-
Medical journals
- Career
Postural Reflexes in Conditions of Visual Disturbance
Authors: K. Pawlak-Osińska 1,2; H. Kaźmierczak 3; W. Kaźmierczak 3
Authors‘ workplace: Nicolaus Copernicus University, Poland Collegium Medicum, Bydgoszcz 1; Nicolaus Copernicus University, Poland Department of Hearing and Vestibular Pathophysiology of the Chair of Otolaryngology, Bydgoszcz 2; Nicolaus Copernicus University, Poland Clinic of Otolaryngology of the Chair of Otolaryngology, Bydgoszcz 3
Published in: Cesk Slov Neurol N 2011; 74/107(6): 669-674
Category: Original Paper
Overview
Introduction:
Tests of balance are routine elements of neurological and otolaryngological examination. What follows is a general study of the effectiveness of balance control when visual-vestibular integrity is disturbed, conducted by means of ascertaining the influence of vertical and horizontal visual stimulation on postural reflexes as measured by craniocorpography.Goal:
The purpose of the study was to observe the effect of visual, horizontal and vertical optokinetic and sinusoidal stimulation on postural reflexes.Material and method:
A group of 40 healthy subjects (20 female, 20 male) aged 18–52 with no pathological otoneurologial history or signs was tested. Horizontal and vertical optokinetic and sinusoidal stimulation was administered in order to observe balance disturbances, as measured by craniocorpography in the course of a Romberg test.Results:
It was revealed that visual stimulation (optokinetic and sinusoidal) in the horizontal plane induced stronger postural disturbances than visual stimulation in the vertical plane. Both horizontal and vertical optokinetic stimulation resulted in high longitudinal body sway. Lateral body displacement was better controlled during vertical visual disturbances. The authors observed that the differences in postural reflexes were dependent on the direction of both horizontal and vertical visual stimulation. Balance control was better when the optokinetic incitement was directed to the left and downward. Increased intensity of visual stimulus (target velocity) did not worsen balance.Key words:
postural reflexes – balance –craniocorpography – visual stimulation – otolithsIntroduction
Tests of balance are routine elements of neurological and otolaryngological examination. The Romberg test and the Unterberger-Fukuda test (walking on the spot) are quick ways to examine the ability to maintain balance while standing and moving [1–3]. The Romberg test is not considered to be very sensitive – a little swaying is normal, but the range of body oscillations is not well-defined, and comparison of the deviations in position between the first and following examinations is difficult when the estimation is only approximate [1]. In the Unterberger-Fukuda test, a high number of inter - and intra-individual variations may be noted [4]. Among other balance assessment tools that allow differentiation of balance deficit, craniocorpography, is easy and quick, both as an ambulatory screening examination and as a component of more sophisticated vestibular research [5–7]. Adding some interesting parameters to routine analysis of the Romberg and Unterberger-Fukuda tests, craniocorpography is capable of measuring head stability (on the basis of torticollis angle) and a widened base of support while stepping (on the basis of shoulder oscillation) [8]. It can be also used for monitoring recovery and for balance training when sensory system integration is deliberately distracted, for example by additional visual stimulus [9].
It is known from Wood [10] and Dichgans et al [11] that visual optokinetic stimulation breaks up vestibulo-visual integration. The signs of travel sickness, without motion but after visual signal, were first described in 1895. Similarly, when the pseudo-Purkinje effect is provoked, real postural disturbances (apart from vegetative symptoms) may be observed. [12]. The irritation of otoliths and ocular motor signals following visual stimulation and burst neurons in the brainstem are believed to change the well-known efference copy, contributing to postural instability [12–15].
The authors of this study wished to ascertain the influence of vertical and horizontal visual stimulation on postural reflexes measured by craniocorpography, in order generally to study the effectiveness of balance control when visual-vestibular integrity is disturbed.
The aims of the investigation were:
- To observe balance control in healthy people on the basis of craniocorpography during a static test disturbed by visual stimulation (horizontal and vertical optokinetic stimulation and sinusoidal stimulation).
- To compare the types and intensities of visual deficiency that have the greater effects on postural control, the better to facilitate the process of rehabilitation.
Material and methods
A group of 40 healthy people (20 female, 20 male) aged 18–52 with no history of otoneurological abnormalities was recruited for testing. Firstly, postural balance was observed in the whole group and measured by craniocorpography in a static Romberg test (standing erect for 60 seconds, with feet together and the arms extended straight in front) with eyes closed, and a stepping Unterberger-Fukuda test (50 steps on the spot for 60 seconds) with eyes closed and both arms stretched forward.
Craniocorpography (Craniocorpograph, Zebris Medical GmbH, Germany) allows the measurement of trunk and head oscillations and turns by means of movement detectors fixed on the patient’s shoulders and on a helmet that is placed on the head (Fig. 1).
Fig. 1. Craniocorpography: movement detectors are fixed on the patient’s shoulders and on the helmet. 
Craniocorpography during the static Romberg’s test allows the following parameters to be evaluated: longitudinal body oscillations (cm), lateral body oscillations (cm), forehead area (cm2) and the angular deviation of the head in relation to the trunk (º). The Unterberger-Fukuda test was analysed during craniocorpography on the basis of: the track that the subject covered from the starting point (the patient is asked to step on the spot, but walks ahead without being aware of it) (cm), lateral sway of the shoulders when stepping (higher values for this measurement often indicate a wider base of support, as in cerebellar syndrome) (cm), angular deviation in the line of stepping from the start point (º), and angular deviation of the body (º) (Fig. 2).
Fig. 2. Craniocorpography: Unterberger-Fukuda test. 
After these tests, the trial group was divided into two equal subgroups to observe changes in postural balance when normal testing is disturbed by visual stimulation (this part of the test appears to have applications in the rehabilitation of vertigo patients). The observations of these two subgroups allow us to compare how a person controls balance during standing and walking when the visual receptor is completely excluded from postural control (first step of the study, described above) and when the vision is active, but disturbed. The subgroups were selected to avoid any mechanism of habituation should visual stimulation be frequently repeated [16]. To check whether the subgroups were compatible, statistical evaluation of differences between them was carried by means of Student’s t-test (Table 1).
1. Results of Romberg test and Unterberger test in terms of craniocorpography for two selected groups of healthy subjects. 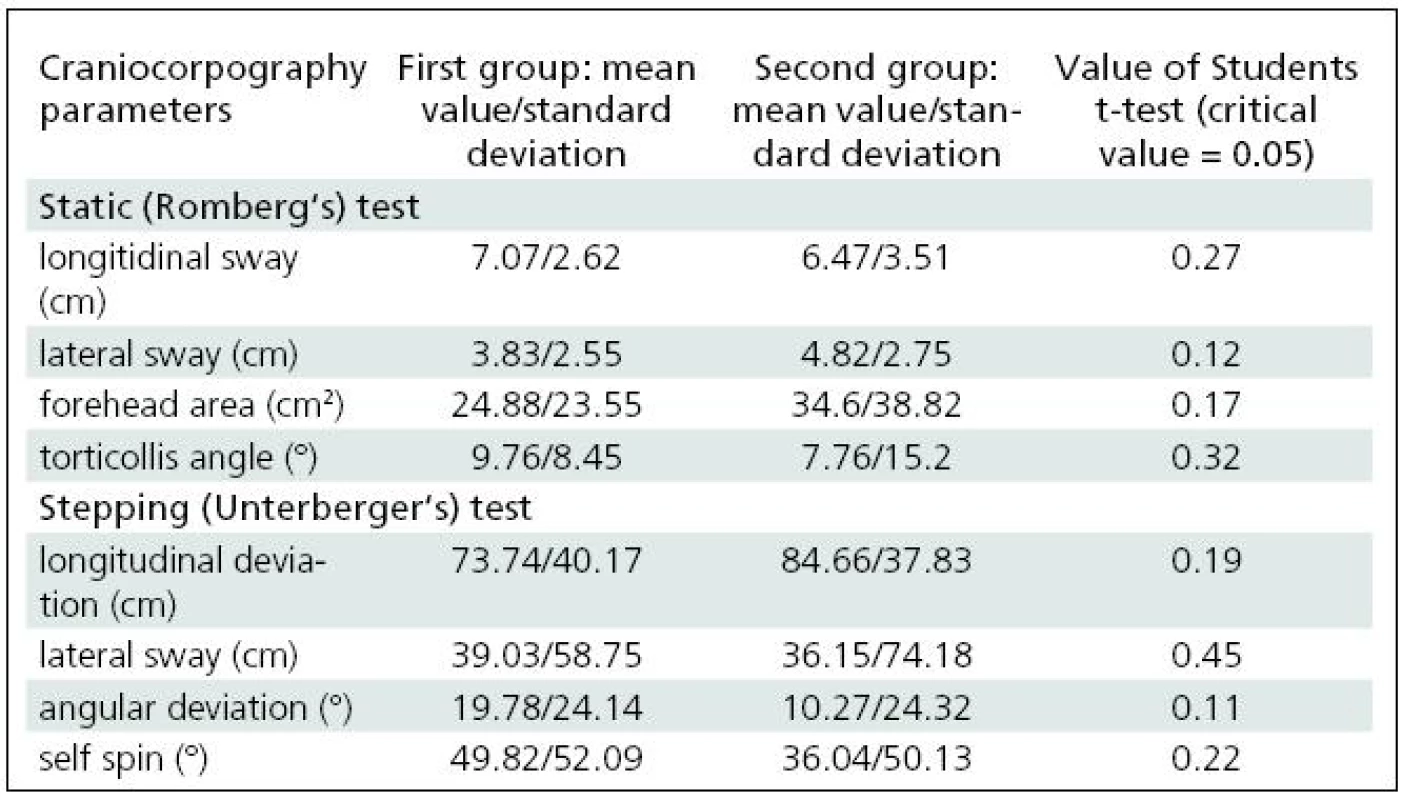
The first subgroup consisted of 20 people (10 female, 10 male) aged 18–46 (mean: 32.1). Horizontal (to the left, then to the right) optokinetic and sinusoidal horizontal stimulations took place with the patient standing with the hands extended ahead and eyes open. The patient was asked to follow a moving target presented two metres in front, with both eyes. Optokinetic and sinusoidal stimulation lasted for 60 seconds at velocities of 10º/s and 20º/s for optokinetic stimulation and 20º/s during the sinusoidal.
The second subgroup consisted of 20 people (10 female, 10 male) aged 18–52 (mean: 34.2). Balance was tested during the same standing test, with the eyes open, as in the first group, but with vertical rather than horizontal stimulation for 60 seconds, upwards then downwards at rates of 10º/s and 20º/s and sinusoidal vertical stimulation at a velocity of 20º/s.
The data were compared by Student’s t-test; the threshold for significance was set at <0.05.
Results
The two selected subgroups were compared in order to confirm that they were statistically identical. None of the craniocorpographical parameters either during the Romberg test or the Unterberger-Fukuda test were different (Table 1). The results of cranicorpography during static Romberg test on visual stimulation – horizontal and vertical – are presented in Table 2.
2. Results of craniocorpography during static test with eyes open and visual disturbance induced by horizontal and vertical optokinetic and sinusoidal stimulation. 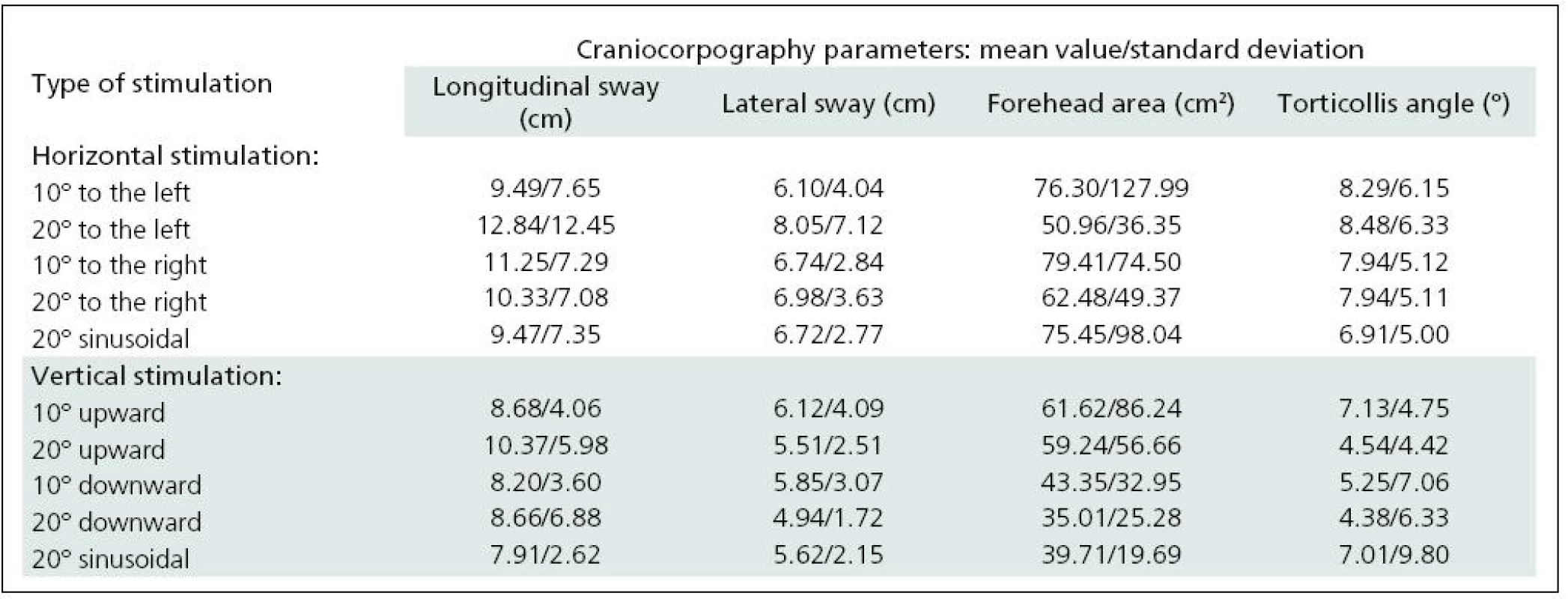
A comparison of the parameters derived from craniocorpography in the course of the various stimulations, optokinetic and sinusoidal, horizontal and vertical, was made. Table 3 presents the results of the Student’s t-test.
3. Comparison of craniocorpographical parameters during Romberg’s test when eyes are closed and under optokinetic and sinusoidal stimulation (significant differences in grey). 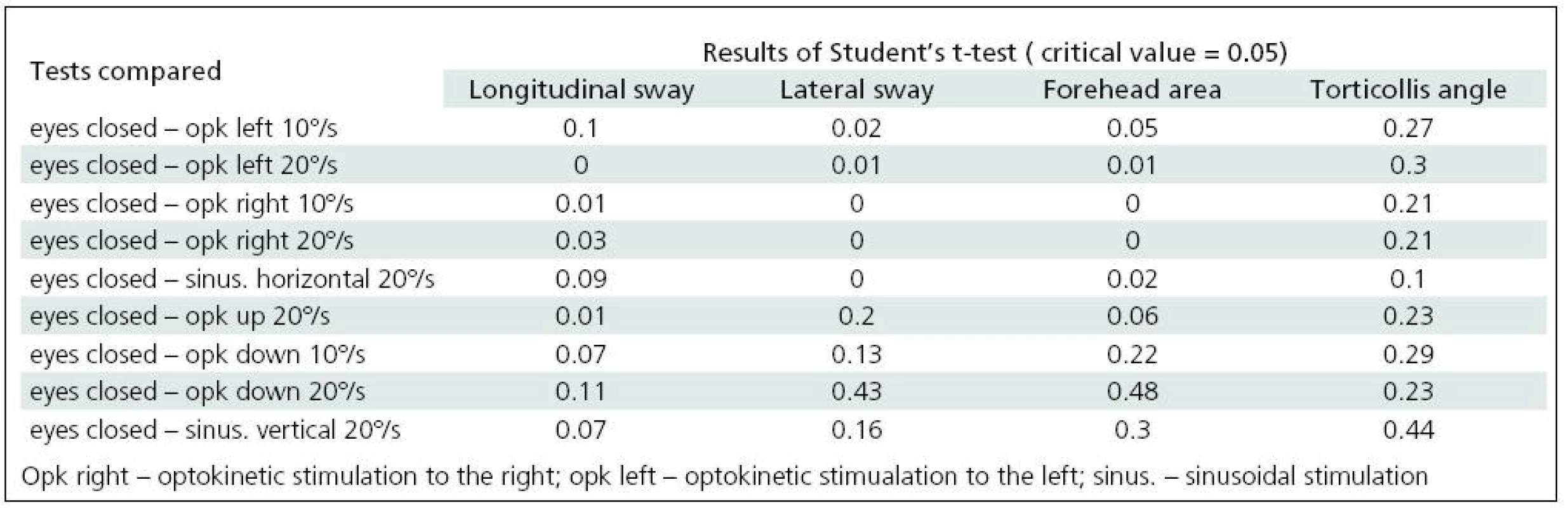
Significant difficulties in balance control were clearly evident when horizontal visual disturbance was applied. Both optokinetic and sinusoidal stimulation resulted in postural instability. Balance control was worst in the frontal plane. Lateral body sway was high for every type of disturbance: slow and fast optokinesis, both to the right and to the left and during sinusoidal impulse. Longitudinal sway and forehead area were disturbed (increased) during optokinetic stimulation to the left at a velocity of 20º/s and during optokinetic stimulation to the right at both 10º/s and 20º/s velocity. Torticollis angle remained normal during each horizontal stimulation. The only effect of vertical disturbance of visual field on postural control was an increase in longitudinal sway during upward optokinetical stimulation (Table 3). Differences in body balance were not observed when stimulation strength was increased (Table 4).
4. Comparison of craniocorpographical parameters during Romberg’s test when the intensity of visual stimulation is varied and when optokinetic and sinusoidal stimulation of the same velocity takes place (no significant differences). 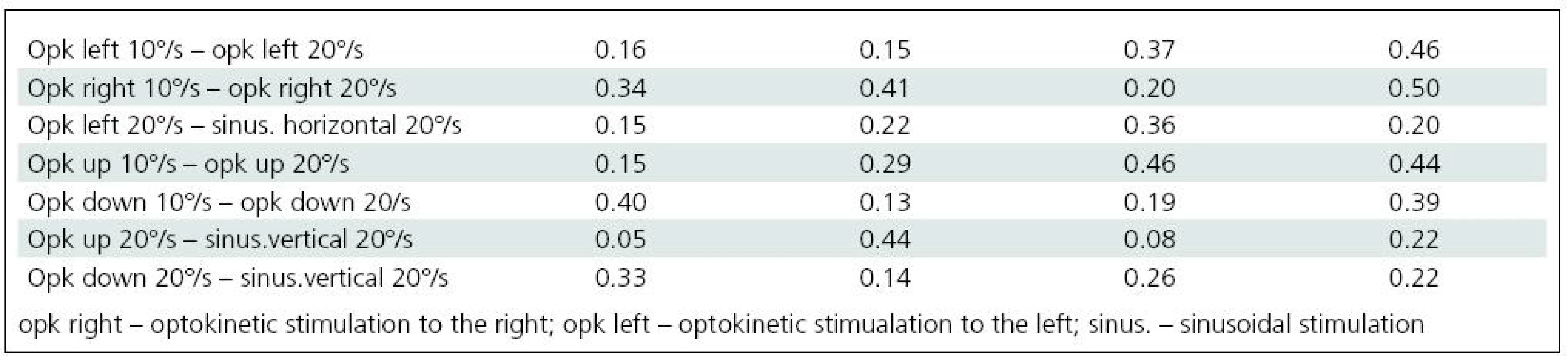
Discussion
The main purpose of the study was to observe the ability to maintain balance coordination when one of the essential receptors, the visual, is excluded or its function disturbed. Craniocorpography was chosen for quantitative and qualitative balance study [5,9]. It allows description of axis, direction and intensity in balance disturbance.
Our results indicated that visual disturbances have a negative effect on postural control. This supports observations by Glasauer et al [15] that eye movements increase sway and that suppression of the ocular motor component reduces postural imbalance. Intersensory interaction with remembering the previous efference copy is one reason for balance deficit: the drive to rebuild afference control (ocular, skeletal muscles) is the key to recovering equilibrium [13,17–19]. The active involvement of gravity – the essential role of the otoliths – may play a role in correcting this linkage, as will be explained below [17].
When visual failures are present, their control is essential for postural stability. A lack of balance control was clearly observed when visual stimulation was moving horizontally. Such visual field disturbance is followed largely by greater lateral sway, independently of whether it was stimulated to the right or to the left. Lateral sway was greater during sinusoidal stimulation.
Instability in the saggital axis was also observed during horizontal visual stimulation but was particularly associated with rightwards direction of disturbance. Optokinetic nystagmus to the left normally follows optokinetic stimulation to the right with a tendency to body declination to the slow phase of nystagmus. Right-handed people with suspected greater tonus of the right-side muscles may not be able to oppose the force drawing them to the right. An increase in balance disturbances – both lateral and longitudinal sway – are consequences of such a situation.
It was very interesting that vertical visual field disturbance (optokinetic and sinusoidal) generally affects balance only weakly. Vertical stimulation is not frequent in daily life. It might therefore be suspected that visual failure in this plane would cause deeper balance discoordination than horizontal visual field movement. This was not observed in our study. The effect of developing a major sensitivity to a frequently repeated stimulation (in the horizontal plane), such as the greater sensitivity of the vestibulo-sympathetic reflex in the familiar head-down prone position (exact horizontal position of the utricular maculas), may be a possible explanation [20]. Moreover, Murofushi et al [21] suggested that the utricle is more respondent to acute perturbation than the sacculus. Taking into consideration the utricular responsibility for subjective horizontal vision, its disorientation may play a role in the resultant increase in lateral postural sway. Further, one must bear in mind not only the direct influence of retinal scanning of visual field movement but also extra-ocular (afferent and efferent) memory implies sensitivity as a forerunner of executive body sway [15].
Slight differences were presented when comparing upward and downward vertical stimulation. The balance problems that occurred on upward optokinetic stimulation were assumed to have appeared because upward and downward optokinetic nystagmus in the physiological state are not equal (contrary to horizontal-induced optokinetic eye movements). Downward nystagmus in healthy individuals is stronger than upward nystagmus [22]. This may be explained by the role of the positions of the maculae with reference to gravity. According to our observations, balance control was worse (greater longitudinal sway) when optokinetic stimulation was set upward, creating optokinetic nystagmus beating downward. The increase in previously-existing asymmetry of eye movement control in the vertical plane was followed by an increase in balance disturbances. Crevits et al [23], using a vertical pendulum, observed that forward body sway is accompanied by upward eye deviation. In humans, the range of upward eye movement is wider than it is downward. The slow phase of nystagmus induced by an upward optokinetic target is directed upward, so the longitudinal body sway may be expected to be greater, as shown in our study.
The effect of greater longitudinal sway could be observed both during horizontal optokinetical stimulation and after vertical incitement. No lateral instability was registered during vertical visual disturbance. In general, lateral displacement was better controlled under various directions of visual disturbances than forward-backward body movement. Brandt et al [24] estimated that the cooperation of otoliths in the frontal plane is well-developed. When the otoliths from one side decrease impulse generation, their counterparts increase it in compensation, allowing the contraction of particular postural muscles to maintain balance. In the saggital plane, such contraction in reaction to the otoliths is slighter [24].
Consideration of the facts above leads to a list of several conditions that might be trained by means of craniocorpography with visual feedback. These are: unilateral labyrinth weakness with delayed compensation; lack of proprioceptive control; disturbance of visual input following, for example, constricted visual field or routine work with a machine in motion. Any condition in which cooperation of balance inputs is disrupted (even under physiological circumstances such as motion sickness), an improvement in postural control may be facilitated by use of individual, purpose-selected visual impulse generation. Craniocorpography appears to be good tool for monitoring the recovery.
Conclusions
- Visual stimulation (optokinetic and sinusoidal) in a horizontal plane induced stronger postural disturbances than visual stimulation in the vertical plane.
- Both horizontal and vertical optokinetic stimulation resulted in major longitudinal body sway; lateral body displacement was well-controlled during vertical visual disturbances.
- Differences in the influence of horizontal and vertical visual disturbances were noted: body control was better during the optokinetic stimulation to the left and downward.
- No significant effect of intensity of visual stimulus on balance was observed.
Assoc. Prof. Katarzyna Pawlak-Osińska, MD
11 Bratkowa Street
85-361 Bydgoszcz, Poland
e-mail: osinskak1@wp.pl
Accepted for review: 16. 9. 2010
Accepted for print: 9. 5. 2011
Sources
1. Van Allen MW, Rodnitzky RL. Pictorial manual of neurologic tests. Chicago London: Year Book of Medical Publishers Inc 1985.
2. Unterberger S. Neue registrierbare Vestibularis-Korperdreh-Reaktionen, erhalten durch Treten auf der Stelle. Der Tretversuch. Arch Ohr Nas Kehlk Heilk 1938; 140 : 273–282.
3. Haid CT. Schwindel aus interdisziplinärer Sicht. New York: Thieme Stuttgart 2003.
4. Kuipers-Upmeijer J, Oosterhuis HJ. Unterberger’s test not useful in testing of vestibular function. Ned Tijdschr Geneesks 1994; 138(3): 136–139.
5. Claussen CF. Craniocorpography a simple photo-optic registration method for vestibulo-spinal reactions. Z Laryngol Rhinol Otol 1970; 49(10): 634–639.
6. Hahn A, Sejna I, Stolbova K, Cocek A. Visuo-vestibular biofeedback in patients with peripheral vestibular disorders. Acta Otolaryngol 2001; 545 (Suppl): 88–91.
7. Mancini M, Horak FB. The relevance of clinical balance assessement tools of differentiate balance deficits. Eur Phys Rehabil Med 2010; 46(2): 239–248.
8. Alpini D, Ciavarro GL, Zinnato C, Andreoni G, Santambrogio GC. Evaluation of head-to-trunk control in whiplash patients using digital Craniocorpography during a stepping test. Gait Posture 2005; 22(4): 308–316.
9. Soto Varela A, Santos Pérez S, Vaamonde Lago P, Labella Caballero T. The usefulness of craniocorpography in the patients with dizziness and increasing muscle tension in neck. Acta Otorhinolaringol Esp 2001; 52(5): 398–403.
10. Wood RW. The haunted swing illusion. Psychol Rev 1895; 2 : 277.
11. Dichgans J, Brandt T. Visual-vestibular interaction: effects on self-motion perception and postural control. In: Held R, Leibowitz HW (eds). Handbook of sensory physiology. Berlin Heidelberg: Springer 1978.
12. Brandt T. Visual vertigo and acrophobia. In: Dix MR, Hood JD (eds). Vertigo. Chichester: Wiley 1984.
13. Jahn K, Strupp M, Krafczyk S, Schuler O, Brandt T. Suppression of eye movements improves balance. Brain 2002; 125(9): 2005–2011.
14. Ramat S, Leigh RJ, Zee DS, Optican LM. Ocular oscillations generated by coupling of brainstem excitatory and inhibitory saccadic burst neurons. Exp Brain Res 2005; 160(1): 89–106.
15. Glasauer S, Schneider E, Jahn K, Strupp M, Brandt T. How the eyes move the body. Neurology 2005; 65(8): 1291–1293.
16. Pfaltz CR. Vestibular habituation and central compensation. Adv Otorhinolaryng 1977; 22 : 136–142.
17. Mergner T, Rosemeier T. Interaction of vestibular, somatosensory and visual signals for postural control and motion perception under terrestrial and microgravity conditions – a conceptual model. Brain Res Rev 1998; 28(1–2): 118–135.
18. Macpherson JM, Horak FB, Dunbar DC, Dow RS. Stance dependence of automatic postural adjustments in humans. Exp Brain Res 1989; 78(3): 557–566.
19. Henry SM, Fung J, Horak FB. Effect of stance width on multidirectional postural responses. J Neurophysiol 2001; 85(2): 559–570.
20. Sauder CL, Leonard O, Ray CA. Greater sensitivity of the vestibulo-sympathetic reflex in the upright posture in humans. J Appl Physiol 2008; 105(1): 65–69.
21. Murofushi T, Ushio M, Takai Y, Iwasaki S, Sugasawa K. Does acute dysfunction of saccular afferents affect the subjective visual horizontal in patients with vestibular neurolabyrinthitis. Acta Otolaryngol 2007; 559 (Suppl): 61–64.
22. Pawlak-Osinska K. The examination of the influence of the macular perception on the vertical optokinetic nystagmus. Bydgoszcz: CpiR ReMedia 2005.
23. Crevits L, Reynaert C. Posture dependent direction reversal of spontaneous vertical nystagmus. Neuro Opht 1991; 11(5): 285.
24. Brandt T, Dietrich M. Vestibular falls. J Vestib Res 1993; 3 : 3.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2011 Issue 6-
All articles in this issue
- Surgical Treatment of Brachial Plexus Injury
- Questionnaires of Activities of Daily Living in Patients with Alzheimer Disease
- Parkinsonian Phenotypes – towards New Nosology of Atypical Parkinsonian Syndromes
- Updated Insight into the Pathophysiology of Migraine – an Update
- Motor Aspects of Speech Imparment in Parkinson‘s Disease and their Assessment
- Tractography in Neuronavigation during Intraaxial Brain Tumour Surgery Near Corticospinal Tract
- Amendment of the Czech Addenbrooke’s Cognitive Examination (ACE-CZ)
- Miller Fisher Syndrome – Four Case Reports and Review of Current Concept
- Parkinson’s Disease with a Phenotype of Progressive Supranuclear Palsy – a Case Report
- Cognitive and Emotional Changes Five Years after SAH – a Case Report
- Amisulpride in the Treatment of Schizophrenia
- The Reasons and the Process of Amendment of the Czech Addenbrooke’s Cognitive Examination (ACE-CZ)
- Postural Reflexes in Conditions of Visual Disturbance
- Extension of the Therapeutic Time Window for Intravenous Thrombolysis Should Not Lead to Prolongation of Door-to-Needle Time
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Miller Fisher Syndrome – Four Case Reports and Review of Current Concept
- Updated Insight into the Pathophysiology of Migraine – an Update
- Amendment of the Czech Addenbrooke’s Cognitive Examination (ACE-CZ)
- Surgical Treatment of Brachial Plexus Injury
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

