligoastrocytomas [4]. In our case series, all tissue samples taken from nonfluorescing tumor parts had average Ki-67 LI corresponding to grade II lesions (Fig. 3c). In contrast, average Ki-67 LI of tissue samples taken from fluorescing tumor parts corresponded to high - -grade tumors in all cases (Fig. 3d). The weak intensity of fluorescence in the pink fluorescing areas within the tumor in patient 5 correlated well with Ki-67 LI (5%), which was higher than the level of KI-67 LI of nonfluorescing tissue (average 2%) and lower than the level of KI-67 LI of fluorescing parts (average 11%) of the same tumor. Histopathological diagnosis of two samples taken from pink fluorescing areas of this tumor was grade II oligodendroglioma. The absence of 5-ALA fluorescence in patient 3 correlated well with the average Ki-67 LI (3%) corresponding to a low-grade lesion.
As a result of anaplastic foci identification, patients 1, 2, 4, and 5 were referred to oncological treatment after the surgery. Because of a small number of the fluorescing parts in these tumors, and because of their small size (all but one under 10 mm), we strongly believe that at least some of these lesions would be at a high risk of undergrading if 5-ALA was not used as a marker for anaplastic foci detection.
According to our results 5-ALA can be used as a useful marker in infiltrative gliomas with nonsignificant CE. However, our study is limited by the small number of patients followed. Larger series are necessary to confirm the effectiveness of using 5-ALA as a marker for anaplasia.
In our small case series 5-ALA was used for gliomas with nonsignificant CE only. However, nonsignificant CE is just one of MRI features indicating that glioma could be partially malignant; elevated MRI perfusion and “malignant” spectroscopic analysis are more reliable, as well as increased 18-FDG, 11C-methionine or 3‘-deoxy-3‘ - [(18)F]-fluorothymidine uptake on PET investigation [1,9–11,15]. A new study examinig whether 5-ALA can be used as a marker for anaplasia in all cases of suspected focal malignancy should be conducted.
Conclusions
According to the results of histopathological examination and proliferation rate of samples taken from five glioma cases, we conclude that 5-ALA may be a useful method for detecting anaplastic foci within infiltrative gliomas with nonsignificant CE.
Accepted for review: 1. 8. 2011
Accepted for publication: 4. 10. 2011
Andrej Šteňo, M.D.
Department of Neurosurgery
Derer’s Hospital, Comenius University
Limbová 5
833 04 Bratislava
e-mail: andrej.steno@gmail.com
Sources
1. Widhalm G, Wolfsberger S, Minchev G, Woehrer A, Krssak M, Czech T et al. 5-Aminolevulinic acid is a promising marker for detection of anaplastic foci in diffusely infiltrating gliomas with nonsignificant contrast enhancement. Cancer 2010; 116(6):1545–1552.
2. Nimsky C, Ganslandt O, Cerny S, Hastreiter P, Greiner G, Fahlbusch R. Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery 2000; 47(5):1070–1079.
3. Pallud J, Capelle L, Taillandier L, Fontaine D, Mandonnet E, Guillevin R et al. Prognostic significance of imaging contrast enhancement for WHO grade II gliomas. Neuro Oncol 2009; 11(2): 176–182.
4. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of tumours of the central nervous system. 4th ed. Lyon, France: IARC 2007
5. Perry A. Pathology of low-grade gliomas: an update of emerging concepts. Neuro Oncol 2003; 5(3): 168–178.
6. Šteňo A, Belan V, Kalina P, Fabian M, Šteňo J. Vplyv chirurgickej liečby na prognózu dospelých pacientov so supratentoriálnymi low-grade gliómami. Cesk Slov Neurol N 2011; 74/107(3): 273–278.
7. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008; 62(4): 753–764.
8. Essig M. Intracranial tumors. In: Reiser MF, Semmler W, Hricak H (eds). Magnetic resonance tomography. Berlin, Heidelberg: Springer 2008 : 243–309.
9. Belan V, Pružincová Ľ, Srbecký M. MR v diagnostike a liečbe primárnych nádorov mozgu. Onkológia (Bratisl.) 2010; 5(3): 138–141.
10. Wagnerová D, Urgošík D, Syrůček M, Hájek M. Využití kombinace metod magnetické rezonance pro diagnostiku tumorů. Cesk Slov Neurol N 2011; 74/107(2): 150–156.
11. Pročka V, Makaiová I, Šteňo J, Kalina P, Belan V, Pružincová Ľ et al. Skúsenosti s využitím 18FDG PET v diferenciálnej diagnostike low-grade a high-grade gliómov. Onkológia (Bratisl.) 2010; 5(3): 156–161.
12. Paulus W, Peiffer J. Intratumoral histologic heterogeneity of gliomas. A quantitative study. Cancer 1989; 64(2): 442–447.
13. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006; 7(5): 392–401.
14. Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 1998; 42(3): 518–525.
15. Price SJ, Fryer TD, Cleij MC, Dean AF, Joseph J, Salvador R et al. Imaging regional variation of cellular proliferation in gliomas using 3‘-deoxy-3‘-[18F]fluorothymidine positron-emission tomography: an image-guided biopsy study. Clin Radiol 2009; 64(1): 52–63.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2012 Issue 2-
All articles in this issue
- Postural Instability, Gait Disorders and Falls in Parkinson’s Disease
- Superior Temporal Sulcus and its Functions
- The Algorithm of CSF Examination according to the Reccomendation of the Committee of CSF and Neuroimmunology of the Czech Neurological Society
- The Use of Percutaneous Endoscopic Gastrostomy – Overview of Indications, Description of the Technique and Current Trends in Neurology
- Imaging Techniques to Evaluate Morphological Correlates of Cognitive Dysfunction in Multiple Sclerosis Patients
- Nutritional and Metabolic Disorders in Parkinson’s Disease
- Measuring of Cognitive Deficit after Cerebral Aneurysm Intervention
- Polysomnographic Findings in Children with Attention-Deficit//Hyperactivity Disorder Investigated for Sleep Disturbances
- Treatment of Neurogenic Detrusor Overactivity after Spinal Cord Injury Using Botulinum A Toxin. Comparison of Endoscopic Submucosal and Intramuscular Route of Application
- Our Experience with Lateral Supraorbital Approach in Surgery of Intracranial Aneurysms
- Visual Functions in Premature Children with Perinatal Brain Injury
- Obstructive Sleep Apnoe and CPAP – is it Reasonable to Solve Nasal Patency?
- Therapeutic Options for Prevention of Cerebrovascular Events in Patients with Carotid Stump Syndrome – Case Studies
- Oligosymptomatic Forms of Myotonic Dystrophy Type 2
- Reversal of Traumatic Pentaplegy after Combined C1–C2 Fracture
- Guideline for the Diagnosis and Therapy of Myasthenia Gravis
- Dementia Diagnosis and Treatment in Czech Neurological and Psychiatric Practices
- Endoscopic Third Ventriculostomy to Treat Hydrocephalus in Children with Brain Tumours – a Single Centre Experience
- Detection of Anaplastic Foci within Infiltrative Gliomas with Nonsignificant Contrast Enhancement using 5-aminolevulic Acid – a Report of Five Cases
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- The Use of Percutaneous Endoscopic Gastrostomy – Overview of Indications, Description of the Technique and Current Trends in Neurology
- Postural Instability, Gait Disorders and Falls in Parkinson’s Disease
- The Algorithm of CSF Examination according to the Reccomendation of the Committee of CSF and Neuroimmunology of the Czech Neurological Society
- Obstructive Sleep Apnoe and CPAP – is it Reasonable to Solve Nasal Patency?
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

