-
Medical journals
- Career
Is Clinical - Diffusion Mismatch Associated with Good Clinical Outcome in Acute Stroke Patients Treated with Intravenous Thrombolysis?
Authors: D. Šaňák 1; D. Horák 2; M. Král 1; R. Herzig 1; J. Zapletalová 3; D. Školoudík 1; T. Veverka 1; A. Bártková 1; I. Vlachová 1; S. Buřval 2; M. Heřman 2; P. Kaňovský 1
Authors‘ workplace: Iktové centrum, Neurologická klinika LF UP a FN Olomouc 1; Radiologická klinika LF UP a FN Olomouc 2; Ústav biometrie a statistiky, LF UP v Olomouci 3
Published in: Cesk Slov Neurol N 2009; 72/105(6): 548-552
Category: Original Paper
Overview
Background and purpose:
Mismatch between stroke severity, assessed by the National Institutes of Health Stroke Scale (NIHSS), and the infarction volume on MRI - DWI (Clinical - Diffusion Mismatch; CDM) may predict follow‑up infarction expansion and early neurological deterioration. The aim was to compare infarction growth, clinical outcomes and incidence of symptomatic intracerebral hemorrhage (sICH) in acute ischemic stroke (IS) patients presented with and without CDM treated with intravenous thrombolysis (IVT) within three hours.Patients and methods:
The set of 125 consecutive hemispheric IS patients (78 males, mean age 66.0 ± 12.1 years) were retrospectively analyzed. CDM was defined as NIHSS ≥ 8 and DWI volume ≤ 25 ml, non‑mismatch as NIHSS ≥ 8 and DWI volume > 25 ml. Infarction volume was measured on admission and after 24 hours. Neurological deficit was evaluated using NIHSS on admission and 24 hours later and the 90-day clinical outcome using modified Rankin Scale (mRS). Mann‑Whitney, Fischer Exact, Chi - Square tests and multiple stepwise linear regression analysis were used for statistical evaluation.Results:
Sixty - one (48.8%) patients presented with CDM and 31 (26.4%) with non‑CDM. Non - CDM patients had significantly higher infarct growth (p < 0.0001) after 24 hours. CDM patients had significantly greater decrease in NIHSS after 24 hours (p = 0.005) and better 90 - day clinical outcome (median mRS 1) than non‑CDM patients (median mRS 5, p < 0.0001). Non - CDM patients (versus CDM) presented with higher incidence of sICH (16.1 versus 0%, p = 0.003) and mortality after IVT (15.8 versus 0%, p = 0.0001).Conclusions:
Patients with CDM before IVT had significantly better clinical outcome than those without CDM.Key words:
ischemický iktus – intravenózní trombolýza – magnetická rezonanceAcknowledgment: This study was supported by the Internal Grant Agency of Ministry of Health of Czech Republic, grant number NR/7985-3/2005 and partially by the Ministry of Education Czech Republic, grant number MSM6198959216.
Introduction
The use of intravenous thrombolysis (IVT) in acute ischemic stroke (IS) patients is recently limited to the 4.5‑hour time window [1,2]. More accurate selection strategies are required, if time window should be extended. The magnetic resonance imaging (MRI) mismatch between the perfusion weighted imaging (PWI) and the smaller diffusion‑weighted imaging (DWI) lesions could indicate potentially salvageable ischemic brain tissue in the risk of infarct growth [3–9]. Patients with PWI/DWI mismatch may have higher benefit from thrombolytic therapy beyond standard therapeutic time window than patients without mismatch [10–14]. However, PWI is time‑consuming and not yet standardized technique and the optimal threshold for the calculation of the volume of the hypoperfused tissue at risk of infarction have not been established [14–17]. Moreover, DWI changes do not represent only irreversible infarct lesion, but also tissue with reversible energy dysfunction [8,18,19].
The stroke severity evaluated by the National Institutes of Health Stroke Scale (NIHSS) was recognized as a predictor of chronic functional outcome after ischemic stroke [20,21]. Baseline NIHSS correlates more closely with the acute abnormal PWI than DWI lesion volume [22,23]. On the basis of this assumption, Daválos et al showed that mismatch between the stroke severity (assessed by NIHSS) and the DWI infarct volume predicts follow‑up infarct expansion and early neurological deterioration. An NIHSS ≥ 8 and DWI infarct volume ≤ 25ml was defined as a clinical‑diffusion mismatch (CDM) [24]. Prosser et al found that CDM predicted PWI/DWI mismatch with high specificity and moderately-high sensitivity [25].
The aim was to compare the infarct growth, clinical outcome and incidence of intracerebral hemorrhage (ICH) in acute (IS) patients with and without CDM treated with IVT within 3-hour time window.
Patients and methods
A retrospective single centre study was used. The set consisted of 125 consecutive patients (78 males, mean age 66.0 ± 12.1 years) with acute hemispheric IS admitted and treated with IVT at our stroke unit between September 2004 and June 2008.
On admission, blood pressure was measured, electrocardiogram was recorded, and blood samples were taken. Clinical status was evaluated using the NIHSS by a certified neurologist. An MRI examination followed immediately in all patients. No additional computed tomography was performed.
The MRI was performed on a Magnetom Symphony 1.5‑T Maestro Class (Siemens, Erlangen, Germany) with quantum gradients (syngo2004A) and a standard head coil (CP head array coil). Protocol contained the following sequences: 1. localizer, 2. T2‑weighted turbo spin echo (TSE), 3. fluid‑attenuated inversion recovery (FLAIR), 4. diffusion‑weighted imaging (DWI), 5. 3‑D time of flight magnetic resonance angiography (TOF MRA). The total acquisition time (AT) was 11 min 28 s. Sequences 2–4 were applied to acquire data from the same set of slices (standard number of slices 19, slice thickness 5mm, distance factor 30%). The standard slice orientation was oblique axial, approximately parallel to skull base in order to minimize susceptibility artifacts in echo‑planar imaging (EPI) sequences. The sequence parameters were as follows: T2‑TSE TR/TE/ETL 4,000/99/9 ms, FOV 230 × 173mm, matrix 256 × 256, AT 1 min 34 s; FLAIR 8,050/112/ETL 21/2 concatenation, FOV 230mm, FOV phase 76.6%, matrix 256 × 151, AT 2 min 26 s. These sequences were used to assess hemorrhage and detect local demyelination changes including sites of ischemic demyelination. The EPI‑DWI sequence parameters were as follows: 3,200/94/EPI factor 128/3 averages, FOV 230 × 230mm, matrix 128 × 128 with interpolation, TA 1 min 20 s. MRI data were acquired with three diffusion weightings: b = 0, DWI b = 500, and DWI b = 1,000. The fourth type of image was an automatically created apparent diffusion coefficient (ADC) map. DWI traces show average local diffusivity in the brain tissue examined when b is 500 and 1,000. This sequence was applied to assess hemorrhage (b = 0: T2*‑EPI, susceptibility-sensitive sequence) and detect sites of reduced diffusion (DWI, b = 500 and 1,000). The 3D‑TOF MRA sequence parameters were as follows: 43/7.15, 3 slabs, 32 partitions/slab, slice thickness 1mm, FOV 200 × 150mm, matrix 512 × 192, AT 5 : 59 min. The images obtained – maximum intensity projection (MIP) and sublayers – would illustrate closure of the main arterial trunk of the circle of Willis or its branches. Infarct volumes were measured on DWI trace images (b = 1,000) and calculated as total hyperintense area in single slices multiplied by effective slice thickness [(actual slice thickness + distance factor)/interslice gap].
All patients underwent standard IVT within a 3-hour time window since stroke onset according to the guidelines valid in the years 2004–2008 [26].
Neurological deficit was evaluated using the NIHSS after 24 and 72 hours, and the 90‑day clinical outcome using modified Rankin Scale (mRS). MRS score 0–2 was considered as good clinical outcome. Follow‑up MRI was performed after 24 hours to evaluate the infarct volume changes and ICH occurrence. Symptomatic ICH was defined as a parenchymal hematoma (PH1 or PH2 type), associated with an increase in ≥ 4 points on the NIHSS score [27].
CDM was defined as NIHSS ≥ 8 and DWI volume ≤ 25ml [22]. Non‑mismatch was defined as NIHSS ≥ 8 and DWI > 25ml. Patients with baseline NIHSS < 8 were excluded from comparison analysis because of previously reported facts, that these patients have high frequency of spontaneous functional recovery without cortical perfusion deficits [28] and post‑hoc analysis showed that almost all these patients had baseline DWI lesion volume ≤ 25ml [24].
According to presence/absence of CDM, patients were divided into two groups.
SPSS software version 10.1 (SPSS Inc., Chicago, USA) was used for statistical analysis. Two‑group comparison of demographic and baseline clinical data, 24‑hour neurological outcomes and infarct growth was performed using Mann‑Whitney test for non‑parametric values. 90‑day clinical outcomes were dichotomized (mRS 0–2 vs 3–6) and compared using Chi‑square test. Fisher’s exact test was used for comparison of the ICH incidence. Multiple stepwise linear regression analysis was used to evaluate if the presence/absence of mismatch and initial NIHSS may predict follow‑up infarct growth.
Results
Out the analyzed 125, 61 (48.8%) patients presented with CDM and 31 (24.8%) with non‑CDM. Thirty-three (26.4%) patients (22 males, mean age 64.2 ± 12.6 years) with baseline NIHSS < 8 points (median NIHSS 6 pts) were excluded from analyzed set due to reasons mentioned in Methods.
Demographic and baseline data of the analyzed patients are showed in Table 1. The age was not significantly different in both groups, while the baseline NIHSS score was significantly higher in non‑CDM group. No difference was found between groups in the time interval from stroke onset to MRI examination and to IVT administration and also in number of arterial occlusions detected on baseline MRA (Table 1).
1. Patient demographic and baseline characteristic. 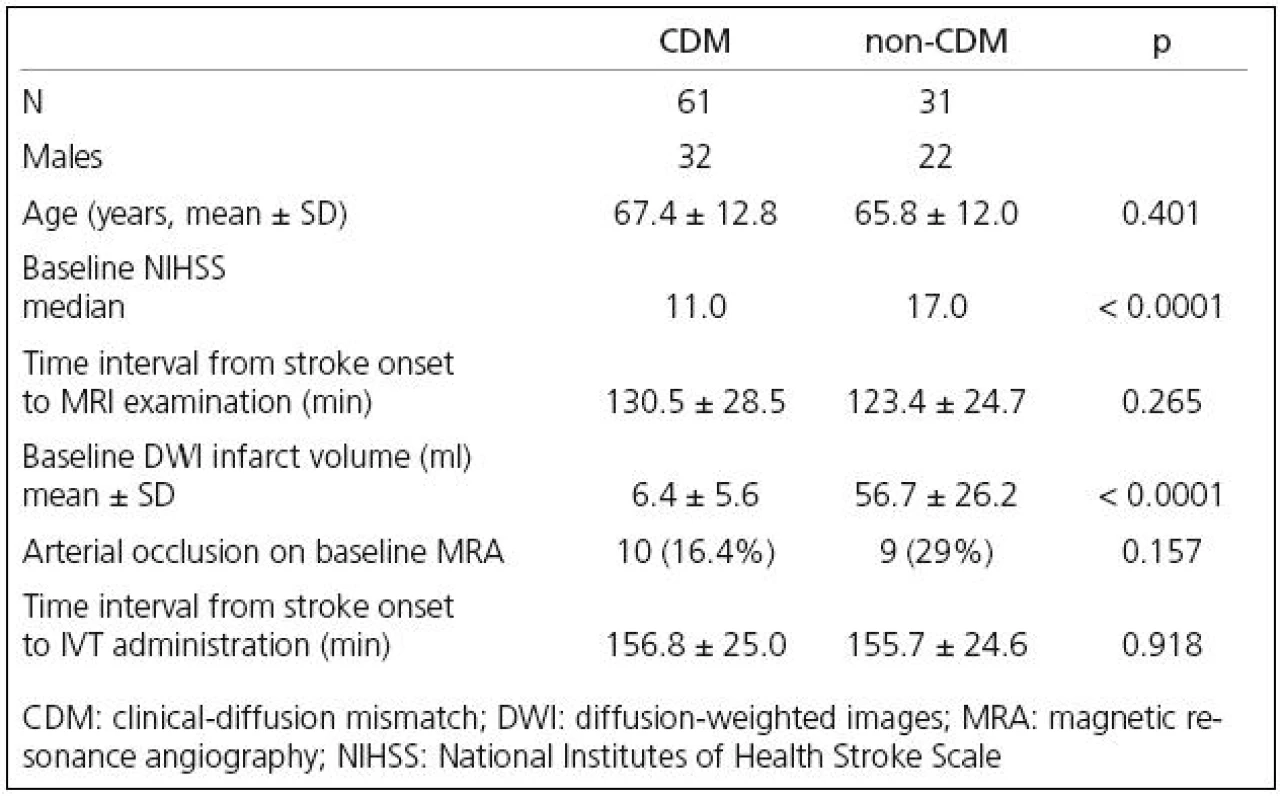
As presented in Table 2, patients with CDM had significantly higher regression of neurological deficit after 24 hours when compared to non‑CDM patients, while patients without CDM had significantly higher infarct growth after 24 hours. The rate of arterial recanalizations was significantly higher in non‑CDM group on the follow‑up MRA after 24 hours (Table 2).
2. Patient clinical outcomes, infarct growth and incidence of ICH. 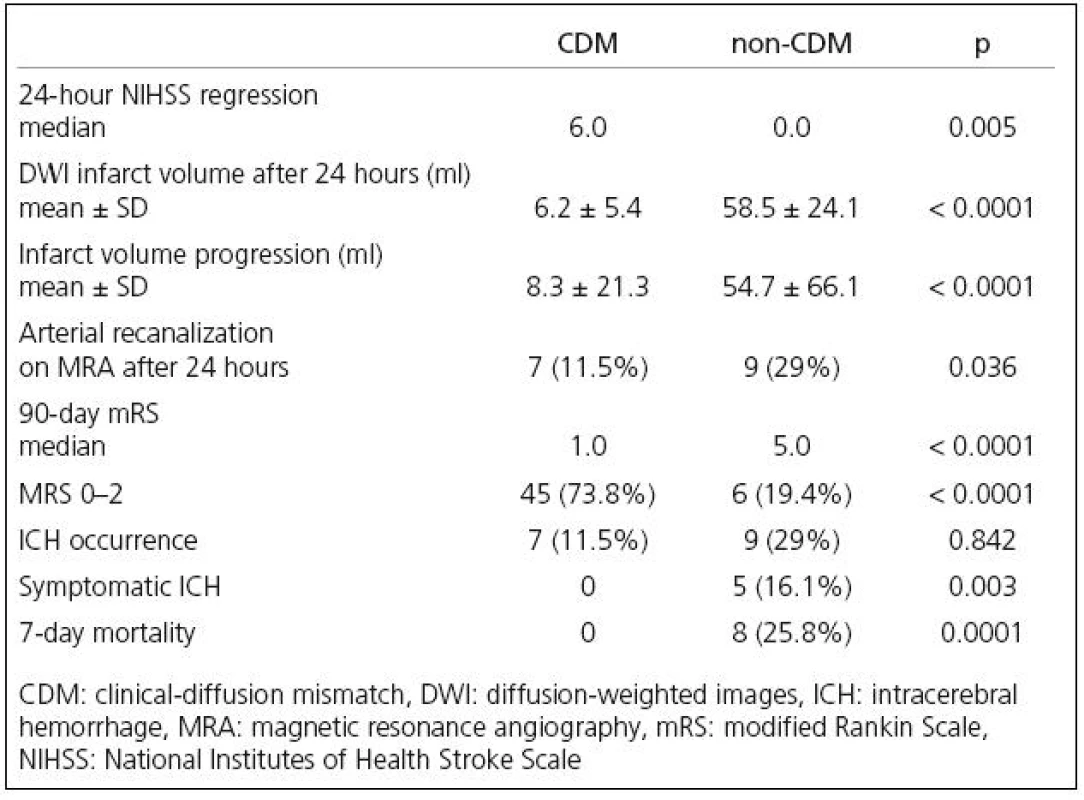
CDM patients had significantly better 90‑day clinical outcome including the percentage of patients with good clinical outcome (Table 2).
No significant difference was found in the ICH occurrence after IVT, while the incidence of symptomatic ICH was significantly higher in non‑CDM patients (Table 2).
The 7‑day mortality was significantly higher in non‑CDM group (0 vs 25.8%).
Multiple stepwise linear regression analysis showed that absence of mismatch was associated significantly with infarct volume increase of 27.4ml (p = 0.034) and the increase by 1 point in baseline NIHSS was associated with infarct volume increase of 2.9ml (p = 0.047).
Discussion
This study demostrated, that presence of CDM before IVT was associated with better clinical outcome after thrombolysis when compared to the absence of CDM. The rate of occluded arteries on baseline MRA was simillar in both groups and the number of arterial recanalizations on follow‑up MRA was even higher in patient group without CDM.
We found that CDM was present in 48.8% of our IVT treated patients within 3 hours of symptom onset, while Lansberg et al found CDM in 62% of patients between 3 and 6 hours since stroke onset in the DEFUSE trial [29]. The different number of CDM patients in presented study might be caused by a lower number of presented occluded arteries of Willis circle on baseline MRA (20.7%) when compared to the DEFUSE trial (66%) [29]. Different time interval between stroke onset to MRI could also play some role. Daválos et al [24] found CDM in 52% of patients examined within 12 hours since stroke onset and Prosser et al [25] found CDM in 42% of acute IS patients examined within 24 hours.
Our results showed that the CDM absence before IVT was associated with follow‑up infarct growth and poor outcome after thrombolysis. On the contrary, Lansberg et al found no association between the CDM and the favorable clinical response after IVT performed between 3 and 6 hours since stroke onset [29]. However in our study, patients treated within 3 hours only were analyzed and different parameters of clinical response after reperfusion therapy were defined when compared to the study by Lansberg et al: 1. change in NIHSS after 24 hours (in the DEFUSE study favorable clinical response was defined as an improvement ≥ 8 points or more in NIHSS between baseline and 30 days), 2. good clinical outcome after 90 days was defined as a score 0–2 in mRS (in the DEFUSE study mRS score as an 0–1 at 30 days after stroke onset), 3. infarct growth and 4. incidence of sICH [30].
Although the PWI/DWI mismatch is largely used in stroke studies and clinical routine, this concept is still considered controversial. Several different definitions of PWI/DWI mismatch are established [3–6,31] and the limitation of accuracy of PWI technique is still being discussed [32]. Prosser et al compared directly CDM and PWI/DWI mismatch in acute IS patients and found statistically significant agreement between CDM and PWI/DWI mismatch with very high specificity and positive predictive value [25]. On the contrary, Lansberg et al showed no agreement between both concepts in patients treated with IVT between 3 and 6 hours after stroke onset [29]. The recent report of Ebinger et al showed no increased benefit from IVT in patients with CDM treated between 3 and 6 hours and similar effects of reperfusion in patients with and without CDM [33].
Mismatch between the clinical deficit and the computed tomography (CT) findings has been suggested as a possible alternative approach [34,35]. Kent et al have recently found that clinical‑CT mismatch using the Alberta Stroke Program Early CT‑Score (ASPECTS) does not reliably identify patients more or less likely benefit from IVT [36]. Also Messé et al showed that mismatch between the ischemic changes on head CT (ASPECTS score) and clinical examination findings did not correlate with the MRI PWI/DWI mismatch [37].
The higher occurrence of ICH in presented non‑CDM patients may be attributed to the fact that initial infarct volume is considered to be an independent predictor of subsequent spontaneous infarct hemorrhagic transformation and also for ICH growth after thrombolysis [38].
The fact, that severe strokes and higher initial infarct volume did not correlate with number of arterial occlusions on baseline MRA in presented study (no difference in number of occluded arteries between groups), may document an important role of the actual state of collateral flow. The state of pretreatment collateral flow is considered as a predictor of infarct volume after thrombolysis [39,40] and may protect brain tissue reducing the volume and intensity of hypoperfusion [41].
The achieved higher recanalization rate in non‑CDM patients after IVT could be explained by different etiology of arterial occlusion in presented study (e.g. fresh cardiac embolus versus atherothrombotic occlusion). This explanation may support also the fact, that strokes caused by cardiac embolisation (atrial fibrillation) have more severe initial neurological deficits and poorer clinical outcomes after IVT [42].
Our study was retrospectively based in a selected patient population from a single center. Patients with CDM had significantly lower baseline NIHSS than patients without CDM. This baseline NIHSS score indifference is partially caused by the initial infarct volume and by its cut‑off value of CDM (25ml).
All patients with initial NIHSS < 8 were excluded from the analysis because of known high spontaneous recovery rate, although the presence of possible cortical perfusion deficit on PWI in these patients was not evaluated [28].
The quantification of infarct volume was performed manually, because no semiautomatic quantification software was available. Therefore the quantification could have been affected by subjective operator error. The T2* (susceptibility) sequence used in this study was the sequence automatically generated from EPI‑DWI software available in MRI machine used (Siemens Symphony). Although this sequence (in b‑0 images) had only some susceptibility effect, and it had limited spatial resolution (128 × 128 matrix), it was used to reduce the time duration of proper examination. The acute stroke patients are usually clinically unstable and MRI examination could be affected by the movement artifacts when T2* sequence with maximum possible susceptibility effect is used. According to author‘s opinion, the safe detection of intracranial hemorrhage (including hemorrhagic transformation of infarction) is possible using the combination of EPI‑DWI (b-0) and other sequences of the protocol (T2 and FLAIR).
Although the score 0–1 of modified Rankin Scale is widely used as an interval for classification of good clinical outcome after thrombolysis, we used the score 0–2 according to register SITS‑MOST [43].
Conclusion
Presented results showed that patients presenting with CDM before thrombolysis had better clinical outcome than those without CDM. This may support the concept that the presence/absence of CDM may contribute to predict the clinical response to intravenous thrombolysis. Nevertheless a large prospective trial with randomized selection between the CDM and the PWI/DWI mismatch would be needed to determine the optimized mismatch criteria.
MUDr. Daniel Šaňák, Ph.D.
Iktové centrum
Neurologická klinika
LF UP a FN Olomouc
I. P. Pavlova 6, 77520 Olomouc
e‑mail: daniel.sanak@centrum.czPřijato k recenzi: 17. 8. 2009
Přijato do tisku: 22. 9. 2009
Sources
1. European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008; 25(5): 457 – 507.
2. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359(13): 1317 – 1329.
3. Barber PA, Parsons MW, Desmond PM, Bennett DA, Donnan GA, Tress BM et al. The use of PWI and DWI measures in “proof ‑ of ‑ concept” stroke trials. J Neuroimaging 2004; 14(2): 123 – 132.
4. Chalela JA, Kang DW, Luby M, Ezzeddine M, Latour LL, Todd JW et al. Early magnetic resonance imaging findings in patients receiving tissue plasminogen activator predict outcome: Insights into the pathophysiology of acute stroke in the thrombolysis era. Ann Neurol 2004; 55(1) 105 – 112.
5. Hacke W, Albers G, Al Rawi Y, Bogousslavsky J, Dávalos A, Eliasziw M et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI‑based 9 ‑ hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 2005; 36(1): 66 – 73.
6. Ribo M, Molina CA, Rovira A, Quintana M, Delgado P, Montaner J et al. Safety and efficacy of intravenous tissue plasminogen activator stroke treatment in the 3 ‑ to 6 ‑ hour window using multimodal transcranial Doppler/ MRI selection protocol. Stroke 2005; 36(3): 602 – 606.
7. Derex L, Nighoghossian N, Hermier M, Adeleine P, Berthezène, Philippeau F et al. Influence of pretreatment MRI parameters on clinical outcome, recanalization and infarct size in 49 stroke patients treated by intravenous tissue plasminogen activator. J Neurol Sci 2004; 225(1 – 2): 3 – 9.
8. Schlaug G, Benfield A, Baird AE, Siewert B, Lövblad KO, Parker RA et al. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology 1999; 53(7): 1528 – 1537.
9. Schellinger PD, Fiebach JB. The penumbra and the mismatch concept. In: Fiebach JB, Schellinger PD (eds). Stroke MRI. Darmstadt: Steinkopff Verlag 2003 : 31 – 34.
10. Alberts GW. Expanding the window for thrombolytic therapy in acute stroke. The potential role of acute MRI for patient selection. Stroke 1999; 30(10): 2230 – 2237.
11. Parsons MW, Barber PA, Chalk J, Darby DG, Rose S, Desmond PM et al. Diffusion ‑ and perfusion weighted MRI response to thrombolysis in stroke. Ann Neurol 2002; 51(1): 28 – 37.
12. Röther J, Schellinger PD, Gass A, Siebler M, Villringer A, Fiebach JB et al. Effect of intravenous thrombolysis on MRI parameters and functional outcome in acute stroke 6 hours. Stroke 2002; 33(10): 2438 – 2445.
13. Hacke W, Brott T, Caplan L, Meier D, Fieschi C, von Kummer R et al. Thrombolysis in acute ischemic stroke: controlled trials and clinical experience. Neurology 1999; 53 (Suppl 4): S3 – S14.
14. Shih LC, Saver JL, Alger JR, Starkman S, Leary MC, Vinuela F et al. Perfusion ‑ weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke 2003; 34(6): 1425 – 1430.
15. Røhl L, Ostergaard L, Simonsen CZ, Vestergaard ‑ Poulsen P, Andersen G, Sakoh M et al. Viability thresholds of ischemic penumbra of hyperacute stroke defined by perfusion ‑ weighted MRI and apparent diffusion coefficient. Stroke 2001; 32(5): 1140 – 1146.
16. Butcher K, Parsons M, Baird T, Barber A, Donnan G, Desmond P et al. Perfusion thresholds in acute stroke thrombolysis. Stroke 2003; 34(9): 2159 – 2164.
17. Thijs VN, Somford DM, Bammer R, Robberecht W, Moseley ME, Albers GW. Influence of arterial input function on hypoperfusion volumes measured with perfusion ‑ weighted imaging. Stroke 2004; 35(1): 94 – 98.
18. Fiehler J, Foth M, Kucinski T, Knab R, von Bezold M, Weiller C et al. Severe ADC decreases do not predict irreversible tissue damage in humans. Stroke 2002; 33(1): 79 – 86.
19. Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G et al. Diffusion ‑ perfusion MR evaluation of perihematomal injury in hyperacute intracerebral hemorrhage. Neurology 2001; 57(11): 2015 – 2021.
20. Frankel MR, Morgenstern LB, Kwiatkowski T, Lu M,Tilley BC, Broderick JP et al. Predicting prognosis after stroke: a placebo group analysis from the National Institute of Neurological Disorders and Stroke rt ‑ PA Stroke Trial. Neurology 2000; 55(7): 952 – 959.
21. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJA, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19(5): 604 – 607.
22.Tong DC, Yenari MA, Albers GW, O’Brien M, Marks MP, Moseley ME. Correlation of perfusion ‑ and diffusion ‑ weighted MRI with NIHSS score in acute (<6.5 hour) ischemic stroke. Neurology 1998; 50(4): 864 – 870.
23. Neumann‑Haefelin T, Wittsack HJ, Wenserski F, Siebler M, Seitz RJ, Mödder U et al. Diffusion ‑ and perfusion ‑ weighted MRI. The DWI/ PWI mismatch region in acute stroke. Stroke 1999; 30(8): 1591 – 1597.
24. Dávalos A, Blanco M, Pedraza S, Leira R, Castellanos M, Pumar JM et al. The clinical ‑ DWI mismatch: a new diagnostic approach to the brain tissue at risk of infarction. Neurology 2004; 62(12): 2187 – 2192.
25. Prosser J, Butcher K, Allport L, Parsons M, MacGregor L, Desmond P et al. Clinical ‑ diffusion mismatch predicts the putative penumbra with high specificity. Stroke 2005; 36(8): 1700 – 1704.
26. European Stroke Initiative Executive Committee; EUSI Writing Committee. European Stroke Initiative Recommendations for Stroke Management – update 2003. Cerebrovasc Dis 2003; 16(4): 311 – 337.
27. Hacke W, Kaste M, Fieschi C, von Kummer R, Dávalos A, Meier D et al. Randomised double‑blind placebo ‑ controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European ‑ Australasian Acute Stroke Study Investigators. Lancet 1998; 352(9136): 1245 – 1251.
28. DeGraba TJ, Hallenbeck JM, Pettigrew KD, Dutka AJ, Kelly BJ. Progression in acute stroke: value of the initial NIH stroke scale score on patient stratification in future trials. Stroke 1999; 30(6): 1208 – 1212.
29. Lansberg MG, Thijs VN, Hamilton S, Schlaug G, Bammer R, Kemp S et al. Evaluation of the clinical ‑ diffusion and perfusion ‑ diffusion mismatch models in DEFUSE. Stroke 2007; 38(6): 1826 – 1830.
30. Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006; 60(5): 508 – 517.
31. Barber PA, Davis SM, Darby DG, Desmond PM, Gerraty RP, Yang Q et al. Absent middle cerebral artery flow predict the presence and evolution of the ischemic penumbra. Neurology 1999; 52(6): 1125 – 1132.
32. Kane I, Carpenter T, Chappell F, Rivers C, Armitage P, Sandercock P et al. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: effect on lesion size, proportion of patients with diffusion/ perfusion mismatch, clinical scores, and radiologic outcomes. Stroke 2007; 38(12): 3158 – 3164.
33. Ebinger M, Iwanaga T, Prosser JF, De Silva DA, Christensen S, Collins M et al. Clinical ‑ diffusion mismatch and benefit from thrombolysis 3 to 6 hours after acute stroke. Stroke 2009; 40(7): 2572 – 2574.
34. Zaharchuk G, Yamada M, Sasamata M, Jenkins BG, Moskowitz MA, Rosen BR. Is all perfusion ‑ weighted magnetic resonance imaging for stroke equal? The temporal evolution of multiple hemodynamic parameters after focal ischemia in rats correlated with evidence of infarction. J Cereb Blood Flow Metab 2000; 20(9): 1341 – 1351.
35. Barber PA, Demchuk AM, Zhang J, Buchan AM.Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet 2000; 355(9216): 1670 – 1674.
36. Kent DM, Hill MD, Ruthazer R, Coutts SB, Demchuk AM, Dzialowski I et al. ‘‘Clinical ‑ CT mismatch’’ and the response to systemic thrombolytic therapy in acute ischemic stroke. Stroke 2005; 36(8): 1695 – 1699.
37. Messé SR, Kasner, SE, Chalela, JA, Cucchiara B, Demchuk AM, Hill MD et al. CT ‑ NIHSS mismatch does not correlate with MRI diffusion ‑ perfusion mismatch. Stroke 2007; 38(7): 2079 – 2084.
38. Selim M, Fink JN, Kumar S, Caplan LR, Horkan C, Chen Y et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion ‑ weighted lesion volume. Stroke 2002; 33(8): 2047 – 2052.
39. Kim JJ, Fischbein NJ, Lu Y, Pham D, Dillon WP. Regional angiographic grading system for collateral flow: correlation with cerebral infarction in patients with middle cerebral artery occlusion. Stroke 2004; 35(6): 1340 – 1344.
40. Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra ‑ arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 2005; 26(7): 1789 – 1797.
41. Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2008; 79(6): 625 – 629.
42. Kimura K, Minematsu K, Yamaguchi T. Atrial fibrillation as a predictive factor for severe stroke and early death in 15,831 patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2005; 76(5): 679 – 683.
43. Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Dávalos A et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials Safe Implementation of Thrombolysis in Stroke ‑ Monitoring STudy (SITS ‑ MOST). Stroke 2008; 39(12): 3316 – 3322.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2009 Issue 6-
All articles in this issue
- The Correlation of Transcranial Colour‑ Coded Duplex Sonography, CT Angiography and Digital Subtraction Angiography in Patients with Atherosclerotic Disorders of Cerebral Arteries in Common Clinical Practice
- Mental Nerve Neuropathy as a Manifestation of Systemic Malignancy
- Carpal Tunnel Syndrome
- Microdialysis in Neurosurgery
- The Variants of the Catatonia
- Rett Syndrome
- Resection of Insular Gliomas – Volumetric Assessment of Radicality
- Intracranial Hematoma in Patients Receiving Warfarin – Case Reports and Recommended Therapy
- The International Classification of Functioning, Disability and Health (ICF) – Quantitative Measurement of Capacity and Performance
- Is Clinical- Diffusion Mismatch Associated with Good Clinical Outcome in Acute Stroke Patients Treated with Intravenous Thrombolysis?
- Short‑term Effects of Botulinum Toxin A and Serial Casting on Triceps Surae Muscle Length and Equinus Gait in Children with Cerebral Palsy
- Extracranial Schwannoma of the Hypoglossal Nerve – a Case Report
- Recurrent Ischemic Stroke in Systemic Sclerosis – a Case Report
- Cavernous Malformation of the Cauda Equina – a Case Report
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- The Variants of the Catatonia
- Rett Syndrome
- Mental Nerve Neuropathy as a Manifestation of Systemic Malignancy
- Carpal Tunnel Syndrome
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

